Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2915
Peer-review started: April 13, 2018
First decision: May 21, 2018
Revised: May 22, 2018
Accepted: June 9, 2018
Article in press: June 9, 2018
Published online: July 14, 2018
Processing time: 90 Days and 23.2 Hours
A depressed lesion was found at a gastric angle of 76-year-old Japanese woman by esophagogastroduodenoscopy. Four years prior, she was diagnosed with a Helicobacter pylori infection but no eradication was performed. The pathological diagnosis of biopsy specimens was signet-ring cell carcinoma. Endoscopic submucosal dissection (ESD) was performed. Histopathological examination of the ESD specimen revealed proliferation of well-differentiated tubular adenocarcinoma mimicking fundic gland cells at the deep layer of the lamina propria mucosae. These tumor cells expressed focally pepsinogen-I, diffusely MUC6, and scattered H+/K+ ATPase according to immunohistochemistry. Therefore, we diagnosed this tumor as gastric adenocarcinoma of fundic gland type (GA-FG). Adjacent to the GA-FG, proliferation of signet-ring cell carcinoma which diffusely expressed MUC 2 and MUC 5AC was observed. Intestinal metaplasia was focally observed in the surrounding mucosa of the signet-ring cell carcinoma. To the best of our knowledge, this is the first case report of GA-FG with a signet-ring cell carcinoma component. The origin of signet-ring cell carcinoma, i.e., whether it accidentally arose from a non-neoplastic mucosa and coexisted with the GA-FG or dedifferentiated from the GA-FG is unclear at present. We expect the accumulation of similar cases and further analysis to clarify this issue.
Core tip: Gastric adenocarcinoma of fundic gland type is a very rare variant of a well-differentiated gastric adenocarcinoma. To the best of our knowledge, this is the first case report of gastric adenocarcinoma of fundic gland type with a signet-ring cell carcinoma component.
- Citation: Kai K, Satake M, Tokunaga O. Gastric adenocarcinoma of fundic gland type with signet-ring cell carcinoma component: A case report and review of the literature. World J Gastroenterol 2018; 24(26): 2915-2920
- URL: https://www.wjgnet.com/1007-9327/full/v24/i26/2915.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i26.2915
Gastric adenocarcinoma showing chief cell differentiation was initially reported by Tsukamoto et al[1] in 2007. In 2010, Ueyama et al[2] reported 10 cases of gastric adenocarcinoma showing chief cell differentiation which expressed pepsinogen-I (a marker for chief cells) and proposed the concept of gastric adenocarcinoma of fundic gland type (GA-FG). Since then, the concept of GA-FG has been widely recognized, and reported cases and studies have been gradually accumulated.
Because GA-FG is thought to originate from the gastric mucosa of the fundic gland region without chronic gastritis or intestinal metaplasia, it has been generally considered that GA-FG develops without Helicobacter pylori (H. pylori) infection[3]. However, cases of GA-FG with current H. pylori infection or post-eradiation therapy were recently reported[4,5]. GA-FG generally presents as a well-differentiated adenocarcinoma with mild nuclear atypia and is generally considered to have a low potential for malignancy, although an extremely rare case of advanced GA-FG showing high-grade malignancy was reported[6]. To the best of our knowledge, no GA-FG case with a poorly differentiated adenocarcinoma or signet-ring cell carcinoma component has been reported.
We recently encountered a case of GA-FG with a signet-ring carcinoma component which developed in a patient with current H. pylori infection, and we report the case as follows.
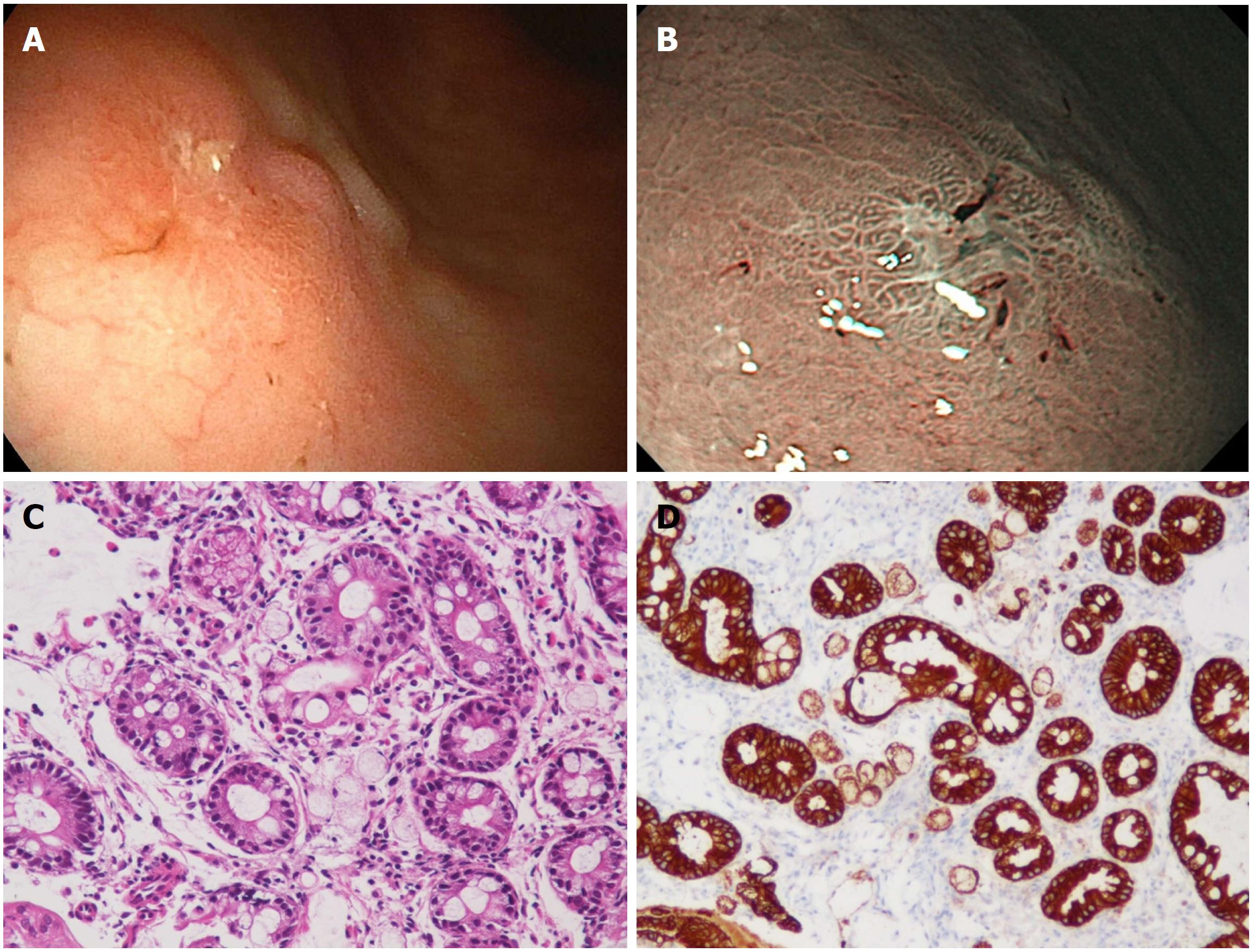
A 76-year-old Japanese woman visited a nearby clinic complaining of a dull feeling in the stomach. Esophagogastroduodenoscopy (EGD) revealed a depressed lesion at a gastric angle of the greater curvature side. She was referred to our hospital for further examination. She had been found to have an H. pylori infection by a urease test four years ago, but no eradication was performed. The depressed lesion was confirmed by an EGD performed at Koga Hospital 21 (Figure 1A) and narrow band imaging (Figure 1B) showed a relatively demarcated lesion with an irregular microsurface pattern. A biopsy of the depressed lesion was performed. Histologically, the biopsy specimens consisted of several fragments of gastric mucosa with intestinal metaplasia. Among the glands with intestinal metaplasia, a small number of atypical cells showing a signet-ring-cell-like appearance were found (Figure 1C). As these atypical cells were positive for immunohistochemistry of pan-cytokeratin (AE1/AE3), a pathological diagnosis of signet-ring cell carcinoma was made (Figure 1D). Endoscopic submucosal dissection (ESD) was performed.
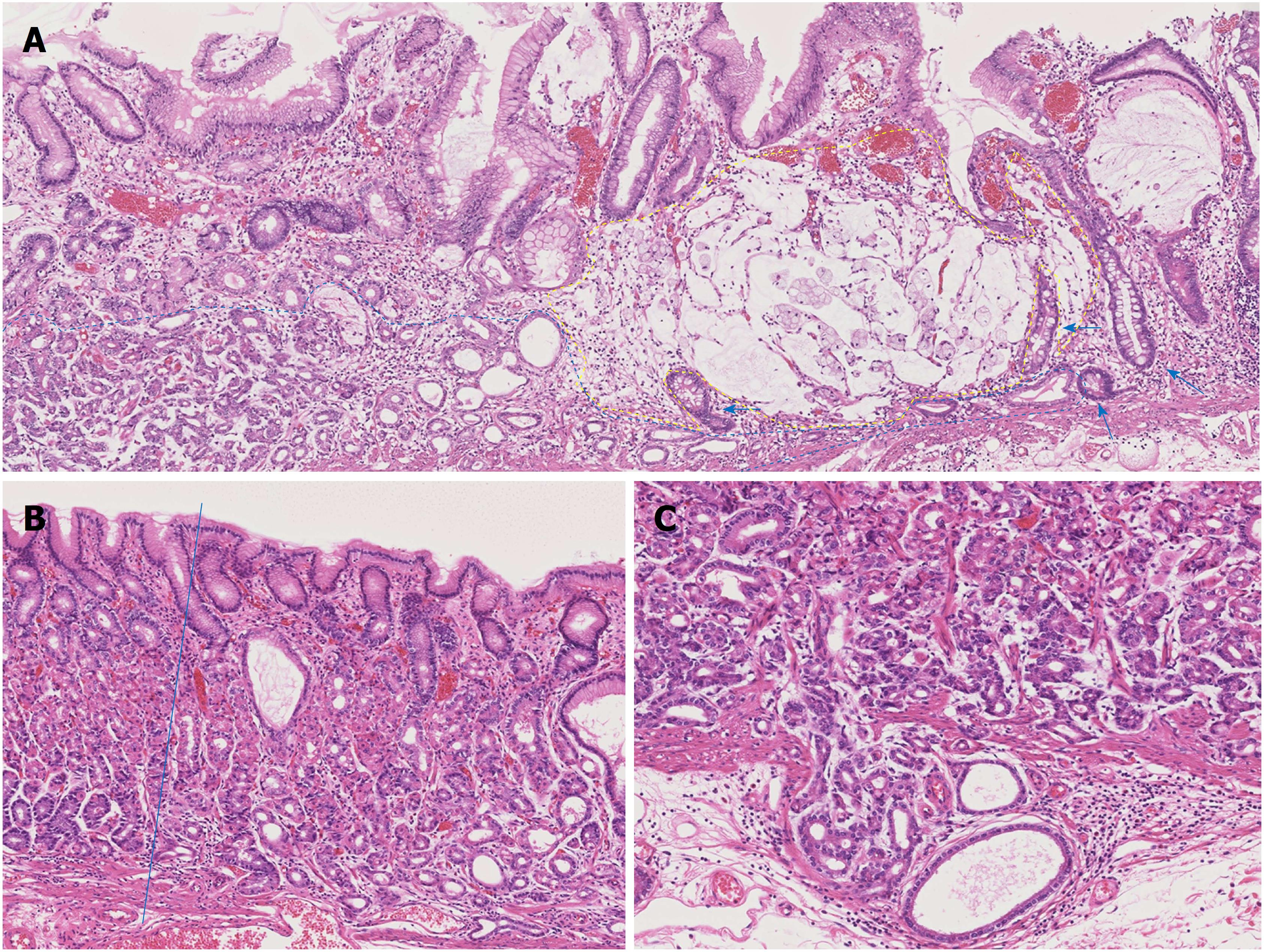
The ESD specimen showed a slightly depressed lesion measuring 28 mm × 14 mm. In that lesion, a deeper depressed lesion measuring 12 mm × 3 mm was found. Histologically, a well-differentiated tubular adenocarcinoma mimicking the fundic gland cells, mainly the chief cells, proliferated at the deep layer of the lamina propria mucosae (Figure 2A). The tumor cells had slightly enlarged nuclei and showed mild nuclear atypia. The structure and differentiation toward the surfaces of the fundic gland were significantly disturbed compared to normal fundic glands (Figure 2B). The tumor had invaded into the submucosal layer, and the maximum depth of invasion was 400 μm (Figure 2C). No lymphatic or venous invasion was observed. The mucosal surface was covered with non-neoplastic foveolar epithelium.
Adjacent to the well-differentiated tubular adenocarcinoma mimicking fundic gland cells, proliferation of a signet-ring cell carcinoma producing intra- and extracellular mucin was observed (Figure 2A). Proliferation of the signet-ring cell carcinoma was restricted within the lamina propria mucosae, and no lymphatic or venous invasion was observed. Focally, intestinal metaplasia was observed at the mucosa surrounding the signet-ring cell carcinoma (Figure 2A).
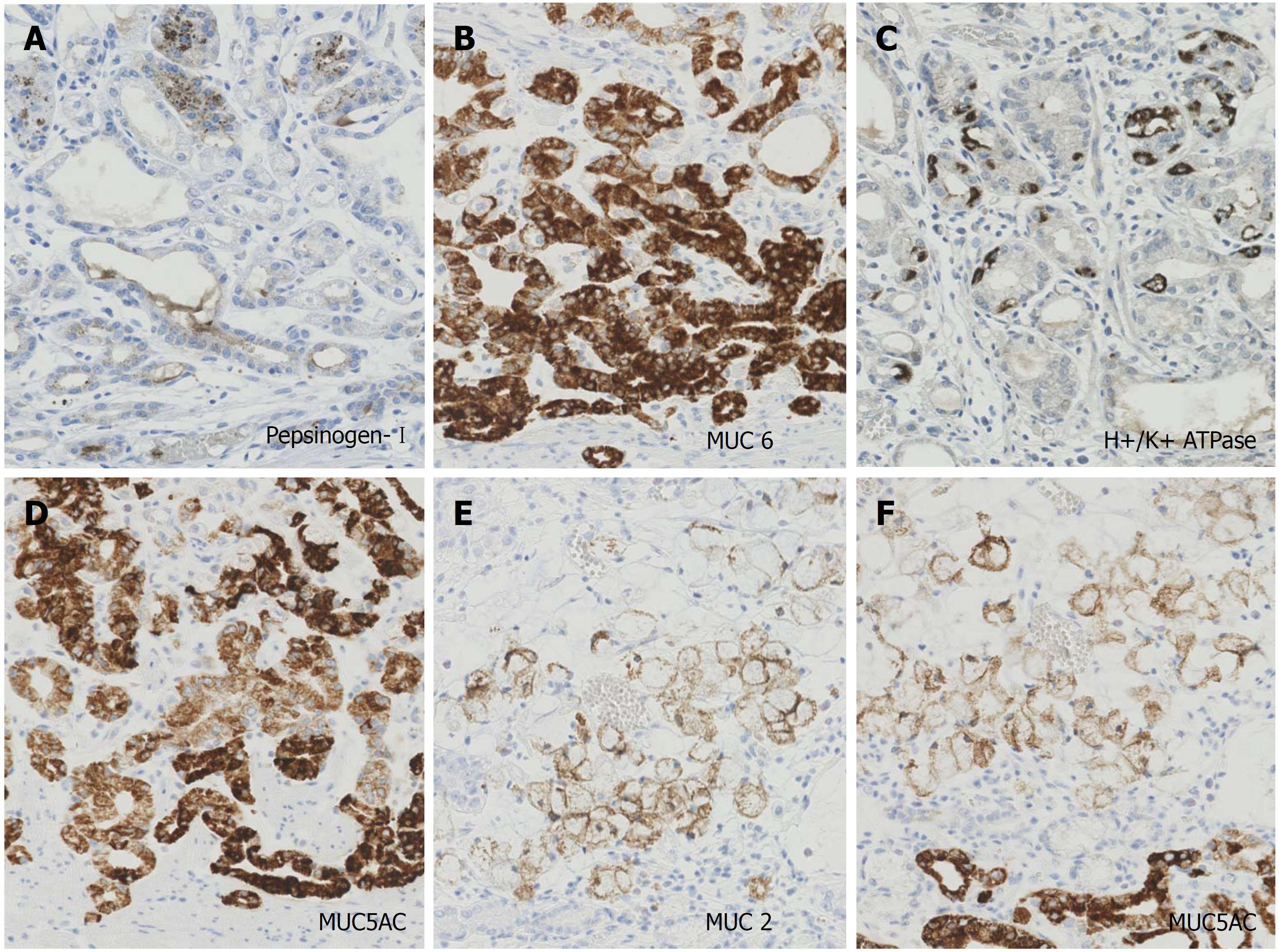
In immunohistochemistry, the tumor cells of well-differentiated tubular adenocarcinoma expressed focally (30%) pepsinogen-I (Figure 3A), diffusely MUC6 (Figure 3B) and scattered (5%) H+/K+ ATPase (Figure 3C). Therefore, we diagnosed this tumor as GA-FG. The tumor cells of GA-FG were negative for MUC 2 but diffusely positive for MUC 5AC (Figure 3D). Meanwhile, the tumor cells of the signet-ring cell carcinoma were diffusely positive for MUC 2 (Figure 3E) and MUC 5AC (Figure 3F) but negative for pepsinogen-I, MUC6, and H+/K+ ATPase. The immunohistochemistry results are summarized in Table 1.
| MUC 6 | H+/K+ ATPase | Pepsinogen-I | MUC5AC | MUC2 | |
| Gastric adenocarcinoma of fundic gland type | + (Diffuse) | + (Scattered, 5%) | + (Focal, 30%) | + (Diffuse) | - |
| Signet-ring cell carcinoma | - | - | - | + (Diffuse) | + (Diffuse) |
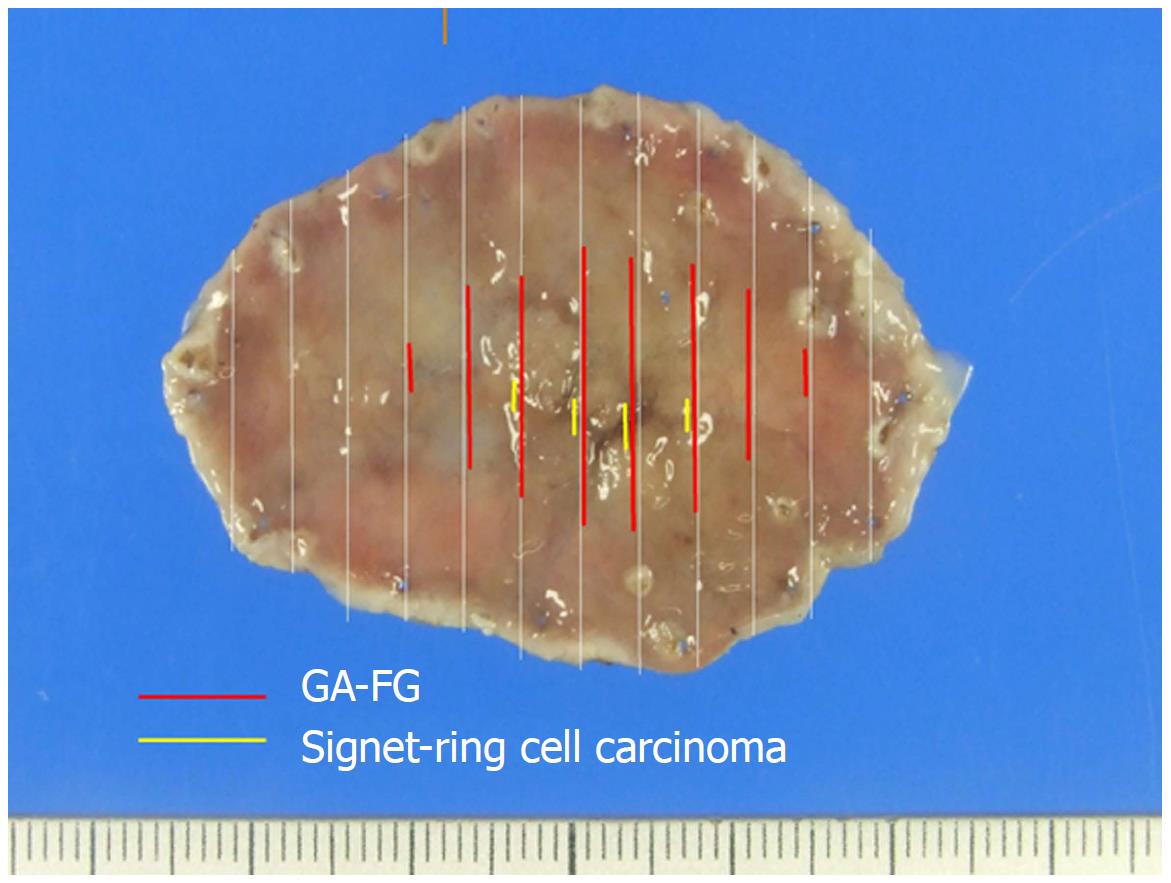
Based on these HE and immunohistochemical findings, we made the final diagnosis of GA-FG with a signet-ring cell carcinoma component. The mapping based on histology revealed that GA-FG was distributed at a slightly depressed lesion (28 mm × 14 mm) and the signet-ring cell carcinoma was distributed at a deeper depressed lesion (12 mm × 3 mm) in the slightly depressed lesion (Figure 4).
GA-FG is a very rare variant of a well-differentiated gastric adenocarcinoma accounting for 1.6% of gastric adenocarcinomas[7]. GA-FGs are characterized by the following: (1) They arise most commonly from the normal gastric mucosa of the fundic gland region without intestinal metaplasia; (2) they are recognized as smooth elevated or depressed lesions; (3) they often invade the submucosal layer, while lymphatic and venous invasion are rare; and (4) the atypia of the tumor cell is usually mild[8]. The Wnt/β-catenin signal signaling pathway and GNAS mutations are considered to contribute to the development and progression of GA-FG[7,9,10].
Immunohistochemically, GA-FG variably express the following biomarkers of fundic gland cells: MUC6 for mucous neck cells; H+/K+ ATPase for parietal cells; and pepsinogen-I for chief cells. Typical cases diffusely express pepsinogen-I and MUC6 and show scattered positivity for H+/K+ ATPase. These cases are referred to as GA-FG of the chief cell predominant type[2]. GA-FGs do not express the intestinal-type mucin of MUC2.
The distinctive feature of present case was the coexistence of the signet-ring cell carcinoma and GA-FG. To the best of our knowledge, no GA-FG case which contains signet-ring cell carcinoma has been reported. The signet-ring cell carcinoma component in our case expressed the intestinal type of MUC2, and intestinal metaplasia was focally observed in the background mucosa. In addition, the present case had a current H. pylori infection. These are unusual findings for GA-FG.
The origin of the signet-ring cell carcinoma is a very interesting subject. We propose two hypotheses regarding this issue. First, these two lesions (GA-FG and the signet-ring cell carcinoma) may have accidentally coexisted. Usually, GA-FGs develop at the fundic gland in a deep layer of the gastric mucosa, and the normal foveolar epithelium remains at the surface. In the present case, intestinal metaplasia due to chronic inflammation caused by the H. pylori infection was focally observed at the surface of the mucosa. Therefore, it seems reasonable that the signet-ring cell carcinoma producing intestinal-type mucin developed at the surface of the mucosa from the intestinal metaplasia and that GA-FG simultaneously developed from the fundic gland of the deep layer of the mucosa. However, the probability for this situation to occur is considered extremely low.
The second hypothesis is that a part of the GA-FG dedifferentiated into signet-ring cell carcinoma. Although dedifferentiation or transformation is often observed in various types of malignant tumors, no GA-FG case showing dedifferentiation or transformation has been reported. Usually, GA-FGs do not express MUC5AC, which is a marker of the foveolar epithelium; however, it is known that GA-FGs rarely express MUC5AC[2,8,11]. In the present case, both the GA-FG and signet-ring cell carcinoma expressed MUC5AC. Ueyama et al[2] speculated that MUC5AC is only expressed in advanced GA-FG lesions with a large diameter and massive submucosal invasion, suggesting that cell differentiation changes from the fundic gland type to the foveolar type during disease progression. This speculation regarding MUC5AC seems to support the potential for the transformation of GA-FG. However, we believe it is impossible to conclusively determine the origin of the signet-ring cell carcinoma in the present case because of a lack of reliable evidence.
In conclusion, we have reported the first case of GA-FG with a signet-ring cell carcinoma component which expressed an intestinal type of mucin. Our case had a current H. pylori infection and showed focal intestinal metaplasia in the background mucosa. The origin of the signet-ring cell carcinoma is unclear at present. We expect the accumulation of the similar cases and further analysis of whether dedifferentiation or transformation can really occur in GA-FG.
This case was presented and discussed at the 359th Kyushu-Okinawa slide conference. We thank the conference participants for their valuable comments and discussion. We are also grateful to the special commentators at that conference, Takashi Yao (Department of Human Pathology, Juntendo University School of Medicine) and Kazuya Akahoshi (Department of Gastroenterology, Aso Iizuka Hospital), for their valuable comments regarding the present case.
A 76-year-old Japanese woman visited a nearby clinic complaining of a dull feeling in the stomach.
Esophagogastroduodenoscopy (EGD) revealed a depressed lesion at a gastric angle of the greater curvature side.
The clinical diagnosis of early gastric cancer was considered by EGD findings.
No specific finding was obtained by laboratory testing.
The narrow band imaging of EGD showed a relatively demarcated lesion with an irregular microsurface pattern.
Pathological findings of endoscopic submucosal dissection (ESD) specimens indicated the diagnosis of gastric adenocarcinoma of fundic gland type (GA-FG) with a signet-ring cell carcinoma component.
Only ESD was performed for treatment.
To the best of our knowledge, no GA-FG case with a poorly differentiated adenocarcinoma or signet-ring cell carcinoma component has been reported.
The term GA-FG describes gastric adenocarcinoma of fundic gland type.
This is the first case report of GA-FG with a signet-ring cell carcinoma component.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abadi AT, Peixoto A S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 2. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Chiba T, Kato K, Masuda T, Ohara S, Iwama N, Shimada T, Shibuya D. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016;28:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Manabe S, Mukaisho KI, Yasuoka T, Usui F, Matsuyama T, Hirata I, Boku Y, Takahashi S. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol. 2017;23:7047-7053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc. 2014;26:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Hidaka Y, Mitomi H, Saito T, Takahashi M, Lee SY, Matsumoto K, Yao T, Watanabe S. Alteration in the Wnt/β-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum Pathol. 2013;44:2438-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of fundic gland type: Five cases treated with endoscopic resection. World J Gastroenterol. 2015;21:8208-8214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Murakami T, Mitomi H, Yao T, Saito T, Shibuya T, Watanabe S. Epigenetic regulation of Wnt/β-catenin signal-associated genes in gastric neoplasia of the fundic gland (chief cell-predominant) type. Pathol Int. 2017;67:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |