Published online Jul 28, 2018. doi: 10.3748/wjg.v24.i28.3120
Peer-review started: April 17, 2018
First decision: June 11, 2018
Revised: June 17, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: July 28, 2018
Processing time: 101 Days and 17.5 Hours
To evaluate the influence of hyperglycemia on the progression of autoimmune pancreatitis.
We induced hyperglycemia by repetitive intraperitoneal (ip) injection of 50 mg/kg streptozotocin in MRL/MpJ mice, which develop autoimmune pancreatitis due to a genetic predisposition. We compared the extent of inflammation (histological score, CD3+ lymphocytes, CD8+ T-cells, CD4+ T-cells, Foxp3+ T-helper cells) in the pancreas of hyperglycemic and normoglycemic mice. We also analyzed the number of leukocytes, lymphocytes, granulocytes and monocytes in the blood. In addition, we determined the percentage of CD3+ lymphocytes, CD8+ T-cells, CD4+ T-cells, Foxp3+ T-helper cells, Foxp3+ CD25+ T-helper and Foxp3- T-helper cells in the spleen by flow cytometry.
Treatment with streptozotocin caused a strong induction of hyperglycemia and a reduction in body weight (P < 0.001). Severe hyperglycemia did not, however, lead to an aggravation, but rather to a slight attenuation of autoimmune pancreatitis. In the pancreas, both the histological score of the pancreas as well as the number of CD3+ lymphocytes (P < 0.053) were decreased by hyperglycemia. No major changes in the percentage of CD8+ T-cells, CD4+ T-cells, Foxp3+ T-helper cells were observed between hyperglycemic and normoglycemic mice. Hyperglycemia increased the numbers of leukocytes (P < 0.001), lymphocytes (P = 0.016), granulocytes and monocytes (P = 0.001) in the blood. Hyperglycemia also moderately reduced the percentage of CD3+ lymphocytes (P = 0.057), significantly increased the percentage of Foxp3+ T-helper cells (P = 0.018) and Foxp3+ CD25+ T-helper cells (P = 0.021) and reduced the percentage of Foxp3- T-helper cells (P = 0.034) in the spleen.
Hyperglycemia does not aggravate but moderately attenuates autoimmune pancreatitis, possibly by increasing the percentage of regulatory T-cells in the spleen.
Core tip: Temporary or sustained hyperglycemia can be observed in about 42%-66% of patients with autoimmune pancreatitis. However, it is unknown to what extent hyperglycemia has an influence on the course of this disease. This preclinical study demonstrates that hyperglycemia does not lead to an aggravation but rather an attenuation of autoimmune pancreatitis. Thus, this result might have the clinical implication that a tight adjustment of blood glucose concentration in patients with autoimmune pancreatitis is not needed, because it might not have a beneficial effect on the progression of this disease.
- Citation: Müller-Graff FT, Fitzner B, Jaster R, Vollmar B, Zechner D. Impact of hyperglycemia on autoimmune pancreatitis and regulatory T-cells. World J Gastroenterol 2018; 24(28): 3120-3129
- URL: https://www.wjgnet.com/1007-9327/full/v24/i28/3120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i28.3120
Autoimmune pancreatitis (AIP) is a chronic and progressive inflammatory disease of the pancreas with an assumed autoimmune etiology, which was first described by Sarles et al[1] in 1961. The disease is classified by two different histopathological patterns called lymphoplasmacytic sclerosing pancreatitis (LPSP; AIP type 1) and idiopathic duct centric pancreatitis (ICDP; AIP type 2). AIP presents as a versatile disease with cholestasis, obstructive jaundice and pancreatic swelling. Besides the lymphoplasmacytic infiltrate, fibrosis is often pronounced, and a strong response to steroids can be seen. Besides these similarities, the two types differ in other characteristics such as serological markers, in particular elevated levels of IgG4 in LPSP, other organ involvement, or disease relapse[2-4]. The differentiation to pancreatic neoplasia is difficult, which sometimes can lead to unnecessary pancreatectomy[5].
The inflammatory infiltrate in the pancreas during AIP is mainly composed of lymphocytes and plasma cells as well as some eosinophil and neutrophil granulocytes and dendritic cells. Frequently, T-cells are observed, in particular CD4+-T-helper cells and CD8+ cytotoxic cells[6]. In addition, regulatory T cells (Tregs) can be found, which are relevant for the immunological self-tolerance by suppressing autoreactive lymphocytes[7]. A specific marker for these cells is the transcription factor forkhead-box-protein P3 (FoxP3), which has a major influence on the development of regulatory T-cells[8,9]. Increased numbers of these cells were found locally in the pancreas as well as in the peripheral blood during AIP[7,10].
A frequent and important complication of AIP is diabetes mellitus (DM). The rate of DM in patients with AIP varies in the literature between 42%-66.7%[11,12]. Of those, 34%-43% already have DM before AIP, 52%-57% show a simultaneous occurrence and 9%-14% develop DM after starting a steroid therapy[13-15]. These findings suggest that both pathologies are present simultaneously in many patients. A correlation between hyperglycemia and pancreatitis in patients is well recognized, which is in general explained by the fact that pancreatitis can trigger DM[16,17]. Investigations into whether DM or transient hyperglycemia can enhance the development of a pancreatitis in return are rare. Previous preclinical studies demonstrate that diabetes dramatically aggravates the course of cerulein-induced acute and chronic pancreatitis[18,19]. Thus, the purpose of this present study was to address the question of whether diabetes can influence the progression of AIP and to analyze which aspects of this disease are affected by diabetes. We chose to use MRL/MpJ mice, an animal model widely used to study AIP. These mice spontaneously develop AIP with an incidence of 74% in female and 36% in male animals at the age of 34-38 wk[20].
All mice were bred in the local SPF facility (the health of the animal stock is routinely checked according to FELASA guidelines) and were exposed to a 12 h artificial light/dark cycle. The mice were caged in groups and were allowed access to water and standard laboratory chow ad libitum. Only female MRL/MpJ mice of the same age (Sham: 210/204-260, STZ: 210/204-224 median/interquartile range in days) were either sham- or streptozotocin-treated (STZ) in the laboratory. Hyperglycemia was induced by intraperitoneal (ip) injection (between 8:00 and 18:00) of 50 mg/kg STZ (Sigma-Aldrich, Steinheim, Germany) on five consecutive days (day 1-5). All control mice were sham-treated with 50 mmol/L sodium citrate pH 4.5 instead of STZ. Animals were allocated in a non-random manner matching the age of both treatment groups. Tissue was harvested on day 113-116 after first STZ injection. For sampling blood and tissue at the indicated time points the animals were anesthetized (by ip injection) with 90 mg/kg ketamine (Bela-Pharm, Vechta, Germany) and 8 mg/kg xylazine (Bayer Health Care, Leverkusen, Germany). The order in which animals were treated and blood and tissue was sampled was arbitrary. All experiments were executed in accordance with the German legislation, the EU-directive 2010/63/EU and approved by the Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern.
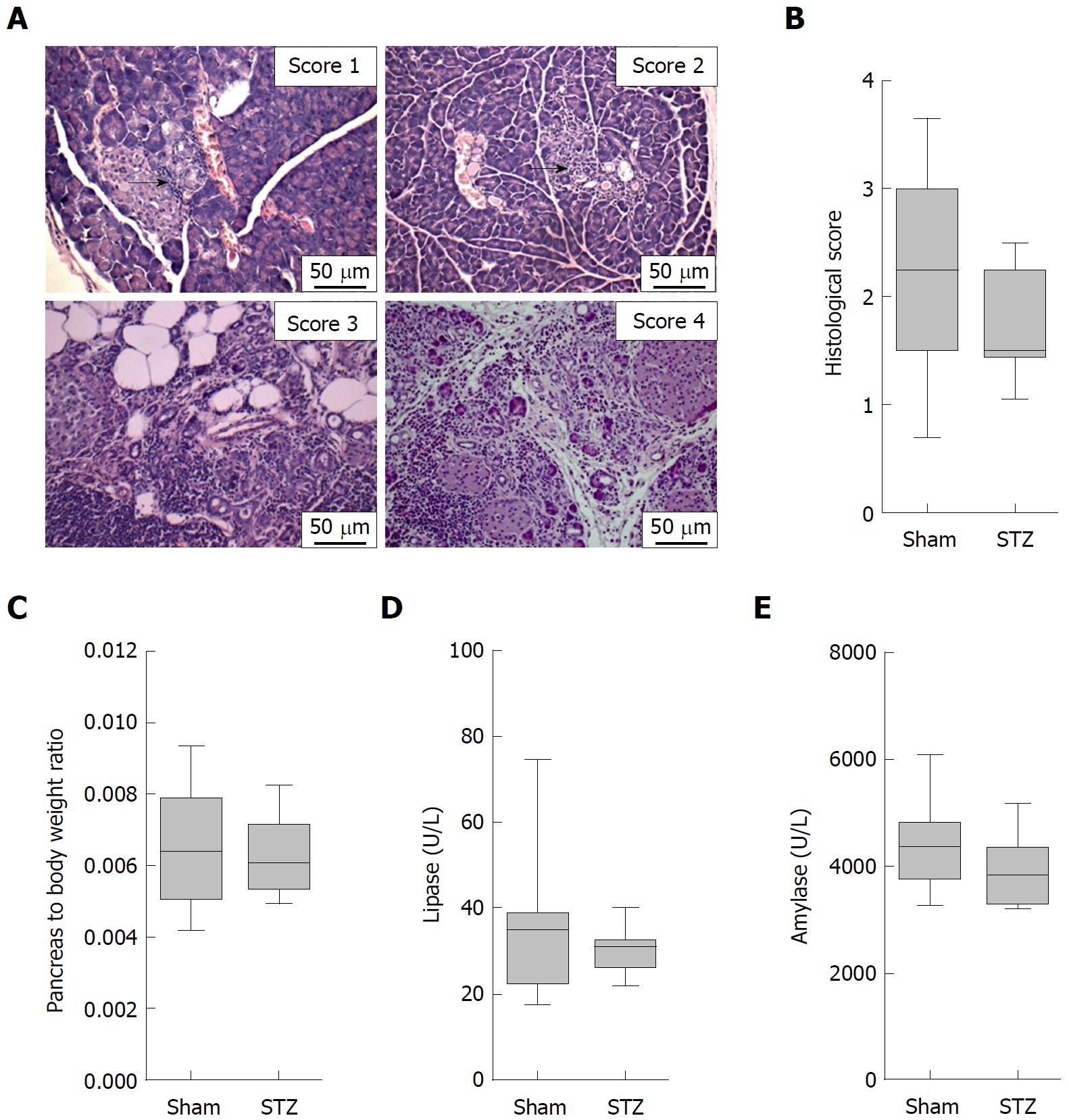
On the day of tissue harvesting, the rostral half of the pancreas was placed lengthwise in a biopsy cassette, fixed in 4% phosphate-buffered formalin, and embedded in paraffin. The second half was embedded in Tissue-Tek OCT Compound (Science Services GmbH, Munich, Germany). Paraffin sections of 4 μm thickness were cut and stained with hematoxylin and eosin to evaluate the organ. The histology of the pancreas was investigated by light microscopy (Olympus Corporation, Tokyo, Japan). The severity of autoimmune pancreatitis was determined by scoring the degree of inflammatory cell infiltration and destruction of the parenchyma as described by Kanno et al[20] (0 = no inflammation, healthy organ; 1 = mild inflammation, mononuclear cells present in the interstitium but no destruction of parenchyma; 2 = moderate inflammation, focal destruction of parenchyma with mononuclear cell infiltration; 3 = moderate and diffuse or severe but focal inflammation, diffuse destruction of parenchyma with residues of intact parenchyma; 4 = severe and diffuse inflammation, extended mononuclear cell infiltrates with destruction of acini and replacement by adipose tissue)[20,21]. The score of two to three sections was averaged to receive a final score by at least two independent reviewers in a blinded manner. In case of discrepancies, a third investigator was consulted for a joint review.
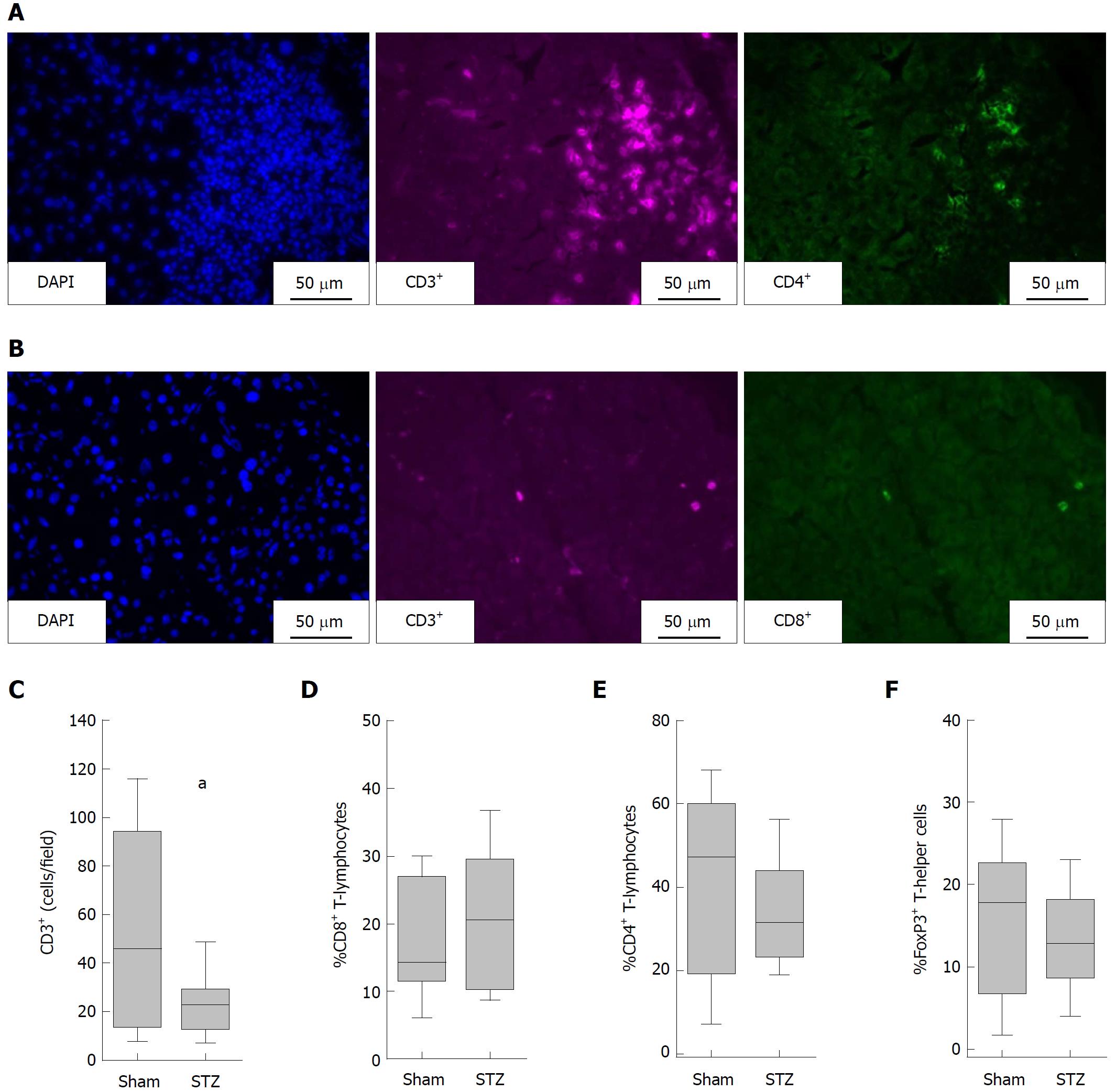
Immunofluorescence was performed using the antibodies eFluor660 conjugated rat anti-CD3 (50-0032; dilution: 600x; eBioscience, Frankfurt, Germany), FITC conjugated rat anti-CD4 (11-0041; dilution: 1200 ×; eBioscience), FITC conjugated rat anti-CD8 (ab25676; dilution: 200 ×; Abcam, Cambridge, United Kingdom) and rabbit anti-FoxP3 (ab54501; dilution: 2100 ×; Abcam) with subsequent secondary antibody Dylight594 goat-anti-rabbit (Dianova, Hamburg, Germany). Immunofluorescence was evaluated on scanned sections with a Leica DMI 4000B scanning microscope using a 40 × objective (Leica Microsystems GmbH, Wetzlar, Germany).
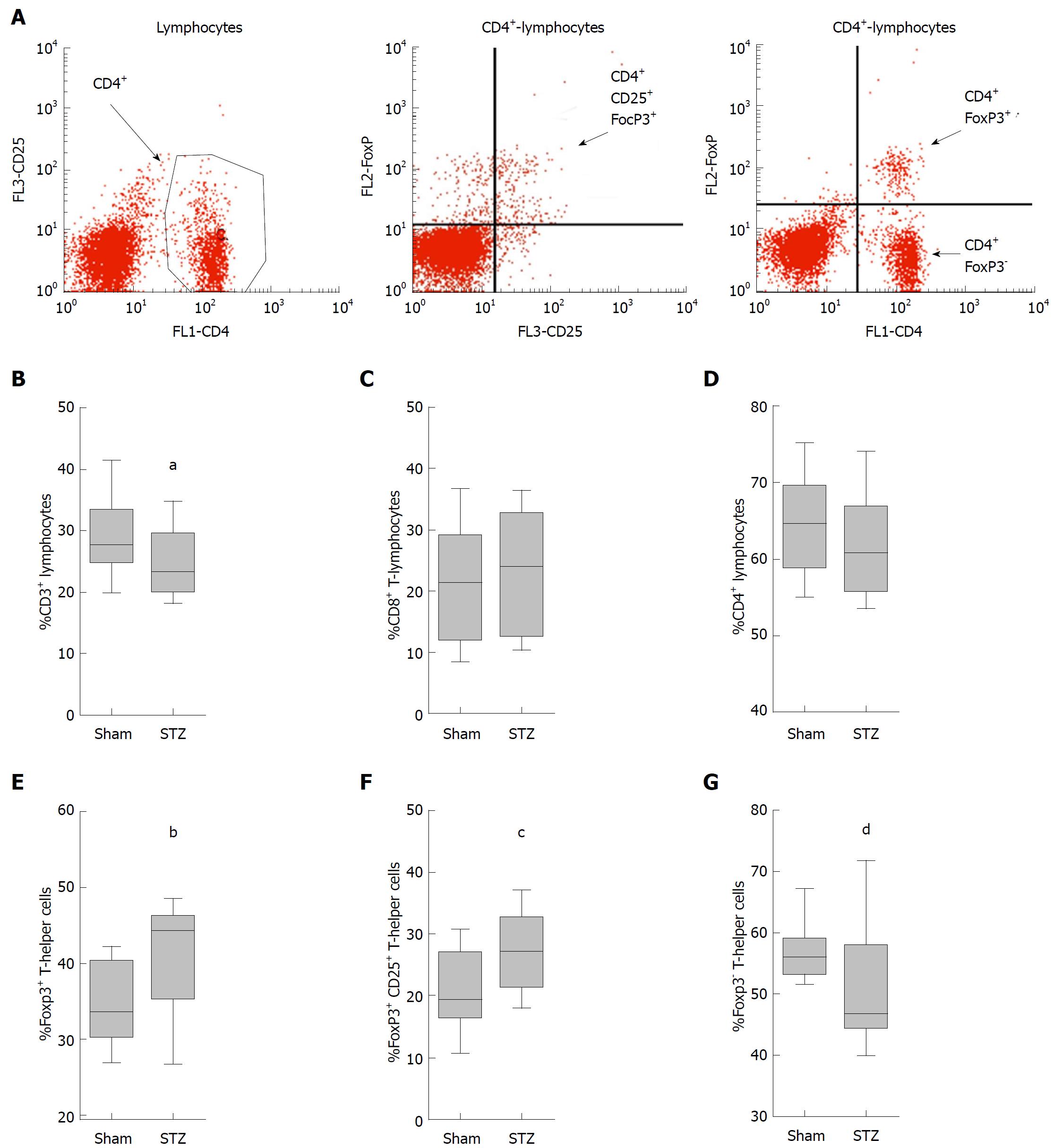
To evaluate subtypes of T-cells, single-cell suspensions were isolated from the spleen of STZ- and sham-treated mice. A total of 1 × 106 leukocytes were stained in a volume of 100 μL PBS pH 7.4 (10010-015, Thermo Fisher Scientific GmbH) containing 0.1% BSA (A9418-10G, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and appropriate antibodies. These antibodies were eFluor660 conjugated rat anti CD3 (50-0032; dilution: 100 ×; eBioscience, Frankfurt, Germany), FITC conjugated rat anti-CD4 (11-0041; dilution, 200 ×; eBioscience), PE conjugated rat anti-CD8a (BD Cat 553032; dilution: 100 ×; BD Bioscience, Heidelberg, Germany) and PE-Cy7 conjugated rat anti-CD25 (25-0251; dilution: 200 ×; eBioscience). Regulatory T-cells were evaluated by staining with FITC conjugated rat anti CD4 and PE conjugated mouse anti-FOXP3 (130-093-014; dilution, 10 ×; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Data were recorded using a BD FACSCalibur and analyzed using the BD CellQuest Pro software (both Becton Dickinson, NJ, United States).
Progression of hyperglycemia was monitored with the blood glucose meter Contour (Bayer Vital, Leverkusen, Germany). A differential blood cell count was performed with the automated hematology analyzer Sysmex KX 21 (Sysmex Cooperation, Kobe, Japan). To assess acinar cell damage, lipase and amylase activity in blood plasma was analyzed using the Cobas c111 spectrophometer (Roche Diagnostics, Mannheim, Germany).
Data are presented as box plots indicating the median, the 25th and 75th percentiles in the form of a box, and the 10th and 90th percentiles as whiskers. Graphs and statistics were made by using SigmaPlot software version 12.0. Three independent replications (always containing animals of both treatment groups) were done for each experiment. The statistical methods of this study were reviewed by Dr. Änne Glass of the Rostock University Medical Center.
The primary outcome was defined at the beginning of the study as number of T-Lymphocytes per field in the pancreas. No sample size calculation was used. Mice that did not develop hyperglycemia after treatment with STZ and mice that died during this long term experiment (an identical number of mice in both treatment groups) were excluded from the analysis. For data acquisition and description, the ARRIVE guidelines were respected. The significance of differences was evaluated using a Mann Whitney rank-sum test. Differences with P ≤ 0.05 were considered to be significant. Differences with P ≤ 0.08 were considered to indicate a tendency.
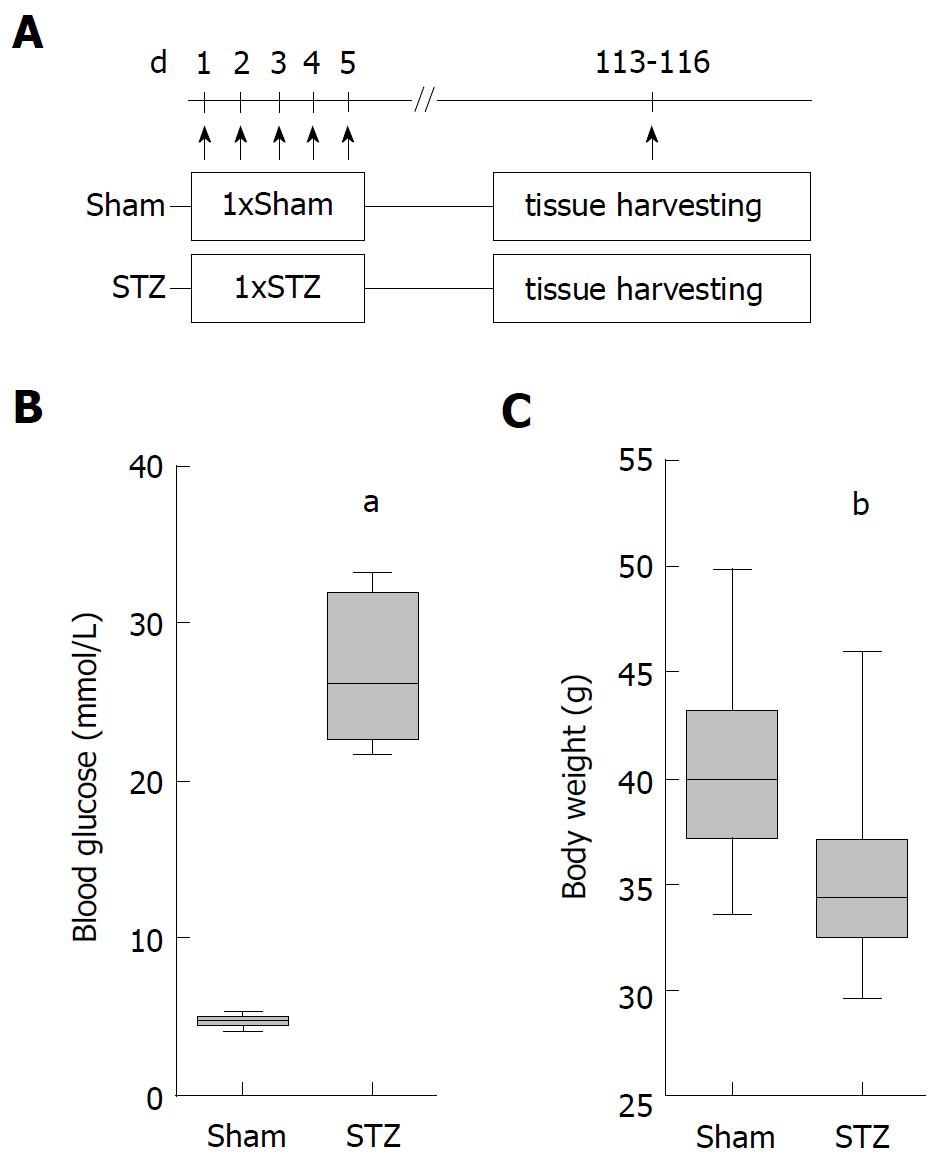
In order to evaluate the influence of hyperglycemia on the course of AIP, we used MRL/MpJ mice due to their spontaneous development of AIP. Distinct cohorts were sham-treated or, in order to induce hyperglycemia, ip injected with STZ for five days and the pancreas was evaluated on day 113-116 (Figure 1A). STZ was effective since it increased the blood glucose concentration on day 22 (Sham: 4.6/4.1-5.5 median/interquartile range in mmol/L. STZ: 22.1/18.2-25.5 median/interquartile range in mmol/L) until the end of the experiment (Figure 1B) and reduced the body weight (Figure 1C). To investigate the severity of AIP, a histological score with numbers from zero to four was used (Figure 2A). Thirteen weeks of hyperglycemia caused a slight decrease in the severity of the histological score and therefore a moderate, non-significant, improvement of AIP when compared to normoglycemic mice (Figure 2B). Little difference was seen in the pancreas to body weight ratio (Figure 2C). Hyperglycemia did not increase the lipase (Figure 2D) or the amylase activity (Figure 2E).
To evaluate the composition of the inflammatory infiltrates in the pancreas, we differentiated between T-lymphocytes (CD3+ cells), T-helper cells (CD3+CD4+cells) and cytotoxic T-cells (CD3+CD8+ cells) by immunofluorescence staining (Figure 3A and B). T-Lymphocytes (defined as number of CD3+ per field) were decreased in diabetic mice compared to non-diabetic mice (Figure 3C). STZ had almost no influence on the percentage of cytotoxic T-cells (CD8+CD3+ cells × 100 divided by the number of CD3+cells) (Figure 3D), the percentage of T-helper cells (CD4+CD3+ cells × 100 divided by the number of CD3+cells) (Figure 3E) and the percentage of regulatory T-cells (FoxP3+CD4+cells × 100 divided by the number of CD4+ cells) (Figure 3F). Thus, no increase, but rather a reduction in the number of CD3+ leukocytes, was observed in the pancreas of hyperglycemic mice.
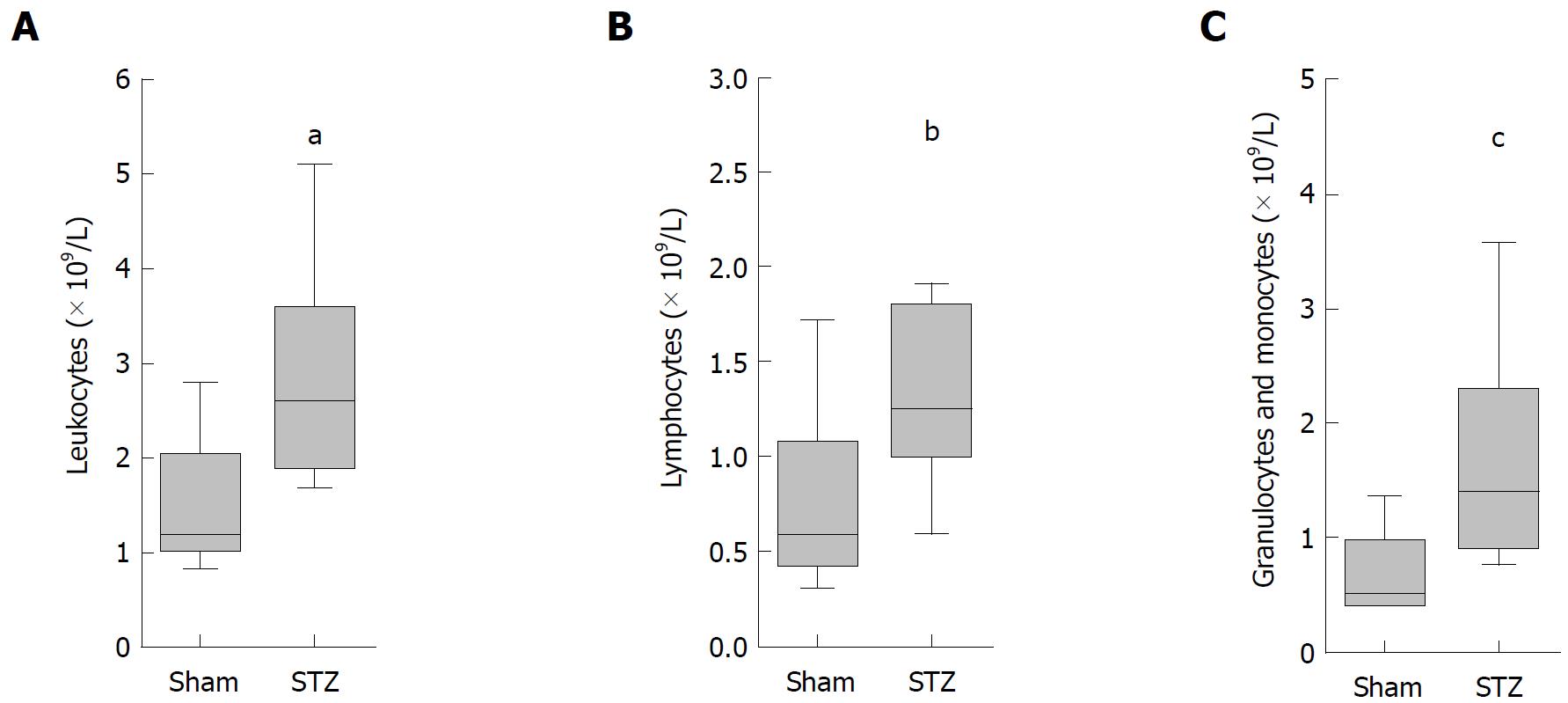
To analyze the systemic effect of hyperglycemia during AIP, we sampled blood on the day of tissue harvesting. A significant increase in the number of leukocytes (Figure 4A), lymphocytes (Figure 4B), as well as granulocytes plus monocytes (Figure 4C), was observed in hyperglycemic mice compared to normoglycemic animals.
Since these major differences in the number of immune cells were seen in the blood, we investigated whether hyperglycemia also had an influence on immune cells in the spleen. For this purpose, we analyzed the splenic cells by fluorescent activated cell sorting, which visualized lymphocytes, their stained subpopulations of T-helper cells (CD3+CD4+ cells) and cytotoxic T-cells (CD3+CD8+ cells) as well as regulatory T-cells (FoxP3+CD4+cells) (data not shown and Figure 5A). The quantification of these cells showed a tendentious decrease in the percentage of T-lymphocytes (defined as number of CD3+ cells × 100 divided by the number of lymphocytes) in mice treated with STZ compared to controls (Figure 5B). STZ also modestly increased the percentage of cytotoxic T-cells (CD8+CD3+ cells × 100 divided by the number of CD3+cells) (Figure 5C), and reduced those of T-helper cells (CD4+CD3+ cells × 100 divided by the number of CD3+cells) (Figure 5D). Hyperglycemia significantly increased the percentage of regulatory T-cells (FoxP3+CD4+cells × 100 divided by the number of CD4+ cells) (Figure 5E). Interestingly, hyperglycemia also significantly increased the percentage of CD25 expressing regulatory T-cells (FoxP3+ CD25+ CD4+ cells v 100 divided by the number of CD4+cells) (Figure 5F) compared to normoglycemic mice, whereas the percentage of FoxP3- T-cells (FoxP3-CD4+cells × 100 divided by the number of CD4+ cells) was significantly reduced in STZ-treated mice (Figure 5G). Thus, a major influence of hyperglycemia on regulatory T-cells was observed in the spleen.
In order to test the influence of hyperglycemia on the course of AIP, we compared the progression of AIP in diabetic and non-diabetic mice. Surprisingly, experimental hyperglycemia didn’t cause an aggravation of AIP in MRL/MpJ mice, but moderately improved autoimmune pancreatitis (Figures 2B and 3C). This is in contrast to our previous studies, where hyperglycemia lead to a major aggravation of cerulein-induced acute and chronic pancreatitis[18,19]. This might be explained by the differences in the pathogenesis of these two distinct types of pancreatitis. It is currently controversial whether cerulein-induced pancreatitis is mediated by premature activation of trypsinogen or by persistent activation of the NF-κB pathway[22,23]. In contrast, AIP is suggested to be caused by an autoimmunological event, which leads to inflammatory infiltration of the organ[1-4]. A beneficial effect of hyperglycemia on autoimmune pancreatitis seems to be counterintuitive, since hyperglycemia has been demonstrated to have a deleterious effect on many organs. However, hyperglycemia can also have an inhibitory effect on the immune system. One could speculate that this impact on the immune system may have a beneficial effect on the progression of some autoimmune diseases.
Our study has several limitations. For example, we cannot completely exclude that instead of high glucose concentration in the blood, the drug we used for inducing hyperglycemia (STZ) influences AIP directly. However, in our opinion it is very unlikely that the alkylating agent STZ, which has been demonstrated to be cytotoxic to cells, directly cures AIP. We also limited our study to evaluate the effect of sustained hyperglycemia on AIP and can therefore not conclude if a transient hyperglycemia also has beneficial effects on the progression of AIP. Another limitation of this study is the use of a histological score, which was defined by the ordinal numbers 0 to 4. Comparing the median of the histological score allows, therefore, only a restricted presentation of complex changes in the histology of the pancreas[20]. We observed that the median of this score was reduced from 2.25 in normoglycemic to 1.50 in hyperglycemic mice (Figure 2B). However, this simple comparison underestimates the observed changes. The same data can also be presented as the percentage of mice with a histological score of ≥ 3. Under normoglycemic conditions, 42% of mice (8 from 19 mice) received a histological score of ≥ 3. Under hyperglycemic conditions, only 6% (1 from 17 mice) received a score of ≥ 3. These high scores were assigned when moderate diffuse or severe focal inflammation plus a diffuse destruction of the acini (score 3) or severe and diffuse inflammation plus extended destruction of acini (score 4) was observed[20,21]. This impressive difference in the percentage of mice with a high histological score suggests that hyperglycemia reduces the damage to the parenchyma, which might indirectly reduce local inflammation. Alternatively, this observation could also suggest that hyperglycemia reduces inflammation and thus minimizes the damage to the parenchyma. Due to the following observations, we prefer the second option. We found increased numbers of regulatory T-cells and decreased FoxP3- T-cells in the spleen. This is consistent with data in another preclinical study, where it has been described, that decreased numbers of regulatory T-cells and increased numbers of FoxP3- T-cells in the spleen lead to an increased severity of AIP[24]. In both studies, the numbers of regulatory T-cells and FoxP3- T-cells in the spleen correlate with the severity of AIP. Indeed, one study demonstrated a suppressive effect of transferred Tregs on autoimmune pancreatitis in MRL/MpJ mice[25]. However, the observed reduction in the histological score was not significant[25].
We observed major differences between hyperglycemic and normoglycemic mice when analyzing immune cells in the blood and the spleen in MRL/MpJ mice. We noticed (1) increased percentage of regulatory T-cells and (2) decreased percentage of FoxP3- T-cells in the spleen, as well as an (3) increased number of leukocytes in the blood of STZ-treated mice (Figures 4A-C and 5E-G). These findings suggest that hyperglycemia might rather influence the overall immune system and only indirectly affect AIP itself. This interpretation is consistent with preclinical studies, which analyzed the influence of hyperglycemia on regulatory T-cells in mice not suffering from AIP. In these animals, hyperglycemia also increased the number of CD4+CD25+Foxp3+ regulatory T-cells in lymph nodes and the spleen of mice[26,27].
For treating AIP in the clinic, guidelines recommend to adjust blood glucose concentration before starting a steroid therapy[28,29]. This is mainly based on publications suggesting that a preexisting DM before the onset of AIP is worsening in 75% of patients after the start of a steroid therapy[28,29]. However, our preclinical study shows that hyperglycemia does not have a negative influence on AIP. Critical attitudes towards adjusting the blood glucose concentration in patients suffering from AIP have been previously published. For example, in one third of all cases, DM even worsened after insulin therapy[15]. Moreover, it was reported that treatment of diabetes with insulin led to hypoglycemic attacks in 10 from 50 AIP patients[13]. In addition, several clinical studies could demonstrate that DM improved in many cases immediately after steroid therapy[15,30]. These clinical studies argue against a tight control of blood glucose in AIP patients. Although our study has the limitation to be only a preclinical study on mice, it also suggests that an aggressive adjustment of blood glucose concentration might not be necessary as a prerequisite for the treatment of AIP. For final clarification of this issue, a clinical study evaluating if a tight adjustment of blood glucose in addition to steroid therapy is beneficial or harmful to patients with AIP might need to be pursued.
In about 42%-66% of patients with autoimmune pancreatitis, hyperglycemia can be observed. Thus, hyperglycemia is a frequent and important complication of this disease. However, it is not known if it merely reflects the severity of pancreatitis or whether it can also influence the progression of autoimmune pancreatitis.
For treating autoimmune pancreatitis in the clinic, guidelines recommend to adjust blood glucose concentration before starting a steroid therapy. However, critical attitudes towards adjusting the blood glucose concentration in these patients have also been published. For example, in one third of all cases, hyperglycemia even worsened after insulin therapy, and treatment of diabetes with insulin sometimes leads to hypoglycemic attacks in patients. In order to decide how important a tight adjustment of blood glucose concentration in patients with autoimmune pancreatitis is, we need to know if hyperglycemia has a positive or negative influence on the progression of this disease.
The purpose of this present study was to address the question of whether diabetes can influence the progression of autoimmune pancreatitis and to analyze which aspects of this disease are affected by hyperglycemia.
We chose to use MRL/MpJ mice, an animal model widely used to study autoimmune pancreatitis. These mice spontaneously develop autoimmune pancreatitis. We induced hyperglycemia by repetitive intraperitoneal (ip) injection of streptozotocin in female mice and compared the extent of inflammation in the pancreas of hyperglycemic and normoglycemic animals. We also analyzed the number of immune cells in the blood. In addition, we determined the percentage of T-cells, especially regulatory T-cells in the spleen.
Surprisingly, experimental hyperglycemia did not cause an aggravation of autoimmune pancreatitis, but moderately improved autoimmune pancreatitis. We noticed an increased percentage of regulatory T-cells in the spleen, as well as an increased number of leukocytes in the blood of hyperglycemic mice. These findings suggest that hyperglycemia might rather influence the overall immune system and only indirectly affect autoimmune pancreatitis itself.
Our preclinical study on mice supports the idea that an aggressive adjustment of blood glucose concentration might not be necessary as a prerequisite for the treatment of patients with autoimmune pancreatitis.
A clinical study that evaluates whether a tight adjustment of blood glucose is beneficial or harmful to patients should be pursued.
We thank Berit Blendow, Dorothea Frenz, Maren Nerowski and Eva Lorbeer-Rehfeldt, (Institute for Experimental Surgery, Rostock University Medical Center) for excellent technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Friedel D, Vagholkar KR, Zhao JB S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | SARLES H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 496] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1058] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 4. | Omiyale AO. Autoimmune pancreatitis. Gland Surg. 2016;5:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Kleeff J, Welsch T, Esposito I, Löhr M, Singer R, Büchler MW, Friess H. Autoimmune pancreatitis--a surgical disease? Chirurg. 2006;77:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, Lankisch P, Stolte M, Lüttges J, Kremer B. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 210] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Miyoshi H, Uchida K, Taniguchi T, Yazumi S, Matsushita M, Takaoka M, Okazaki K. Circulating naïve and CD4+CD25high regulatory T cells in patients with autoimmune pancreatitis. Pancreas. 2008;36:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6389] [Article Influence: 290.4] [Reference Citation Analysis (0)] |
| 9. | Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 10. | Fukumura Y, Takase M, Mitani K, Suda K, Imamhasan A, Nobukawa B, Ueda A, Abe H, Yao T. Amount of CD4+CD25+ regulatory T cells in autoimmune pancreatitis and pilonidal sinus. Pancreas. 2012;41:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ito T, Nishimori I, Inoue N, Kawabe K, Gibo J, Arita Y, Okazaki K, Takayanagi R, Otsuki M. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol. 2007;42 Suppl 18:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kamisawa T, Egawa N, Inokuma S, Tsuruta K, Okamoto A, Kamata N, Nakamura T, Matsukawa M. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas. 2003;27:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ito T, Nakamura T, Fujimori N, Niina Y, Igarashi H, Oono T, Uchida M, Kawabe K, Takayanagi R, Nishimori I. Characteristics of pancreatic diabetes in patients with autoimmune pancreatitis. J Dig Dis. 2011;12:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Miyamoto Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Inaba Y, Kuwata G, Fujiwara T, Egashira H. Short and long-term outcomes of diabetes mellitus in patients with autoimmune pancreatitis after steroid therapy. Gut Liver. 2012;6:501-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Nishimori I, Tamakoshi A, Kawa S, Tanaka S, Takeuchi K, Kamisawa T, Saisho H, Hirano K, Okamura K, Yanagawa N. Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas. 2006;32:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Angelopoulos N, Dervenis C, Goula A, Rombopoulos G, Livadas S, Kaltsas D, Kaltzidou V, Tolis G. Endocrine pancreatic insufficiency in chronic pancreatitis. Pancreatology. 2005;5:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Czakó L, Hegyi P, Rakonczay Z Jr, Wittmann T, Otsuki M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology. 2009;9:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Zechner D, Spitzner M, Bobrowski A, Knapp N, Kuhla A, Vollmar B. Diabetes aggravates acute pancreatitis and inhibits pancreas regeneration in mice. Diabetologia. 2012;55:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Zechner D, Knapp N, Bobrowski A, Radecke T, Genz B, Vollmar B. Diabetes increases pancreatic fibrosis during chronic inflammation. Exp Biol Med (Maywood). 2014;239:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Kanno H, Nose M, Itoh J, Taniguchi Y, Kyogoku M. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol. 1992;89:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Fitzner B, Holzhueter SA, Ibrahim S, Nizze H, Jaster R. Interferon-gamma treatment accelerates and aggravates autoimmune pancreatitis in the MRL/Mp-mouse. Pancreatology. 2009;9:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Frossard JL, Pastor CM. Experimental acute pancreatitis: new insights into the pathophysiology. Front Biosci. 2002;7:d275-d287. [PubMed] |
| 23. | Sah RP, Dudeja V, Dawra RK, Saluja AK. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144:1076-1085.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Schwaiger T, van den Brandt C, Fitzner B, Zaatreh S, Kraatz F, Dummer A, Nizze H, Evert M, Bröker BM, Brunner-Weinzierl MC. Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut. 2014;63:494-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Ehlers L, Rohde S, Ibrahim S, Jaster R. Adoptive transfer of CD3+ T cells and CD4+ CD44 high memory T cells induces autoimmune pancreatitis in MRL/MpJ mice. J Cell Mol Med. 2018;22:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kaminitz A, Mizrahi K, Askenasy N. Surge in regulatory T cells does not prevent onset of hyperglycemia in NOD mice: immune profiles do not correlate with disease severity. Autoimmunity. 2014;47:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Zhen Y, Sun L, Liu H, Duan K, Zeng C, Zhang L, Jin D, Peng J, Ding W, Zhao Y. Alterations of peripheral CD4+CD25+Foxp3+ T regulatory cells in mice with STZ-induced diabetes. Cell Mol Immunol. 2012;9:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Kamisawa T, Okazaki K, Kawa S, Ito T, Inui K, Irie H, Nishino T, Notohara K, Nishimori I, Tanaka S. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 30. | Farris AB 3rd, Lauwers GY, Deshpande V. Autoimmune pancreatitis-related diabetes: quantitative analysis of endocrine islet cells and inflammatory infiltrate. Virchows Arch. 2010;457:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |