Published online Jun 21, 2018. doi: 10.3748/wjg.v24.i23.2508
Peer-review started: March 14, 2018
First decision: March 30, 2018
Revised: April 5, 2018
Accepted: April 26, 2018
Article in press: April 26, 2018
Published online: June 21, 2018
Processing time: 93 Days and 0.5 Hours
To detect the expression of Raf kinase inhibitory protein (RKIP) in gastrointestinal stromal tumors (GISTs) and to analyze its relationship with clinicopatholgical characteristics and prognosis of this disease.
Sixty-three patients with pathologically diagnosed GISTs who underwent surgical resection at the Shengjing Hospital of China Medical University from January 2011 to January 2015 and had complete clinical, pathological, and follow-up data were included. Immunohistochemical method was used to detect the expression of RKIP in GIST tissue samples from these patients. Kaplan-Meier method was used to calculate the survival rate of 60 patients with complete follow-up data, and Cox regression analysis was performed to identify factors affecting the prognosis of patients GISTs to evaluate further the diagnostic and prognostic value of RKIP in GISTs.
In GIST tissues, RKIP positive signals, manifesting as brownish yellow or brown granules, were located in the cytoplasm or on the membrane. Of 63 tissue samples included in this study, 34 (54%) were positive and 29 (46%) were negative for RKIP expression. Statistical analysis showed that RKIP expression in GISTs was significantly associated with tumor size, National Institutes of Health (NIH) risk grade, and mucosal invasion, but had no significant association with age, gender, tumor location, or the number of mitotic figures. Univariate Kaplan-Meier analysis revealed that the 1-, 3-, and 5-year survival rates were 94.4%, 89.2%, and 80.5% for patients with positive RKIP expression, and 88.6%, 68.2%, and 48.2% for patients with negative RKIP expression, suggesting that patients with high RKIP expression had significantly higher survival rates than those with low expression (Log-rank test, P = 0.0015). Cox regression analysis demonstrated that NIH risk grade was significantly associated with the prognosis of GISTs (P = 0.037), suggesting that NIH risk grade is a significant predictor of the prognosis of GISTs. RKIP expression had a tendency to predict the survival of GISTs (P = 0.122), suggesting that RKIP expression may have appreciated value to predict the prognosis of GISTs.
This study demonstrated that: (1) RKIP expression in GISTs is associated with tumor size, NIH risk grade, and mucosal invasion, and low or no expression of RKIP predicts a high malignancy potential; (2) high RKIP correlates positively with the survival of patients with GISTs; and (3) RKIP expression has appreciated value for predicting the survival of patients with GISTs, although it is not an independent prognostic factor in GISTs.
Core tip: In this study, the expression of Raf kinase inhibitory protein (RKIP) in gastrointestinal stromal tumors (GISTs) was examined by immunohistochemistry. We explored the relationship between RKIP protein expression and survival and prognosis in a large sample of GIST patients in China. RKIP protein expression was correlated with tumor growth, differentiation, malignancy, and the prognosis of the tumor. Our findings provide evidence for the diagnosis and prognosis assessment of GIST and offer new tumor treatment targets in GIST.
- Citation: Wang Y, Chen JJ, Wang XF, Wang Q. Clinical and prognostic significance of Raf kinase inhibitory protein expression in gastrointestinal stromal tumors. World J Gastroenterol 2018; 24(23): 2508-2517
- URL: https://www.wjgnet.com/1007-9327/full/v24/i23/2508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i23.2508
Gastrointestinal stromal tumors (GISTs) have previously been classified or pathologically diagnosed as leiomyosarcomas, leiomyomas, or leiomyoblastomas. For many years, surgical resection was the only effective treatment for GISTs. Until 1983, Mazur and Clark, two professors of pathology at the State University of New York, found that these “leiomyomas” have neither smooth muscle characteristics nor Schwann characteristics, and they for the first time proposed the concept of GISTs[1,2]. In 1998, Hirota et al[3] at the Osaka University reported that GISTs contained activated c-kit mutations. Further immunohistochemical examination showed that GIST tissue was positive for CD34 and CD117. Since then, the diagnosis of GISTs entered the standardization phase[4].
GISTs are now considered the most common gastrointestinal mesenchymal tumors, accounting for about 1%-4% of all gastrointestinal neoplasms. With the application of endoscopic ultrasound (EUS) in the gastrointestinal tract, more GISTs have been detected and distinguished form other subepithelial lesions[5-7]. The annual incidence of GISTs is about 2/100000[8,9]. Approximately 90% of GISTs are located in the stomach and small intestine, with gastric lesions being the most common (~60%). Geneticists estimate that about 10% of all individuals suffer from deleterious gene mutations[10], and current research shows that the development of GISTs is associated with multiple gene mutations, such as c-kit and PDGFRα (platelet derived growth factor receptor alpha) mutations. C-kit gene mutations generally occur in exon 11 (~60%) and include deletions, point mutations, and insertions. In some GISTs without c-kit mutations, PDGFRα mutations may occur, which account for about 8% of all GISTs. PDGFRα mutations occur mainly in exon 18 (~6% of all GISTs) and occasionally in exons 12 and 14 (~1.5% and 0.5% of all GISTs, respectively). Exons 18 and 12 encode the intracellular tyrosine kinase domain and jaxtamembrane domain, respectively. Of note, a small portion of GISTs are wild-type tumors, without c-kit or PDGFRα abnormalities.
GISTs are basically a malignancy, and there are almost no absolutely benign GISTs. Seemingly benign GISTs have the potential to be malignant. For many years, treatment of GISTs was limited to surgical resection and the application of chemotherapy regimens for sarcomas, but with poor efficacy. In 2000, the first successful case using the targeted drug “imatinib” in GISTs was reported. Imatinib is a small molecule tyrosine kinase inhibitor that targets c-kit and PDGFRα and can prevent the initiation of the downstream oncogenes by inhibiting theses tyrosine kinases.
The application of the targeted drug imatinib in GISTs was a milestone in the understanding and gene therapy of GISTs. However, with the increased application of imatinib in clinical cases, drug resistance is beginning to emerge. Thus, it is important to investigate further the pathogenesis of GISTs and discover new therapeutic targets for this malignancy.
Raf kinase inhibitory protein (RKIP), also known as phosphatidylethanolamine-binding protein 1 (PEBP1), is an important endogenous modulator of many kinases and the star protein of recent oncology research. This protein was initially identified in the brain of cattle[11]. RKIP belongs to a highly conserved family of phospholipid-binding proteins, which contains more than 400 members and is widely distributed in microbes, plants, and mammals. RKIP can regulate multiple signaling transduction pathways, including the Raf/MAP kinase (MAPK) pathway, the β-adrenergic (β-AR) pathway, and the NF-kappa B pathway[12-14]. RKIP is not only a kinase inhibitor but also a target of phosphorylation, having important and complex functions, such as regulating tumor growth and metastasis and affecting cell cycle and apoptosis.
Recently, there have been many studies on RKIP, including in gastric cancer, colon cancer, esophageal cancer, and gynecological tumors. However, there have been very few reports on RKIP in GISTs. The role of this protein in the growth and metastasis of GISTs, the relationship between RKIP expression and clinical characteristics of GISTs, and the effect of RKIP expression on the prognosis of GISTs remain unclear. To address these problems, we detected the expression of RKIP in GISTs by immunohistochemistry and analyzed the clinical and prognostic significance of RKIP expression in GISTs, with an aim to provide a basis for GIST diagnosis, prognosis evaluation, and identification of new tumor therapeutic targets.
Sixty-three patients with pathologically diagnosed GISTs who underwent surgical resection at the Shengjing Hospital of China Medical University from January 2011 to January 2015 and had complete clinical, pathological, and follow-up data were included in this study. There were 33 men and 30 women, with a mean age of 56.2 years (range, 21-83 years). The location of GISTs included the stomach (n = 35, 55.6%), duodenum (n = 12, 19.0%), jejunoileum (n = 14, 22.2%), and colon (n = 2, 3.1%).
The diagnostic criteria for GISTs were histopathological features consistent with GISTs and immunohistochemical positivity for CD117, immunohistochemical negativity for CD117 but positivity for CD34, or immunohistochemical negativity for CD117 and CD34 as well as smooth muscle actin (SMA), desmin, and S-100 (to exclude smooth muscle tumors and neurogenic tumors). There were 59 (90.8%) cases positive for CD117 and 50 (76.9%) cases positive for CD34 (Table 1).
| Characteristic | Patients (n = 63) | |
| Number | Percentage (%) | |
| Gender | ||
| Male | 33 | 52.4 |
| Female | 30 | 47.6 |
| Age (yr) | ||
| ≥ 56 | 32 | 50.8 |
| < 56 | 31 | 49.2 |
| Tumor location | ||
| Stomach | 35 | 55.6 |
| Duodenum | 12 | 19.0 |
| Jejunoileum | 14 | 22.2 |
| Colon | 2 | 3.2 |
| Tumor size (cm) | ||
| < 2 | 11 | 17.5 |
| 2-5.9 | 20 | 31.7 |
| 6-10 | 18 | 28.6 |
| > 10 | 14 | 22.2 |
| NIH risk grade | ||
| Very low | 10 | 15.9 |
| Low | 19 | 30.1 |
| Moderate | 10 | 15.9 |
| High | 24 | 38.1 |
| Mitotic figures per 50 HPFs | ||
| 0 | 11 | 17.5 |
| 1-4 | 35 | 55.6 |
| 5-9 | 6 | 9.4 |
| >10 | 11 | 17.5 |
| Mucosal invasion | ||
| Yes | 33 | 52.4 |
| No | 30 | 47.6 |
GISTs were graded based on the National Institutes of Health (NIH) consensus on defining the risk of aggressive behavior: (1) Very low risk of aggressive behavior (grade I), tumor size < 2 cm and mitotic figures < 5/50 high-power fields (HPFs); low risk (grade II), tumor size of 2-5 cm and mitotic figures < 5/50 HPFs; moderate risk (grade III), tumor size of 5-10 cm and mitotic figures < 5/50 HPFs, or tumor size < 5 cm and mitotic figures of 6-10/50 HPFs; and high risk (grade IV), tumor size > 5 cm and mitotic figures > 5/50 HPFs, or tumor size > 10 cm and mitotic figures > 10/50 HPFs.
Of 63 cases of GISTs included in this study, three died from other reasons and the remaining 60 had complete follow-up data, with a mean follow-up period of 48 mo (range, 5-61 mo). Tumor-adjacent tissue samples were collected as controls.
GIST tissue samples were fixed in formalin, embedded in paraffin, and cut into 4 μm sections. The sections were then routinely dewaxed and rehydrated. After endogenous peroxidase was inactivated with H2O2, heat-mediated antigen retrieval was performed. The sections were then blocked with 5% bovine serum albumin (BSA) blocking solution for 20 min at room temperature. Subsequently, the sections were incubated with primary antibody (rabbit anti-RKIP antibody) for 12 h at room temperature, followed by incubation with secondary antibody (biotinylated goat anti-rabbit IgG) for 20 min at 25 °C. After that, the sections were incubated with SABC-AP/BCIP reagents to develop the color. Finally, the sections were counterstained with nuclear fast red, dehydrated, mounted, and observed under a microscope.
Positive immunohistochemical staining signals for RKIP were present in the cytoplasm or on the membrane. A semi-quantitative method that combines staining intensity and the percentage of positive cells was adopted to evaluate the expression of RKIP. Ten HPFs were randomly selected from a slide, and 100 cells were counted in each HPF to calculate the percentage of positive cells. The staining intensity was graded as follows: 0, no staining; 1, yellow; 2, brownish yellow; and 3, brown. The percentage of positive cells was scored as follows: 0, < 25%; 1, 25% to 50%; 2, 51% to 75%; and 3, > 75%. The overall score was the product of the staining intensity and the percentage of positive cells and graded as negative (0-2), mildly positive (+, 3), moderately positive (++, 4-6), or strongly positive (+++, 9). RKIP expression was judged to be either negative (0-2) or positive (3-9).
The patients were followed by telephone, outpatient visits, or letters. Survival time was defined as the period from the date of surgery to the date of the last follow-up or death. Of the 63 cases included, 60 had complete follow-up data and three died of other diseases or accidents. The follow-up period was between January 2013 and December 31, 2017.
SPSS17.0 software was used for all statistical analyses. Percentages were compared using the chi-square test. Survival curves were plotted with GRAPHPAD software, and survival analysis was performed using the Kaplan-Meier method and the log-rank test. Multivariate prognostic analysis was performed using a Cox regression model. P-values < 0.05 were considered statistically significant.
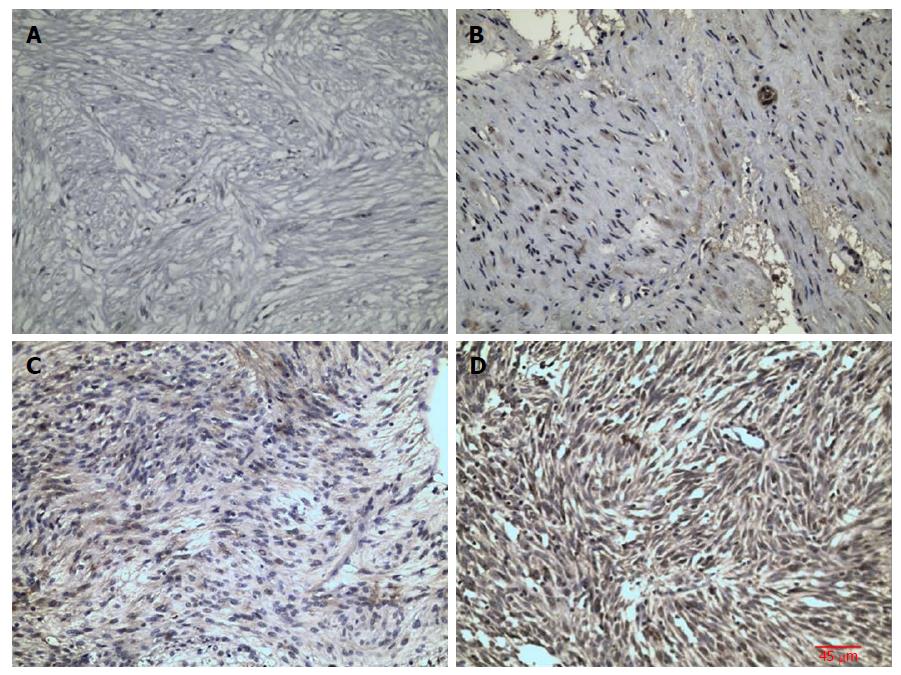
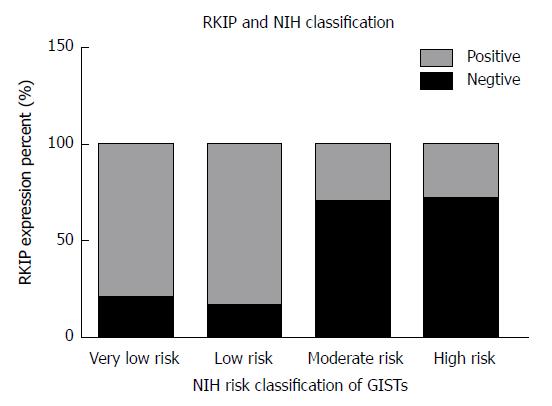
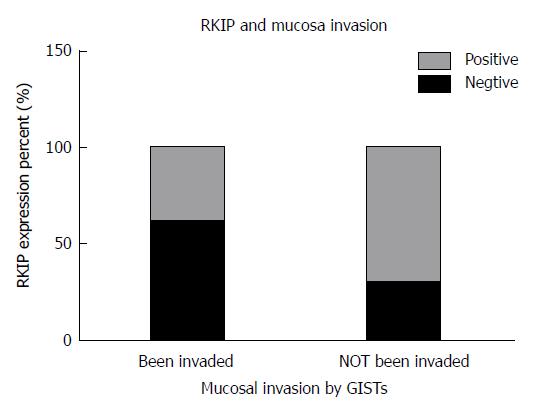
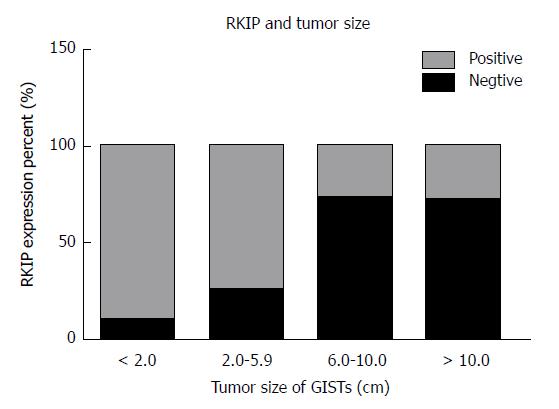
In GIST tissue, RKIP positive signals, manifesting as brownish yellow or brown granules, were located in the cytoplasm or on the membrane (Figure 1). Of 63 tissue samples included in this study, 14 were strongly positive (+++), 16 moderately positive (++), four mildly positive (+), and 29 negative for RKIP expression (Figure 1A-D, respectively). In total, 34 (54%) cases were positive and 29 (46%) were negative for RKIP expression. Statistical analysis showed that RKIP expression in GISTs was significantly associated with tumor size, NIH risk grade, and mucosal invasion. The positive rates of RKIP expression were 80.0%, 84.2%, 30.0%, and 29.2% in the very low risk, low risk, moderate risk, and high risk groups, respectively (P < 0.01); 90.9%, 75.0%, 27.8%, and 28.6% in tumors of < 2 cm, 2-5.9 cm, 6-10 cm, and > 10 cm, respectively (P < 0.01); and 39.4% and 70.0% in tumors with and without mucosal invasion, respectively (P = 0.015). However, RKIP expression had no significant association with age, gender, tumor location, or the number of mitotic figures (Table 2, Figures 2-4).
| Characteristic | Number | RKIP expression | P value | |
| Positive (≥ 3) | Negative (< 3) | |||
| Gender | ||||
| Male | 33 | 19 | 14 | 0.547 |
| Female | 30 | 15 | 15 | |
| Age (yr) | ||||
| ≥ 56 | 32 | 20 | 12 | 0.167 |
| < 56 | 31 | 14 | 17 | |
| Tumor location | ||||
| Stomach | 35 | 16 | 19 | 0.503 |
| Duodenum | 12 | 8 | 4 | |
| Jejunoileum | 14 | 9 | 5 | |
| Colon | 2 | 1 | 1 | |
| Tumor size (cm) | ||||
| < 2 | 11 | 10 | 1 | < 0.011 |
| 2-5 | 20 | 15 | 5 | |
| 6-10 | 18 | 5 | 13 | |
| > 10 | 14 | 4 | 10 | |
| NIH risk grade | ||||
| Very low | 10 | 8 | 2 | < 0.011 |
| Low | 19 | 16 | 3 | |
| Moderate | 10 | 3 | 7 | |
| High | 24 | 7 | 17 | |
| Mitotic figures per 50 HPFs | ||||
| 0 | 11 | 8 | 3 | 0.218 |
| 1-4 | 35 | 20 | 15 | |
| 5-9 | 6 | 2 | 4 | |
| > 10 | 11 | 4 | 7 | |
| Mucosal invasion | ||||
| Yes | 33 | 13 | 20 | 0.0151 |
| No | 30 | 21 | 9 | |
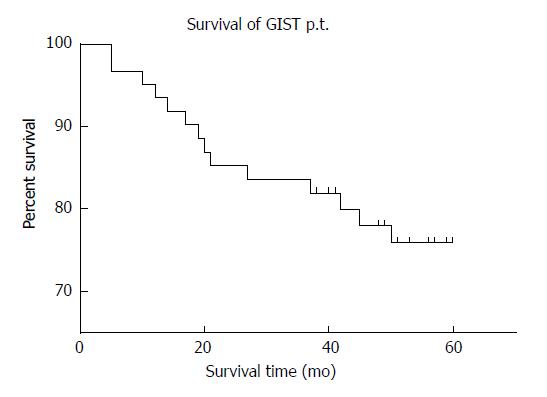
Of 60 patients who were followed, two were lost to follow-up, and the rate of follow-up was 92.1%. Fourteen patients died of GISTs. The median survival time was 54.96 mo. The 1-, 3-, and 5-year survival rates for all patients were 91.7%, 83.3%, and 71.7%, respectively, and the mean survival time was 47.66 ± 16.61 mo (Figure 5).
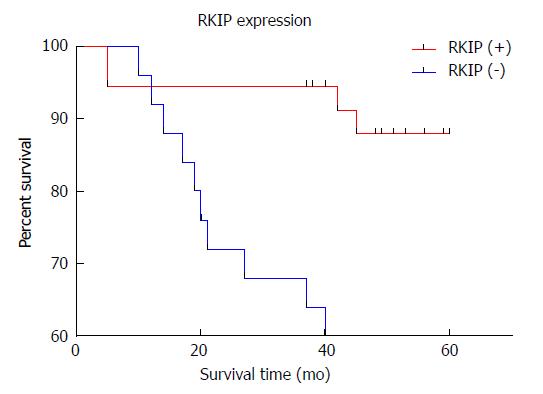
Univariate Kaplan-Meier analysis revealed that the survival rates differed significantly between patients with positive and negative RKIP expression (Log-rank test, P = 0.0015) (Figure 6). The 1-, 3-, and 5-year survival rates were 94.4%, 89.2%, and 80.5% for patients with positive RKIP expression, and 88.6%, 68.2%, and 48.2% for patients with negative RKIP expression, suggesting that patients with high RKIP expression had higher survival rates than those with low expression.
Cox regression analysis demonstrated that NIH risk grade was significantly associated with the survival of GISTs (P = 0.037) (Table 3), suggesting that NIH risk grade is a significant predictor of the prognosis of GISTs. Of note, RKIP expression had a tendency to predict the survival of GISTs (P = 0.122), suggesting that RKIP expression may have appreciated value to predict the prognosis of GISTs. In contrast, other factors, including age, gender, number of mitotic figures, and tumor size, did not significantly predict the prognosis of GISTs (Table 3).
| B | SE | Wald | df | significance | Exp (B) | |
| NIH risk grade | 1.299 | 0.624 | 4.337 | 1 | 0.037 | 3.664 |
| Age | -0.008 | 0.019 | 0.186 | 1 | 0.667 | 0.992 |
| Sex | -0.500 | 0.561 | 0.795 | 1 | 0.373 | 0.606 |
| RKIP expression | 1.049 | 0.678 | 2.395 | 1 | 0.122 | 2.855 |
| Mitotic figures | 0.087 | 0.283 | 0.095 | 1 | 0.758 | 1.091 |
| Tumor size | -0.121 | 0.435 | 0.078 | 1 | 0.781 | 0.886 |
| Tumor location | 0.269 | 0.351 | 0.589 | 1 | 0.443 | 1.309 |
GISTs are the most common gastrointestinal mesenchymal neoplasms. In the past 30 years, the pathogenesis of GISTs has gradually been elucidated, and their diagnosis and treatment has become standardized[15-19]. However, there is still a significant number of recurrent or metastatic GISTs. Because of their resistance to radiation and chemotherapy, recurrent or metastatic GISTs were once considered an incurable disease. GIST patients with metastasis have a median survival period as low as 20 mo, and for patients with locally recurrent GISTs, the median survival period is only 9-12 mo[20,21]. Imatinib, which is a drug that targets c-kit and PDGFRα gene mutations, is the first choice of molecular therapy for GISTs. However, there are currently no other targets for therapy and prognosis monitoring in GISTs.
RKIP is a structurally complex protein that can act as a “multidirectional switch” to regulate multiple signaling transduction pathways, including the Raf/MAPK pathway, the β-AR pathway, and the NF-kappa B pathway[12,13]. RKIP protein is not only a kinase inhibitor but also a target of phosphorylation. For example, in the MAPK pathway, RKIP binds and inhibits Raf protein, and in the G protein-coupled pathway, RKIP binds to GRK2. This functional shift is achieved through the phosphorylation of the S153 residue by protein kinase C (PKC)[22]. Recently, the regulatory function of RKIP protein in tumors has gradually become a hot research topic, but the data on its expression in GISTs are limited.
In this study, we found in GIST tissue that RKIP was mainly expressed in the cytoplasm and on the membrane, presenting as brownish yellow or brown granules. Such an expression pattern is consistent with many previous reports. RKIP is mainly expressed in the human cytoplasm or on the membrane. The RKIP gene is located on chromosome 12q24.23 and contains four exons, spanning a length of 1435 bp and encoding a 21 KDa protein containing 187 amino acid residues[23]. Immunohistochemical results showed that the expression of RKIP in GISTs tissue was correlated with tumor size; in tumors of < 2 cm, 3-5.9 cm, 6-10 cm, or > 10 cm, the positive expression rates of RKIP were 90.9%, 75%, 27.8%, and 28.6%, respectively (P < 0.01). As the tumor size increased, the degree of malignancy increased, and the expression of RKIP was gradually reduced or absent. This result suggests that the expression of RKIP may be associated with the growth of GISTs. Consistent with this finding, Eves et al[24] reported the lack of RKIP protein expression in hepatoma cells, which can lead to rapid cell division and increased cell proliferation instability[25]. As far as the mechanism is concerned, RKIP protein is an important regulatory factor in the MAPK signaling transduction pathway, and its ligand Raf-1 can regulate the cell cycle of mitotic cells[26]. In addition, the activation of downstream protein ERK1/2 in the MAPK pathway can control important structures that are related to cell division, such as centromeres, spindles, and intermediates[27,28]. Collectively, RKIP can regulate tumor cell cycle and mitosis by regulating the phosphorylation and activation of Raf-1 and the activation of downstream MEK/ERK. In GIST cells, the low or no expression of RKIP protein can reduce its inhibitory effect on the MAPK pathway, thus resulting in the proliferation of tumor cells[29]. Therefore, there is a negative correlation between tumor volume and RKIP expression.
When analyzing the relationship between RKIP protein expression and tumor invasion, we found that there was a significant difference in the positive rate of RKIP between GISTs with and without mucosal invasion (P = 0.015). Previously, many reports on RKIP in other tumor types have found that RKIP protein was related to invasion or metastasis suppression, suggesting a general role of RKIP protein in tumor metastasis and invasion. In the earliest research on RKIP in prostate cancer, Fu et al[30] performed a gene chip analysis of mRNA expression in a prostate cancer cell line with a low metastatic potential (LNCaP cells) and a prostate cancer cell line with a high metastatic potential (C42B cells) and found that the expression level of RKIP was lower in C42B cells than in LNCaP cells. The study of specimens from patients with prostate cancer also showed that the expression of RKIP was lower in metastatic prostate cancer. These results suggest that there is a correlation between RKIP expression and tumor metastasis. Furthermore, an in vitro tumor invasion assay demonstrated that down-regulation of RKIP expression in LNCaP cells can enhance their invasion ability, while restoring RKIP expression in C42B cells can weaken their ability of invasion[31]. In both in vivo and in vitro studies of prostate cancer and melanoma, high expression of RKIP can reduce vessel invasion and reduce metastasis risk[32]. Similar results have also been reported in studies on breast cancer with lymph node metastasis[33], insulinoma[34], colon cancer[35,36], liver cancer[25], ovarian cancer[37], and thyroid cancer[38].
In the present study, the positive rate of RKIP expression differed significantly among different NIH risk grades (very low risk: 80.0%; low risk: 84.2%; moderate risk: 30.0%; high risk: 29.2%; P < 0.01). Fletcher discovered that tumor size and the number of mitotic figures are the main prognostic factors to evaluate the malignant potential of GISTs; and, therefore, proposed a risk grading system (NIH grading), which is of great importance in clinical and pathological diagnosis. In this study, the expression of RKIP was correlated with the size of GISTs: the larger the tumor and the higher the malignancy, the higher the possibility of negative expression of RKIP. However, our results indicated that the expression of RKIP did not significantly correlate with the number of mitotic figures. This result is similar to the study of Miettinen, who found that small intestinal stromal tumors had higher malignant potential than gastric stromal tumors. Gastric stromal tumors had good biological behavior as long as the number of mitotic figures was no more than 5/50 HPFs, and duodenal stromal tumors had good prognosis only when the number of mitotic figures was less than 2/50 HPFs[39]. These findings suggest that other factors (such as tumor location) may also affect the weight of the number of mitotic figures as one of the only two indicators of the NIH risk grading system. To overcome this problem, Miettinen further proposed a new risk assessment system to use anatomical site as an independent assessment factor. In 2013, Sebatian et al[40] performed an immunohistochemical analysis of 161 surgical specimens and a survival analysis based on the clinical and pathological data of these surgical patients and found that the low expression of RKIP was significantly correlated with both high NIH risk grade (P = 0.033, moderate vs low risk group) and high Miettnen grade (P = 0.044, moderate vs low risk group).
In order to have a more direct understanding of the impact of RKIP expression on the prognosis of patients with GISTs, we followed 60 GISTs patients (median follow-up period, 54.96 mo) and plotted the survival curve of these patients according to RKIP expression using univariate Kaplan-Meier method. The results showed that the survival rate differed significantly between patients with positive and negative expression of RKIP (Log-rank test, P = 0.0015). The 1-, 3-, and 5-year survival rates were 94.4%, 89.2%, and 80.5% for patients with positive RKIP expression, and 88.6%, 68.2%, and 48.2% for patients with negative RKIP expression, suggesting that patients with high RKIP expression had higher survival rates than those with low expression. Thus, RKIP expression has appreciated value for evaluating the prognosis of patients with GISTs.
Further, Cox regression analysis showed that the prognosis of GISTs patients was related to NIH risk grade (P = 0.037), indicating that NIH risk grade is an important parameter to evaluate the prognosis of patients. RKIP expression had a tendency to predict the prognosis of GISTs (P = 0.122), suggesting that RKIP expression may have appreciated value to predict the survival of GISTs. RKIP as a prognostic factor has been reported in many other tumors. For example, Fu et al[41] used prostate cancer tissue chip to detect RKIP expression in non-tumor tissues, primary tumor tissues, and metastasis tumor tissues and found that RKIP was strongly expressed in the majority of non-tumor tissues. Although RKIP was weakly expressed in advanced prostate cancer (Gleason score, 6-7), it was strongly expressed in the rest prostate cancer tissues. RKIP expression, however, almost disappeared in metastasis tumor tissues. These results are consistent with the above-mentioned observation that RKIP may act as a metastasis suppressor gene, and also indicate that RKIP can be used as a prognostic factor for prostate cancer. In gliomas, the absence of RKIP correlated with a higher malignancy and a shorter survival period. Yu et al[42] performed a meta-analysis of RKIP expression in gastrointestinal tumors, in which they systematically summarized and analyzed 28 articles that met the criteria, and found that low expression of RKIP is associated with a poor prognosis and short survival time.
Although both univariate and multivariate analyses indicated that RKIP expression had appreciated significance in prognosis evaluation in patients with GISTs in this study, multivariate analysis demonstrated that RKIP expression cannot be an independent prognostic factor in GISTs (P = 0.122). Similarly, Marcus Valadao concluded that RKIP expression does not affect the overall survival (P = 0.73), progression free survival (P = 0.22), or objective response rate (P = 0.30)[43]. Sebatian et al[40] performed a multiple regression analysis of factors affecting overall survival (age, gender, imatinib chemotherapy, Flecher grade, etc.) in patients with GISTs and suggested that low expression of RKIP does not have prognostic significance. This may be because current studies are all limited to detection of RKIP expression at the protein level using immunohistochemical method. Because of the qualitative nature and limited number of experimental samples, there is a lack of quantitative research and detection of RKIP expression at the genetic level.
In conclusion, this study demonstrated that: (1) RKIP expression in GISTs is associated with tumor size, NIH risk grade, and mucosal invasion, and low or no expression of RKIP predicts a high malignancy potential; (2) high RKIP correlates positively with the survival of patients with GISTs; and (3) RKIP expression has appreciated value for predicting survival of patients with GISTs, although it is not an independent prognostic factor in GISTs. These findings suggest that RKIP expression in GISTs is closely related to the diagnosis and treatment of this disease and RKIP may be used as a novel target for therapy and prognostic evaluation in GISTs. Future studies should further investigate the role and underlying mechanism of RKIP in GISTs.
Gastrointestinal stromal tumors (GISTs) are the most common gastrointestinal mesenchymal tumor. Raf kinase inhibitory protein (RKIP) protein is both a kinase inhibitor and a phosphorylation target. It has important and complex functions in regulating tumor growth, metastasis, cell cycle, and apoptosis. Many recent articles on RKIP proteins have reported a link to various cancers, including gastric cancer, bowel cancer, esophageal cancer, and gynecologic oncology. However, there are only a few references on the expression of RKIP protein in gastrointestinal stromal tumors.
The following problems, which we need to solve urgently, are also the research motivation of this article: (1) The function of the RKIP in the GISTs neoplastic generation and metastasis; (2) the relationship between the RKIP expression and GIST clinical data; and (3) the relationship between the prognosis of GIST and RKIP.
In this study, we examined the expression of RKIP in GISTs by immunohistochemistry to explore the clinical significance of GIST expression and its prognosis. This research will provide evidence and support for the diagnosis of GIST, prognostic evaluation, and new tumor treatment targets
The study included 63 cases of paraffin-embedded specimens of surgically resected and pathologically confirmed clinical specimens from Shengjing Hospital Affiliated to China Medical University. All the cases are clinically and pathologically complete, dated from January 2011 to January 2015. The expression of RKIP protein was analyzed by immunohistochemistry. The Kaplan-Meier method was used to calculate the overall survival of 60 patients followed up for survival analysis. The prognostic significance of each index was analyzed by COX multiple regression to clarify further the value of RKIP protein level in the diagnosis and prognosis of GISTs.
In GIST tissues, RKIP protein was positively expressed in the cytoplasm and cell membrane with brownish-yellow or brown granules. RKIP protein positive expression was found in 34 (54%) of the 63 specimens in this experiment, and the total negative expression was 29 (46%). The RKIP positive expression was related with GIST tumor size, NIH grade, and mucosal invasion. RKIP and age, gender, tumor location, and how many mitotic figures were not related. Kaplan-Meier method was used to draw the survival curves related to RKIP differential expression. The results showed that the 1, 3, and 5-year survival rates of RKIP positive group were 94.4%, 89.2% and 80.5%, respectively. The survival rates of RKIP negative group at 1, 3, and 5 years were only 88.6%, 68.2%, and 48.2%, respectively. Comparing with the RKIP negative Group, the RKIP high expression group was correlated with a better survival rate (Log-Rank analysis, P = 0.0015). The results of the COX multivariate analysis showed that the prognosis of patients with GISTs was related to NIH grade (P = 0.037) and Exp (B) was 3.664, indicating that NIH risk grade was an important factor to evaluate the prognosis of patients. However, the expression of RKIP correlated with the prognosis of patients (P = 0.122). The Exp (B) value was 2.855, suggesting that RKIP expression may have some reference value for the survival of GIST.
The expression of RKIP protein in GISTs correlated with tumor size, NIH stage, and invasiveness of the mucosa (invasion degree), and each index suggested that the higher the degree of malignancy was associated with lower or more loss of RKIP expression. When compared with other factors (age, sex, tumor location, etc.), there was no relationship with RKIP level. RKIP overexpression was positively correlated with the survival of patients with GISTs, which has some implications for the prognosis of patients. RKIP expression has certain reference value for the survival of GIST, but it cannot be used as an independent factor to evaluate the prognosis of GISTs.
As a complex protein, RKIP has a “multi-directional switch” function on many cell conduction pathways. RKIP remains a hot topic in recent cancer research. However, data on the relationship between RKIP and the pathogenesis and treatment of GIST still remains unclear. Additional studies on the mechanisms underlying RKIP expression disorder will possibly find that RKIP protein is marker protein for prognostication of GIST. In addition to its potential as a monitoring indicator, regulation and target treatment of RKIP could be a treatment option for GIST. Thus, RKIP may have practical value in understanding the biological characteristics and expression of GIST.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Michael Z, Janus O, Yukihiko T S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [RCA] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Ersan V, Kutlu R, Erdem C, Karagul S, Kayaalp C. Colorectal Stenting for Obstruction due to Retrorectal Tumor in a Patient Unsuitable for Surgery. J Transl Int Med. 2017;5:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (NY). 1998;279:577-580. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3111] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 4. | Shi Y. Gastrointestinal stromal tumor. People’s Medical Publishing House. 2011;. |
| 5. | Butt MO, Luck NH, Hassan SM, Abbas Z, Mubarak M. Gastritis profunda cystica presenting as gastric outlet obstruction and mimicking cancer: A case report. J Transl Int Med. 2015;3:35-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhou C, Wang S, Tang W, Qian M, Cheng G, Chen G, Hu D. A rare case of spherical calcifications presenting as a submucosal lesion on the stomach wall: An EUS analysis. Endosc Ultrasound. 2016;5:137-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Park HY, Jeon SW, Lee HS, Cho CM, Bae HI, Seo AN, Kweon OK. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc Ultrasound. 2016;5:384-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 864] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 9. | Wu D, Li JN, Qian JM. Endoscopic Diagnosis and Treatment of Precancerous Colorectal Lesions in Patients with Inflammatory Bowel Disease: How Does the Latest SCENIC International Consensus Intersect with Our Clinical Practice? J Transl Int Med. 2017;5:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Grouse L Md PhD. Galileo in Bethesda: The future of gene editing. J Transl Int Med. 2016;4:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bernier I, Jolles P. Purification and characterization of a basic 23 kDa cytosolic protein from bovine brain. BBA-BIOMEMBRANES. 1984;790:174-181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Odabaei G, Chatterjee D, Jazirehi AR, Goodglick L, Yeung K, Bonavida B. Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis. Adv Cancer Res. 2004;91:169-200. [RCA] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Skinner JJ, Rosner MR. RKIP structure drives its function: a three-state model for regulation of RKIP. Crit Rev Oncogenesis. 2014;19:483-488. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Montagnani F, Di Leonardo G, Pino M, Perboni S, Ribecco A, Fioretto L. Protracted Inhibition of Vascular Endothelial Growth Factor Signaling Improves Survival in Metastatic Colorectal Cancer: A Systematic Review. J Transl Int Med. 2017;5:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ignee A, Jenssen C, Hocke M, Dong Y, Wang WP, Cui XW, Woenckhaus M, Iordache S, Saftoiu A, Schuessler G. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017;6:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Ge N, Zhang S, Jin Z, Sun S, Yang A, Wang B, Wang G, Xu G, Hao J, Zhong L. Clinical use of endoscopic ultrasound-guided fine-needle aspiration: Guidelines and recommendations from Chinese Society of Digestive Endoscopy. Endosc Ultrasound. 2017;6:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Sandhu DS, Holm AN, El-Abiad R, Rysgaard C, Jensen C, Gerke H. Endoscopic ultrasound with tissue sampling is accurate in the diagnosis and subclassification of gastrointestinal spindle cell neoplasms. Endosc Ultrasound. 2017;6:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Okasha HH, Naguib M, El Nady M, Ezzat R, Al-Gemeie E, Al-Nabawy W, Aref W, Abdel-Moaty A, Essam K, Hamdy A. Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc Ultrasound. 2017;6:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1680] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 21. | Yamane S, Katada C, Tanabe S, Azuma M, Ishido K, Yano T, Wada T, Watanabe A, Kawanishi N, Furue Y. Clinical Outcomes in Patients with Cancer of Unknown Primary Site Treated By Gastrointestinal Oncologists. J Transl Int Med. 2017;5:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Granovsky AE, Clark MC, McElheny D, Heil G, Hong J, Liu X, Kim Y, Joachimiak G, Joachimiak A, Koide S. Raf kinase inhibitory protein function is regulated via a flexible pocket and novel phosphorylation-dependent mechanism. Mol Cell Biol. 2009;29:1306-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Hori N, Chae KS, Murakawa K, Matoba R, Fukushima A, Okubo K, Matsubara K. A human cDNA sequence homologue of bovine phosphatidylethanolamine-binding protein. Gene. 1994;140:293-294. [PubMed] |
| 24. | Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell. 2006;23:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Ziogas A, Lorenz IC, Moelling K, Radziwill G. Mitotic Raf-1 is stimulated independently of Ras and is active in the cytoplasm. J Biol Chem. 1998;273:24108-24114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, Ahn NG. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142:1533-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Zecevic M, Catling AD, Eblen ST, Renzi L, Hittle JC, Yen TJ, Gorbsky GJ, Weber MJ. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J Cell Biol. 1998;142:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Yesilkanal AE, Rosner MR. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: regulation of signaling networks in cancer. Crit Rev Oncogenesis. 2014;19:447-454. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Yang KG. Progress in research of RKIP in tumors. Lingnan Mod Clin Surg. 2015;15:514-515. |
| 32. | Schuierer MM, Bataille F, Hagan S, Kolch W, Bosserhoff AK. Reduction in Raf kinase inhibitor protein expression is associated with increased Ras-extracellular signal-regulated kinase signaling in melanoma cell lines. Cancer Res. 2004;64:5186-5192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, García JJ, Kolch W. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res. 2005;11:7392-7397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Zhang L, Fu Z, Binkley C, Giordano T, Burant CF, Logsdon CD, Simeone DM. Raf kinase inhibitory protein inhibits beta-cell proliferation. Surgery. 2004;136:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Minoo P, Zlobec I, Baker K, Tornillo L, Terracciano L, Jass JR, Lugli A. Loss of raf-1 kinase inhibitor protein expression is associated with tumor progression and metastasis in colorectal cancer. Am J Clin Pathol. 2007;127:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Lossignol D. A little help from steroids in oncology. J Transl Int Med. 2016;4:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Li HZ, Wang Y, Gao Y, Shao J, Zhao XL, Deng WM, Liu YX, Yang J, Yao Z. Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res. 2008;6:917-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Akaishi J, Onda M, Asaka S, Okamoto J, Miyamoto S, Nagahama M, Ito K, Kawanami O, Shimizu K. Growth-suppressive function of phosphatidylethanolamine-binding protein in anaplastic thyroid cancer. Anticancer Res. 2006;26:4437-4442. [PubMed] |
| 39. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 40. | Schoppmann SF, Beer A, Nirtl N, Ba-Ssalamah A, Brodowicz T, Streubel B, Birner P. Downregulation of phosphatidylethanolamine binding protein 1 associates with clinical risk factors in gastrointestinal stromal tumors, but not with activation of the RAF-1-MEK-ETV1 pathway. Cancer Lett. 2013;335:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Martinho O, Granja S, Jaraquemada T, Caeiro C, Miranda-Gonçalves V, Honavar M, Costa P, Damasceno M, Rosner MR, Lopes JM. Downregulation of RKIP is associated with poor outcome and malignant progression in gliomas. PLoS One. 2012;7:e30769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Yu M, Wang Q, Ding JW, Yang Z, Xie C, Lu NH. Association between raf kinase inhibitor protein loss and prognosis in cancers of the digestive system: a meta-analysis. Cancer Biomark. 2014;14:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Valadão M, Braggio D, Santos AF, Pimenta-Inada HK, Linhares E, Gonçalves R, Romano S, Vilhena B, Small I, Cubero D. Involvement of signaling molecules in the prediction of response to imatinib treatment in metastatic GIST patients. J Surg Res. 2012;178:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |