Published online Jun 28, 2018. doi: 10.3748/wjg.v24.i24.2640
Peer-review started: March 22, 2018
First decision: April 24, 2018
Revised: May 12, 2018
Accepted: May 26, 2018
Article in press: May 26, 2018
Published online: June 28, 2018
Processing time: 95 Days and 17.5 Hours
Primary hepatic neuroendocrine tumor (PHNET) is an extremely rare liver tumor. Patients often have no clinical symptoms or have only non-specific symptoms, such as abdominal pain and abdominal mass. The clinical manifestations, disease development, treatment methods, and treatment outcomes of PHNET vary greatly among cases. Here we report a case of PHNET with a confirmed 26-year survival before surgery. The patient was a 56-year-old female. A large right hepatic mass was detected when the patient was 30 years old. The tumor could not be removed during exploratory laparotomy, and constriction of the right hepatic artery and biopsy were conducted. Pathological results indicated a diagnosis of benign tumor, but a confirmed diagnosis was not reached. Twenty-six years after the patient had been living with the tumor, she sought treatment again because of tumor progression. After systematic evaluation of the resectability, the tumor was resected. Based on the examination results of the gastrointestinal tract and lungs, intraoperative examination results, pathological findings, and long-term follow-up results, the diagnosis of PHNET was confirmed. This case represents the longest reported survival time for a PHNET patient before removal of the tumor.
Core tip: In this work, we reported a rare case of primary hepatic neuroendocrine tumor. To our knowledge, the total number of cases does not exceed 150. The diagnosis, radiological features, treatment, and survival conditions vary among cases. Our case has two features that have not yet been reported in the literature: (1) The patient had a confirmed preoperative disease course of 26 years-the longest preoperative course among the reported cases; and (2) the lesion contained multiple calcifications, which is not reported previously.
- Citation: Meng XF, Pan YW, Wang ZB, Duan WD. Primary hepatic neuroendocrine tumor case with a preoperative course of 26 years: A case report and literature review. World J Gastroenterol 2018; 24(24): 2640-2646
- URL: https://www.wjgnet.com/1007-9327/full/v24/i24/2640.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i24.2640
Primary hepatic neuroendocrine tumor (PHNET) is a rare hepatic space-occupying lesion. Thus far, the English literature mostly contains case reports, with the total number of cases not exceeding 150[1]. In these case reports, the lesion size, location, imaging manifestations, treatment methods, and treatment effects present a rich diversity. Here, we report a case with a huge PHNET with a preoperative course of 26 years and present a brief review of the literature. To the best of our knowledge, this PHNET case has the longest preoperative course, and it represents the only reported case with multiple calcifications in the tumor.
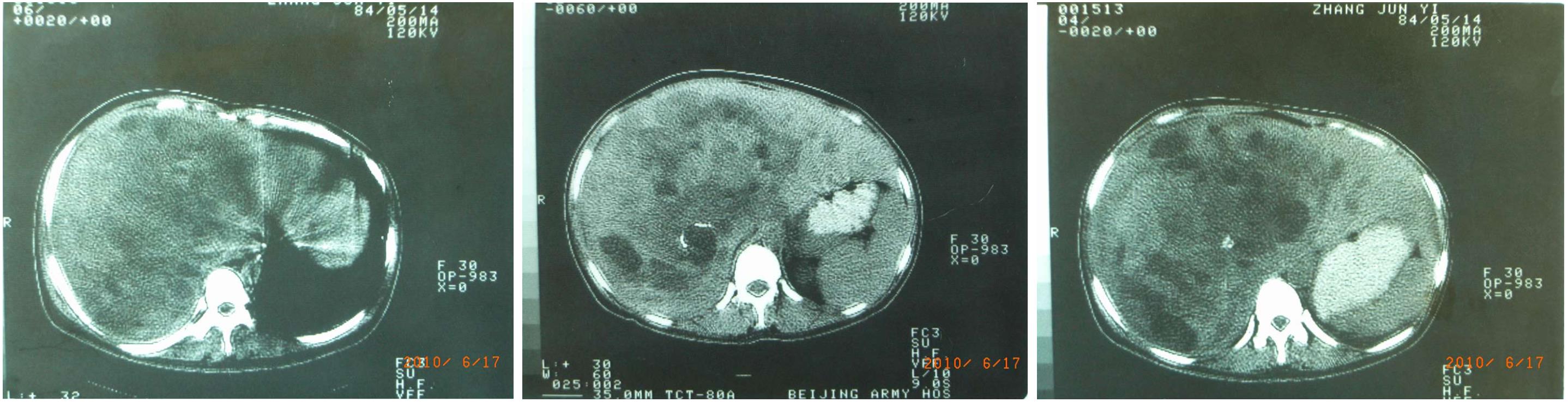
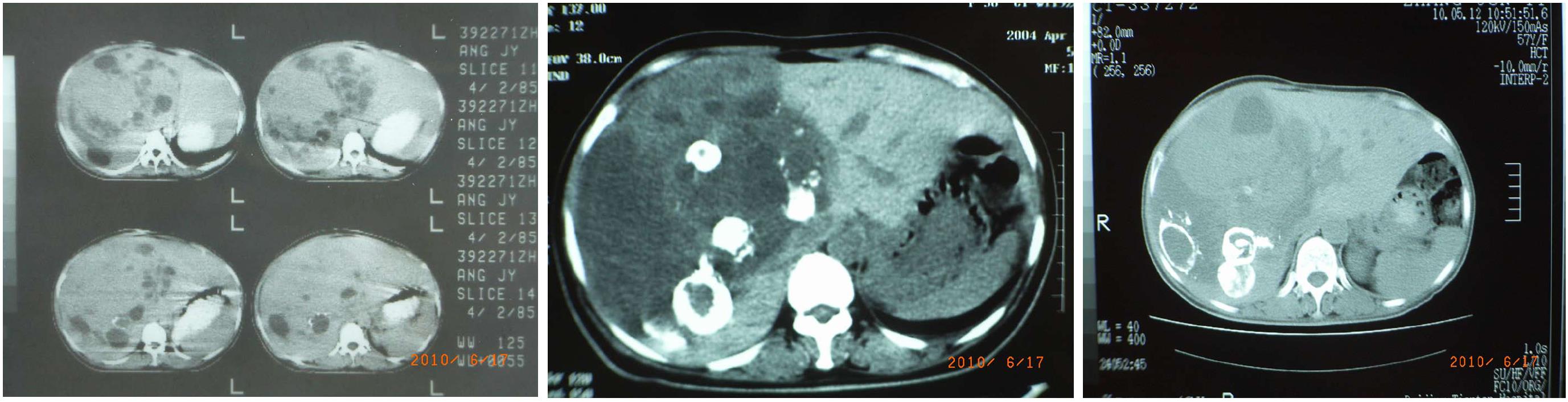
A 56-year-old female patient sought treatment on her own in our hospital due to “liver space-occupying lesion for 26 years and bloating for six months” on June 2, 2010 and walked into the ward herself. In April 1984, the patient self-reported the symptoms of abdominal distension and intermittent abdominal pain but without nausea, vomiting, fever, yellow skin, sclera, increased heart rate, diarrhea, or flushing. B-mode ultrasound and computed tomography (CT) examination (Figure 1) found a liver mass, approximately 20 cm × 16 cm × 11 cm in size. The nature of the mass was uncertain, and there were no abnormalities identified in the complete blood test and in tests for liver function and alpha-fetoprotein. In July 1984, a famous surgeon in China conducted an exploratory laparotomy on the patient. During the surgery, it was found that the right hepatic artery was surrounded by a tumor and the hepatic vein was invaded. The tumor could not be removed, and constriction of the right hepatic artery and liver biopsy were performed. Postoperative recovery was uneventful. Postoperative pathology failed to determine the diagnosis but suggested the following possibilities: (1) Hepatocellular adenoma; (2) highly differentiated hepatocellular carcinoma; or (3) sinusoidal dilatation and hemorrhage. The long-diameter of the tumor was reduced to 18 cm after surgery, and the patient was followed up intermittently for 26 years (Figure 2). No significant increase in liver mass was observed and no significant discomfort was reported. But at the beginning of 2010, the patient reported gradually aggravated abdominal distention with occasional abdominal pain. An examination revealed increased liver tumor size, and the patient exhibited a weight loss of 10 kg in 6 mo.
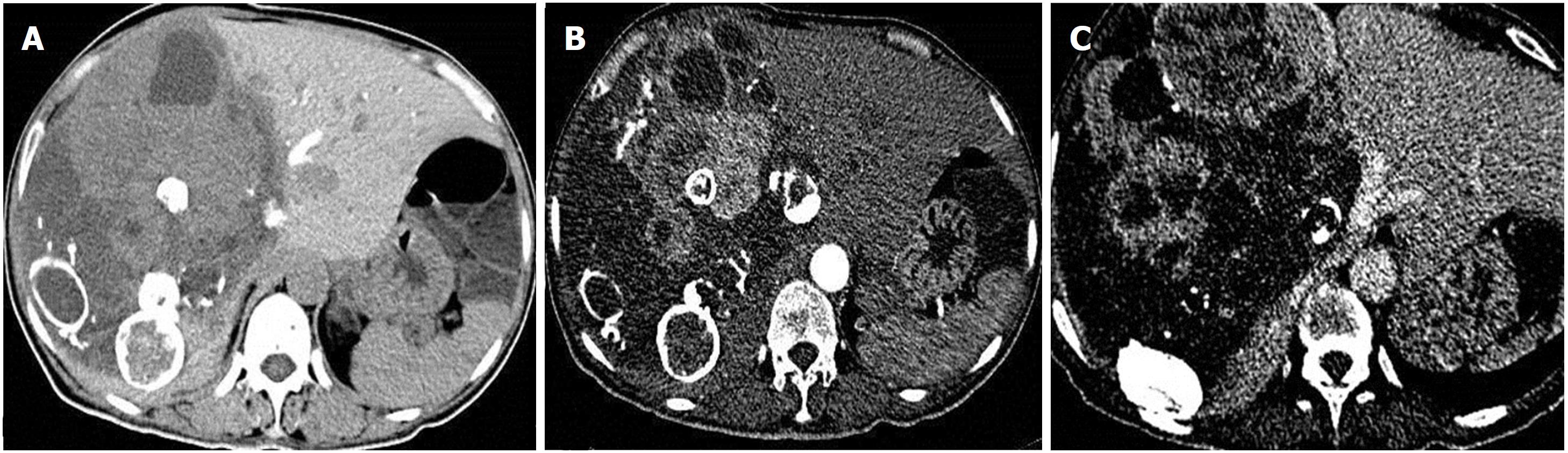
Upon physical examination, a hard mass with an uneven surface was identified in the right upper abdomen; the boundary of the mass was not clear, there was no significant tenderness, the mass could not be moved easily, and there were no other positive findings (Figure 3).
After admission, the patient underwent routine blood tests, including examinations of complete blood count, liver and renal function, electrolytes, coagulation function, and tumor markers, along with gastrointestinal endoscopy and pulmonary imaging. No abnormal findings were found.
Although the nature of the tumor was not clarified following the examination conducted in 1984, the previous pathological results indicated a benign liver tumor. Therefore, surgery should have been the first choice of treatment. Although the tumor was not successfully resected in 1984, liver surgery has made tremendous progress over the past 26 years owing to great advances in radiology and liver transplantation. Therefore, the resectability of the case was re-evaluated systematically.
In accordance with the preoperative assessment strategy for a large-scale liver resection, the team of doctors further evaluated the hepatic vascular anatomy, liver volume, and hepatic reserve function of the patient. The patient’s CT was used for three dimensional (3D) visualization of the liver and the design of virtual surgeries (Myrian, France) to derive liver volume information and to assist in surgical decisions. Liver reserve function was evaluated by Indocyanine Green (ICG) test and 99mTc-galactosyl human serum albumin scintigraphy (GSA) test. Two clinicians with more than 10 years of liver surgery experience independently reviewed tomographic images and reconstructed 3D images to evaluate the feasibility of lesion resectability and then discussed for last decision.
Both surgeons unanimously confirmed that the arterial, portal, and hepatic veins in the left lateral lobe of the liver could be fully displayed on the tomographic images and that their distances to the tumor allowed safe manipulation for the surgical procedures. Liver volume calculations revealed Future Liver Remnant (FLR) 1060 ml according to the design of right hepatic trisegmentectomy. The ICG reserved in 15 min (R15) was 1.4%. 99mTc-GSA showed a valid functional liver volume of 749 mL and a functional liver volume of 660 mL in the left lobe, accounting for 88.1% of the total functional liver volume. Based on the above results, right hepatic trisegmentectomy was determined to be safe and feasible.
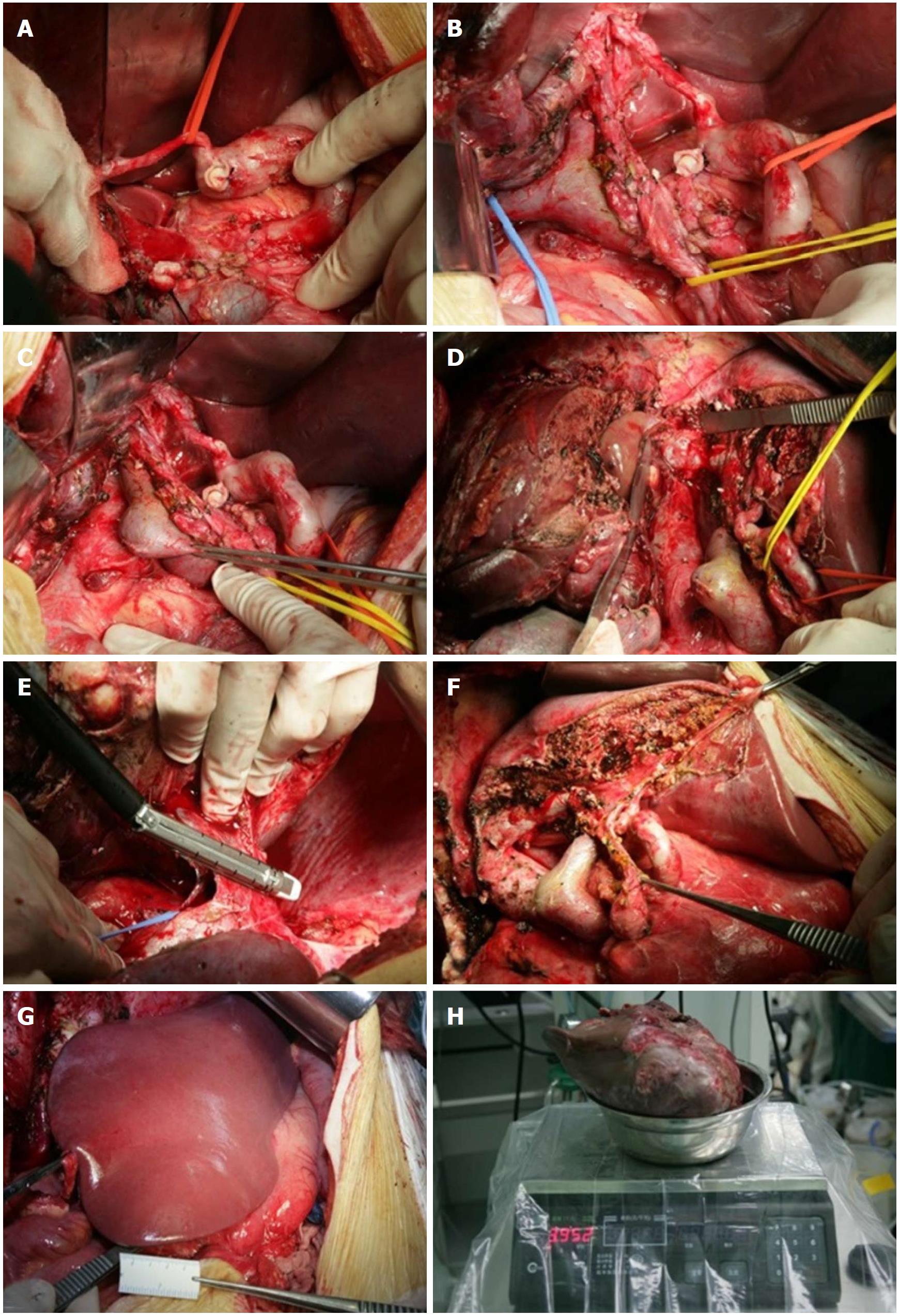
The surgery was conducted on June 30, 2010. After careful exploration of the stomach, small intestine, colon, and pancreas after laparotomy, no tumor lesions were found. The surgery was conducted in accordance with an anatomical right hepatic trisegmentectomy and lasted for 8 h. The intraoperative bleeding was 1600 mL, and the specimen weight was 3952 grams (Figure 4). Postoperative recovery was successful; the patient was discharged after 10 d without complications.
Postoperative pathology: acinar and daisy clumpy structures could be seen under light microscope, and some areas were beam-like and had rich sinusoids. The tumor size was 26 cm × 23 cm × 8 cm, and mitotic phases were rare (0-1/10 high power field). Immunohistochemistry revealed cluster of differentiation 56 (+), synaptophysin (+), vimentin (-), P53 (-), chromogranin A (-), Ki-67 index < 1%, and cytokeratin (++) in the tumor. The final diagnosis was PHNET G1; TNM stage: T1b.
The patient was followed up in our hospital 1, 3, 6, and 12 mo after surgery and was then followed by telephone or in-hospital once each year. Follow-up included blood tests and radiological examinations. The last follow-up was conducted on August 2016 in our outpatient department. The patient had no signs of tumor recurrence and had a good quality of life.
Neuroendocrine tumors (NETs) are relatively rare tumors, accounting for roughly 0.46% of the malignant lesions of the gastrointestinal tract and bronchial lungs and approximately 2% of gastrointestinal malignancies[2]. Data from SEER (Surveillance, Epidemiology and End Results Program, United States) show that the incidence of NET has been on the rise in the recent 30 years[3]. This increase may be related to the increasing awareness of NET among clinicians and the progress of diagnostic experience/technology.
Approximately 67% of NETs come from the gastrointestinal tract, and approximately 25% come from the lungs; the rest come from other organs, such as thyroid, pancreas, esophagus, and biliary tract. Among NETs found in the liver, approximately 80% are metastatic, while primary hepatic neuroendocrine tumor (PHNET) is very rare, accounting for roughly 0.4% of all cases of NET[3]. In the current English literature, the number of all reported PHNET is less than 150.
The majority of PHNET cases are found in Asians[4]. The tumor is often found in middle-aged patients, mostly aged 40 and older, and it affects slightly more female than male patients. Upper abdominal pain is the most common symptom, and approximately 5% of patients may have carcinoid syndrome[5]. Some patients are asymptomatic, and the tumors are accidentally found in imaging. Preoperative blood laboratory tests are generally normal, and CEA, AFP, and CA19-9 generally have negative changes. Lesions can occur as single lesions, but there can also be multiple lesions. Single lesions in the liver occur with equal probabilities on the right and left hepatic lobes.
Because of the limited number of cases, there are few articles devoted to the characterization of the imaging features of PHNET. Recently, two reports by Chinese scholars described the ultrasonographic features of ten and six PHNET cases, respectively[6,7]. B-mode ultrasound examination showed that the majority of tumors were mixed hyperechoic, and a few were iso- or hypoechoic. In CEUS, more than 80% of cases can be characterized by an enhancement of the tumor parenchyma at the arterial phase and expurgation at the portal vein/balance phase[6,7]. Enhanced tomographic examination (CT/MR) findings include (1) tumor with heterogeneous mixed signals; (2) slight or significant enhancement of the parenchyma or peripheral enhancement during the arterial phase, followed by subsidence or continuous increase; and (3) huge lesions often presenting cystic areas, which may be bleeding (MR T1 high signal, T2 low signal)[7-10]. On positron emission tomography (PET), PHNET may show high uptake of F-18 deoxyglucose (F-18 FDG). PET may be helpful in diagnosis[11]. However, these features are similar to the features of HCC, lacking the specificity of PHNET, and the imaging performance varies from patient to patient. Therefore, it is difficult to confirm the diagnosis of PHNET using preoperative imaging alone. Currently, the established method to diagnose NET is labeled octreotide radionuclide imaging, with a reported sensitivity of up to 85%-90%. Another function of this approach is to predict the sensitivity of NET to somatostatin treatment[1,12,13].
The confirmation of PHNET diagnosis requires both preoperative and postoperative processes. After considering PHNET diagnosis, we should first rule out primary lesions in other organs. PET-CT, gastrointestinal endoscopy, and a series of examinations are often conducted, focusing on the stomach, small intestine, colon, pancreas, and appendix. After excision of the liver lesion, pathological and immunohistochemical examinations are required to confirm the diagnosis of NET. Long-term follow-up is required, and the absence of NET lesions in any other organs is required to establish the diagnosis of PHNET. This patient underwent gastrointestinal endoscopy and lung CT examination, and no lesions were found. After 26 years of preoperative course and 7 years of postoperative follow-up, no other lesions were found except in the liver. Finally, we confirmed the diagnosis of PHNET.
There is no clear treatment recommendation for PHNET. However, most authors in the literature conducted hepatectomies for resectable lesions[6,14-17]. The 5-year survival rate after hepatectomy ranges from 74%-78%, and the recurrence rate is approximately 18%[18]. Other treatments include trans-arterial embolization, liver transplantation, and radiofrequency ablation. However, no survival data have yet been found because of the limited number of cases. It is noteworthy that the postoperative recurrence of PHNET may not be in the immediate postoperative period, and there are reports of lesion recurrence 10 to 13 years after surgery[19,20]. Therefore, long-term follow-up after surgery is very important.
To the best of our knowledge, our case has two features that have not yet been reported in the literature: (1) The patient had a confirmed preoperative disease course of 26 years-the longest preoperative course among the reported cases; (2) the lesion contained multiple calcifications, which is a previously unreported feature. These findings once again show that G1 PHNETs may be slow-growing tumors with heterogeneous internal components, the characteristics of preoperative imaging examination can vary, and patients can have a long-term survival with the tumor.
Another interesting fact in the treatment of this case is that this patient waited a long time and gained new surgical opportunities after the previous surgery failed to remove the tumor. The tumor progressed very slowly during the waiting period, but improvements in liver surgery occurred rapidly. Since 1984, great advances have been made in radiology, liver transplantation, and liver surgery, both globally and in China. Evaluation of liver volume, evaluation of liver reserve function, isolation and control of liver blood vessels, vascular reconstruction, and other key surgical techniques of liver surgery continue to be established and improved, and more and more Chinese surgeons have mastered and used these new techniques. In the 1980s, China’s top liver surgeons could not surgically remove giant liver tumors, which now can be cured[21-23]. The reported case is a good example demonstrating the technical progress of liver surgery in China.
The female patient suffered a giant but slow-growing, mild symptom right hepatic tumor for 26 years before definitive surgery, and the diagnosis was confirmed as primary hepatic neuroendocrine tumor (PHNET). Surgical removal leads to a good long-term disease free survival.
The female patient had no obvious symptoms but abdominal distension and intermittent abdominal pain.
In the first surgery in 1984, tumor biopsy was performed and three differential diagnoses were considered: (1) Hepatocellular adenoma; (2) highly differentiated hepatocellular carcinoma; and (3) sinusoidal dilatation and hemorrhage.
No abnormal findings were found in preoperative laboratory tests, including blood regular test, liver and renal function, tumor markers, etc.
Computed tomography scan showed a huge space-occupying lesion of the right liver, with the heterogeneous internal density and multiple enveloped calcifications.
Histological and immunochemical exams confirmed acinar and daisy clumpy structures under light microscope. Some areas were beam-like and had rich sinusoids, and mitotic phases were rare (0-1/10 HPF). Cluster of differentiation 56 and synaptophysin were positive, and Ki-67 was < 1% in the tumor.
Patient accepts definitive surgery, and the tumor was removed thoroughly.
PHNET is a kind of very rare primary hepatic lesion. The symptoms, radiological findings, clinical course, therapy methods, and patients’ survival conditions are variable between different cases. Although PHNET should be a differential diagnosis in liver tumors, it is very difficult to confirm this diagnosis before pathological examination. Different therapy methods have been used to treat PHNET. Surgery should be the first choice if possible.
Neuroendocrine Tumor (NET), previously named carcinoid tumor, is a kind of rare tumor. NETs always originate from gastrointestinal tracts or bronchial lungs. NETs in liver are rare, and about 80% of them are metastasis tumors. PHNET is extremely rare, accounting for roughly 0.4% of all cases of NETs, and it is difficult to differentiate from other liver tumors, such as hepatocellular carcinoma and adenoma.
In this rare, slow growing PHNET case, we find that methods and the possibility of treatment may change along with technology progress, such that definitive treatment may be achieved after long-time conservative treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013) statement: The CARE Checklist (2013) has been adopted.
P- Reviewer: Marion R, Raghow R, Scorsetti M S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Yang K, Cheng YS, Yang JJ, Jiang X, Guo JX. Primary hepatic neuroendocrine tumor with multiple liver metastases: A case report with review of the literature. World J Gastroenterol. 2015;21:3132-3138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Estrozi B, Bacchi CE. Neuroendocrine tumors involving the gastroenteropancreatic tract: a clinicopathological evaluation of 773 cases. Clinics (Sao Paulo). 2011;66:1671-1675. [PubMed] |
| 3. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1852] [Article Influence: 84.2] [Reference Citation Analysis (1)] |
| 4. | Ibrahim ME, Abadeer K, Zhai QJ, Nassar A. Primary Hepatic Neuroendocrine Tumor with Unusual Thyroid Follicular-Like Morphologic Characteristics. Case Rep Pathol. 2017;2017:7931975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y, Nishi H, Taguchi KI, Kotoh K, Nawata H. Primary hepatic carcinoid tumor: case report and review of 53 cases. Med Sci Monit. 2001;7:746-750. [PubMed] |
| 6. | Li W, Zhuang BW, Wang Z, Liao B, Hong LY, Xu M, Lin XN, Xie XY, Lu MD, Chen LD. Case Report of Contrast-Enhanced Ultrasound Features of Primary Hepatic Neuroendocrine Tumor: A CARE-Compliant Article. Medicine (Baltimore). 2016;95:e3450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma KS, Guo DY, Ding SY. Primary hepatic neuroendocrine tumors: clinical characteristics and imaging features on contrast-enhanced ultrasound and computed tomography. Abdom Radiol (NY). 2016;41:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ichiki M, Nishida N, Furukawa A, Kanasaki S, Ohta S, Miki Y. Imaging findings of primary hepatic carcinoid tumor with an emphasis on MR imaging: case study. Springerplus. 2014;3:607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Baek SH, Yoon JH, Kim KW. Primary hepatic neuroendocrine tumor: gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging. Acta Radiol Short Rep. 2013;2:2047981613482897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Li RK, Zhao J, Rao SX, Chen CZ, Zeng MS, Qiang JW. Primary hepatic neuroendocrine carcinoma: MR imaging findings including preliminary observation on diffusion-weighted imaging. Abdom Imaging. 2013;38:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Mitamura K, Yamamoto Y, Tanaka K, Sanomura T, Murota M, Nishiyama Y. F-FDG PET/CT Imaging of Primary Hepatic Neuroendocrine Tumor. Asia Ocean J Nucl Med Biol. 2015;3:58-60. [PubMed] |
| 12. | Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Gorla AK, Basher RK, Kaman L, Bal A, Bhattacharya A, Mittal BR. 68Ga-DOTATATE PET/CT in Primary Hepatic Neuroendocrine Tumor. Clin Nucl Med. 2017;42:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Song JE, Kim BS, Lee CH. Primary hepatic neuroendocrine tumor: A case report and literature review. World J Clin Cases. 2016;4:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 15. | Yalav O, Ülkü A, Akçam TA, Demiryürek H, Doran F. Primary hepatic neuroendocrine tumor: Five cases with different preoperative diagnoses. Turk J Gastroenterol. 2012;23:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Park CH, Chung JW, Jang SJ, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Gao J, Hu Z, Wu J, Bai L, Chai X. Primary hepatic carcinoid tumor. World J Surg Oncol. 2011;9:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Nishimori H, Tsuji K, Miyamoto N, Sakurai Y, Mitsui S, Kang JH, Yoshida M, Nomura M, Fuminori I, Ishiwatari H. Recurrence of primary hepatic carcinoid tumor in the remnant liver 13 yr after resection. Int J Gastrointest Cancer. 2005;35:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Abdel Wahab M, Fathy O, Elghwalby N, Sultan A, Mostafa M, El-Baz M, Elsaadany M, Elshobary M, Ezzat F. Primary hepatic carcinoid tumor: one Egyptian center experience. Hepatogastroenterology. 2006;53:33-38. [PubMed] |
| 21. | Dong JH, Yang SZ, Duan WD, Ji WB, Cai SW, Wang J, Shi XJ, Jiang K, Xia HT, He L. [Clinical application of precise liver resection techniques in patients with complicated liver space-occupying lesions]. Chinese Journal of Surgery. 2009;47:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Yang SZ, Zhang WZ, Cai SW, Ji WB, Jiang K, Duan WD, Dong JH, Wang J. Application of computer-assisted operation planning system in precise hepatectomy. Zhonghua Xiaohua Wai Ke Za Zhi. 2010;9:31-34. [DOI] [Full Text] |
| 23. | Dong J, Yang S, Zeng J, Cai S, Ji W, Duan W, Zhang A, Ren W, Xu Y, Tan J. Precision in liver surgery. Semin Liver Dis. 2013;33:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |