Published online Jun 21, 2018. doi: 10.3748/wjg.v24.i23.2457
Peer-review started: April 18, 2018
First decision: April 27, 2018
Revised: May 6, 2018
Accepted: May 18, 2018
Article in press: May 18, 2018
Published online: June 21, 2018
Processing time: 59 Days and 16 Hours
The biologic antitumor necrosis factor alpha (anti-TNFα) agents have revolutionised the treatment of inflammatory bowel disease (IBD). However, some patients experience primary nonresponse, loss of response, or intolerance. Therefore, introducing a newer class of therapy with a mechanism of action that acts on different inflammatory pathways involved in IBD pathogenesis is appealing. Vedolizumab is a fully humanised monoclonal antibody that selectively targets α4β7 integrin. Based on the results of the pivotal clinical GEMINI trials, vedolizumab was approved for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) and Crohn’s disease (CD) refractory or intolerant to either conventional therapy or TNFα inhibitors. This review describes the efficacy, safety, and tolerability of vedolizumab reported in both randomized, controlled, clinical trials and from real-world experience in patients with UC and CD in order to identify its place in treatment algorithms for IBD.
Core tip: Vedolizumab represents an interesting new therapeutic option for the treatment of patients with moderate-to-severe ulcerative colitis and Crohn’s disease that are refractory or intolerant to either conventional treatments or anti-TNFα agents. This review describes the efficacy, safety, and tolerability of vedolizumab demonstrated in the clinical GEMINI trials. In addition, the paper reviews the effectiveness and the safety of vedolizumab in the real-world studies in order to identify its place in treatment algorithms for patients with inflammatory bowel disease.
- Citation: Scribano ML. Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence. World J Gastroenterol 2018; 24(23): 2457-2467
- URL: https://www.wjgnet.com/1007-9327/full/v24/i23/2457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i23.2457
The introduction of biologic, antitumor necrosis factor alpha (anti-TNFα) therapies has transformed the management of patients with moderate-to-severe, active inflammatory bowel diseases (IBD) that are refractory to conventional treatments[1-3]. However, a proportion of patients do not respond to these drugs, loose their response over time, or are intolerant to these treatments[4-6]. Additionally, the efficacy of a second anti-TNFα agent is lower in patients who have previously received an anti-TNFα drug[7]. Therefore, the advent of a newer class of therapy, characterized by a different mode of action, is an attractive option for patients with IBD.
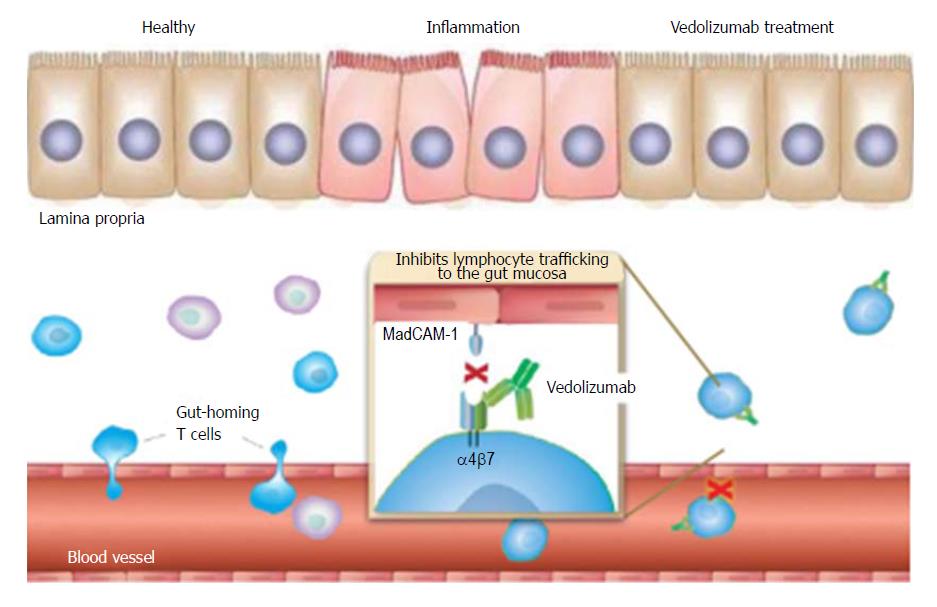
Vedolizumab is a fully humanised monoclonal IgG-1 antibody that selectively inhibits the interaction between α4β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1). It prevents lymphocyte translocation from the blood into the inflamed gut tissue, resulting in a reduction in local inflammation[8,9] (Figure 1).
The efficacy of vedolizumab for the induction and maintenance of remission in patients with IBD was demonstrated in the pivotal phase III GEMINI studies[10-12]. Based on the results of these randomized, double-blind, placebo-controlled trials, vedolizumab was approved for the treatment of adult patients with moderate-to-severe active ulcerative colitis (UC) and Crohn’s disease (CD) who had an inadequate response to either standard therapies or TNFα antagonists by both the European Medicines Agency and the US Food and Drug Administration.
However, all randomized controlled trials (RCTs) have restrictive enrolment criteria and, in order to include a highly selected and homogeneous population, tend to exclude several groups of patients[13]. This limits the generalisation of RCT results to patients commonly seen in general practice.
Patients in real-world cohorts tend to have more complicated diseases, multiple comorbidities, variable treatment regimens applied with flexibility, and follow-up controls that are not fixed. In addition, the goals of therapy in clinical practice are variable and are specific for the single patient (“treat to target”)[14]. Therefore, evaluating biologic therapies is highly relevant in the clinical practice setting.
To date, several real-world studies on the effectiveness and safety of vedolizumab in patients with moderate-to-severe, active UC and CD have been published[15-26]. This paper reviews the efficacy of vedolizumab for the treatment of IBD from the randomized controlled clinical trials (GEMINI program) and in the GEMINI long-term safety (LTS) study[27,28], the effectiveness of vedolizumab in the real-world studies, and the drug’s safety profile.
The efficacy of vedolizumab for inducing and maintaining remission in patients with UC was demonstrated in the GEMINI 1 study, a trial involving more than 800 patients with moderate-to-severe UC, defined as a Mayo score[29] of 6-12, with an endoscopic subscore ≥ 2[10]. The trial consisted of two induction cohorts; a double-blind cohort including 374 patients randomized to receive vedolizumab 300 mg intravenous (iv) or placebo at weeks 0 and 2, and a second additional cohort of 521 patients receiving open-label vedolizumab aimed to generate the needed number of responders to fulfil sample-size requirements for the maintenance phase. Eligible patients had no response to or unacceptable adverse events from steroids, immunosuppressive drugs, or anti-TNFα therapy.
In the first cohort, a significantly higher rate of patients treated with vedolizumab achieved clinical response, clinical remission, and mucosal healing after 6 wk compared to placebo. The primary outcome of the induction phase, clinical response at week 6, was achieved by 47.1% of patients treated with vedolizumab vs 25.5% of patients in the placebo group (P < 0.001) (Table 1).
| Study | n (patients) | Setting of trial | Treatment arms | Clinical response (%) | Clinical remission (%) | CS-free remission (%) | Mucosal healing (%) |
| GEMINI 1[10] 2013 | 374 | Induction | 300 mg | 47.1 | 16.9 | - | 40.9 |
| Placebo | 25.5 | 5.4 | 24.8 | ||||
| Maintenance | 300 mg 4 weekly | - | 44.8 | 45.2 | 56.0 | ||
| 300 mg 8 weekly | 41.8 | 31.4 | 51.6 | ||||
| Placebo | 15.9 | 13.9 | 19.8 | ||||
| GEMINI 2[11] 2013 | 368 | Induction | 300 mg | 31.4 | 14.5 | - | - |
| Placebo | 25.7 | 6.8 | |||||
| Maintenance | 300 mg 4 weekly | 45.5 | 36.4 | 28.8 | - | ||
| 300 mg 8 weekly | 43.5 | 39.0 | 31.7 | ||||
| Placebo | 30.1 | 21.6 | 15.9 | ||||
| GEMINI 3[12] 2015 | 315 | Induction | 300 mg | - | 15.2 | - | - |
| Placebo | 12.1 |
Patients from both cohorts achieving clinical response to vedolizumab at 6 wk were randomized to receive vedolizumab 300 mg iv every 4 wk or 8 wk, or to receive placebo in the maintenance phase for up to 52 wk. The results of the maintenance phase were as impressive as those in the induction phase. The rates of clinical remission at week 52, the primary outcome of the maintenance phase, were significantly higher in patients treated with vedolizumab than in those treated with placebo (44.8% in the vedolizumab 4-weekly group, 41.8% in the vedolizumab 8-weekly group, and 15.9% in the placebo group; P < 0.001). Durable clinical remission (defined as remission at week 6 and week 52) was also reported by significantly more patients in the vedolizumab groups (24.0% in the vedolizumab 4-weekly group, 20.5% in the vedolizumab 8-weekly group, and 8.7% in the placebo group; P = 0.001 and P = 0.008, respectively, vs placebo). Vedolizumab was also associated with greater mucosal healing rates (P < 0.001 for both vedolizumab groups vs placebo) and significantly higher rates of steroid-free remission (P < 0.001 for both vedolizumab groups vs placebo) (Table 1).
A clear difference in efficacy between the 4- and 8-weekly vedolizumab regimens was not observed. Efficacy was reported by both patients with previous exposure to anti-TNFα therapy as well as those who were anti-TNFα therapy-naïve; however, slightly better outcomes were seen in patients who were TNFα-inhibitor-naïve.
The efficacy of vedolizumab in patients with moderately to severely active CD was demonstrated in the GEMINI 2 and GEMINI 3 clinical trials[11,12]. In GEMINI-2, 368 patients were randomized to receive either vedolizumab 300 mg iv or placebo at week 0 and week 2[11]. Additionally, as in the GEMINI 1 trial, a second cohort of 747 subjects was treated with vedolizumab in an open-label fashion. All patients enrolled had active disease defined by a Crohn’s Disease Activity Index (CDAI)[30] of 220-450, and had one of the following: serum C-reactive protein (CRP) > 2.87 mg/L or colonoscopic documentation showing ≥ 3 large ulcers or ≥ 10 aphthous ulcers, or faecal calprotectin concentrations > 250 μg/g in conjunction with computed tomography or magnetic resonance enterography, small-bowel radiography, or capsule endoscopy revealing Crohn’s ulcers. Eligible patients had no response to or unacceptable adverse events from steroids, immunosuppressive drugs, or anti-TNFα drugs.
Two coprimary endpoints in the induction trial, clinical remission and CDA-100 response, were evaluated at week 6. A significantly greater proportion of patients receiving vedolizumab achieved clinical remission at 6 wk with respect to the placebo group (14.5% vs 6.8%; P = 0.02) (Table 1). However, the CDAI-100 response rate was comparable to the placebo (31.4% vs 25.7%; P = 0.23).
During the maintenance phase, 461 patients who were vedolizumab responders were randomized to receive vedolizumab 300 mg iv administered at either 4- or 8-weekly intervals up to week 52. Clinical remission at week 52, the primary endpoint of this phase, was significantly greater in patients assigned to vedolizumab therapy every 4 wk or 8 wk (36.4% and 39.0%) than in the placebo group (21.6%; P = 0.004 and P < 0.001, respectively, vs placebo). The rates of steroid-sparing remission were also significantly higher among patients treated with vedolizumab (P = 0.04 and P < 0.02, respectively, vs placebo), while the rates of durable clinical remission showed no significant differences (Table 1).
Similar results were observed in the GEMINI 3 trial, which evaluated the efficacy of vedolizumab in 315 patients with moderately to severely active CD and inadequate response, loss of response, or intolerance to previous TNFα antagonists[12]. Patients were assigned randomly to receive vedolizumab 300 mg iv or placebo at weeks 0, 2, and 6. Clinical remission at week 6 was observed in 15.2% of vedolizumab patients compared to 12.1% in the placebo group (P = 0.4) (Table 1). Therefore, the primary endpoint of the study was not met. However, the rates of clinical remission at week 10 were significantly higher in patients treated with vedolizumab (26.6% vs 12.1% in the placebo group; p = 0.001). The benefit in this population was therefore observed at week 10, suggesting a delayed response in obtaining clinical remission. In clinical practice, there is an opportunity for a fourth induction dose at week 10 in patients with CD, with insufficient response to the first three administrations of vedolizumab.
A meta-analysis pooling data from the phase II and phase III randomized controlled studies involving patients with active CD showed that vedolizumab increased the rates of clinical remission and CDAI-100 response during the induction phase, although it failed to meet some of the primary endpoints of the GEMINI 2 and GEMINI 3 trials[31].
An interim analysis of the efficacy data from the GEMINI LTS study was recently published[27,28]. The GEMINI LTS study is an ongoing, open-label, extension trial in patients with UC and CD designed to investigate the long-term safety of vedolizumab in patients with IBD. In addition, an exploratory evaluation of long-term clinical efficacy was also performed. Patients were enrolled from the long-term, phase II, C13004 study and from the GEMINI 1, GEMINI 2, and GEMINI 3 trials. A remaining part of the population consisted of patients with IBD who were vedolizumab-naïve who were included directly into the GEMINI LTS trial. A total of 894 patients with UC and 1349 with CD were enrolled in the GEMINI LTS. All patients received vedolizumab 300 mg iv every 4 wk.
Populations evaluated during the efficacy analysis of the GEMINI LTS included only patients with moderate-to-severe UC (532/894) or CD (1297/1349); patients from the C13004 study were excluded because some patients with mild IBD were enrolled in this study.
Outcomes of clinical response and remission, evaluated using a partial Mayo score in UC and the Harvey-Bradshaw Index[32] in CD, were assessed after up to 152 wk of therapy. The results showed that among patients with UC having a response to vedolizumab at week 6 in the GEMINI 1 study, 88% (n = 120/136) and 96% (n = 70/73) were in clinical remission after 104 and 152 wk, respectively. Similarly, the rates of remission reported by the patients with CD who responded to the induction phase of the GEMINI 2 study were 83% (n = 100/120) and 89% (n = 62/70) at the same time points.
An increase in dosing frequency in patients who had withdrawn early from the GEMINI 1 and GEMINI 2 studies, treated every 8 wk to every 4 wk in the GEMINI LTS trial, resulted in remission rates of 28% and 32%, respectively, after 52 wk. Similar improvements were observed regardless of previous anti-TNFα therapy.
A retrospective evaluation of mucosal healing after treatment with vedolizumab in patients with IBD enrolled in the GEMINI LTS study at Leuven University Hospital was recently reported[33]. A total of 58 patients (34 UC, 24 CD), previously exposed to anti-TNFα therapy, were endoscopically followed for a median duration of 3.2 years. Mucosal healing, corrected with non-responder imputation, was reported by 50% of patients with UC and 29% with CD. Additionally, 32.4% of patients with UC and 20.8% with CD achieved histological healing. A significant correlation between mucosal and histological healing was observed in both patients with UC and CD.
Several prospective and retrospective real-life studies of vedolizumab in patients with moderate-to-severe UC and CD have been published by authors from Europe and the United States[15-26].
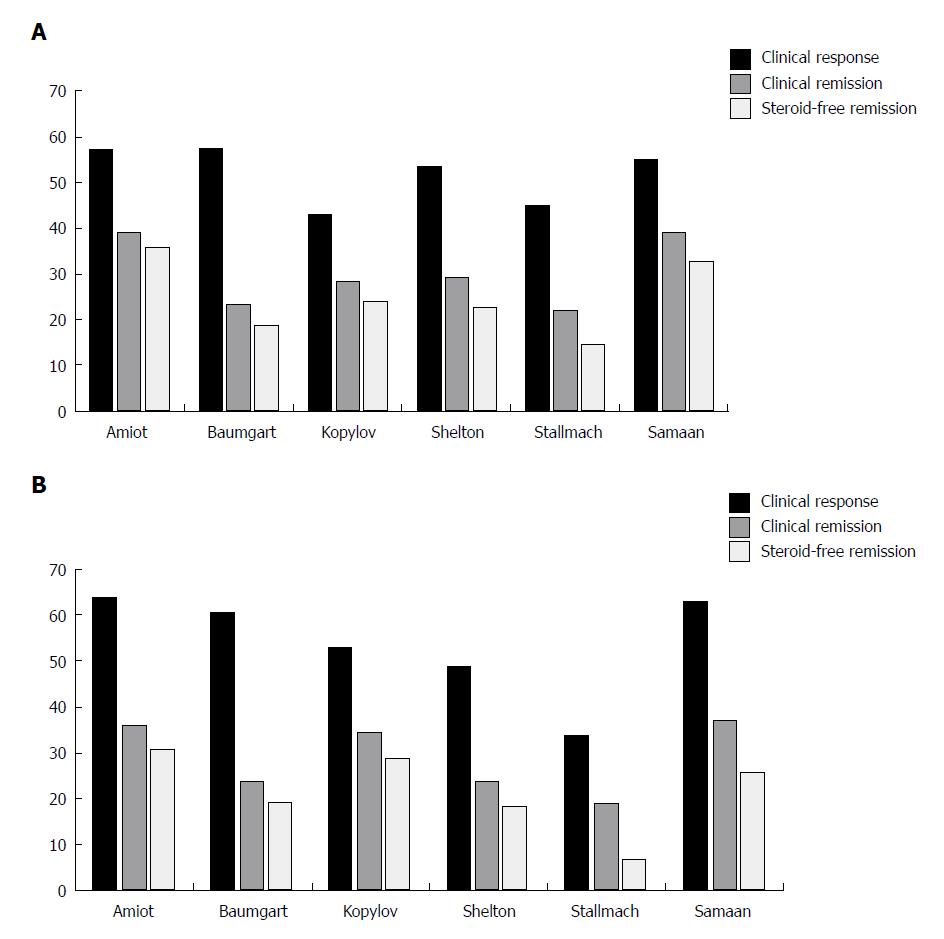
To date, the French GETAID group (Groupe d’ Etude Therapeutique des Affections Inflammatoires du tube Digestif) has published the largest real-world cohort comprising 294 patients with moderately to severely active IBD who were followed prospectively until week 54[15,16] (Table 2). Almost all patients had previously failed at least one anti-TNFα agent, with 91% having failed two. Patients received vedolizumab 300 mg iv at weeks 0, 2, and 6 then every 8 wk afterward up to week 52. At week 6, 32% of patients with UC and 31% with CD were in clinical remission. The primary outcome of the induction study, steroid-free clinical remission at week 14, was reported by 36% and 31% of patients with UC and CD, respectively[15] (Figure 2A and B). At week 54, steroid-free clinical remission rates were 40.5% in patients with UC and 27.2% in patients with CD[16]. Mucosal healing, assessed between weeks 30 and 54, occurred in 54.8% of patients with UC and 29.8% with CD. However, it was evaluated in only a small proportion of the population, and it is possible that patients with active disease were reassessed less frequently. A significant number of patients experienced inadequate response or loss of response during the year of treatment, and vedolizumab therapy was optimized (300 mg every 4 wk) in 54% of patients. Dose optimization induced or restored clinical response in 41% of patients, of whom 30% achieved clinical remission.
| Study author (country) years | IBD | CD | UC |
| Amiot et al[15,16] (France), 2016-2017 | 294 | 173 | 121 |
| Eriksson et al[19] (Sweden), 2017 | 246 | 147 | 991 |
| Baumgart et al[17] (Germany), 2016 | 212 | 97 | 115 |
| Dulai et al[23] (United States, multicentre), 2016 | 212 | 212 | - |
| Kopylov et al[20] (Israel), 2017 | 204 | 130 | 741 |
| Shelton et al[24] (United States, Boston), 2015 | 172 | 107 | 651 |
| Macaluso et al[22] (Italy, Sicily), 2018 | 163 | 84 | 79 |
| Allegretti et al[26] (United States, Boston), 2017 | 136 | 96 | 40 |
| Stallmach et al[18] (German Registry), 2016 | 127 | 67 | 60 |
| Vivio et al[25] (United States, Saint Louis), 2016 | 102 (51)2 | 30 | 21 |
| Samaan et al[21] (United Kingdom), 2017 | 50 | 27 | 231 |
| Total | 1.918 | 1.170 | 697 |
Predictors of clinical effectiveness were assessed by the authors, who found that a clinical response at week 6, baseline CRP > 20 mg/L, and a higher baseline disease activity were predictive of steroid-free remission at week 14 in both groups[15]. In addition, patients with UC and CD who achieved a clinical response at week 6 were more likely to achieve steroid-free clinical remission at week 54 (P < 0.001)[16].
A German National cohort study prospectively included 212 patients with IBD, most of whom were anti-TNFα experienced[17] (Table 2). The results showed that clinical remission at week 6 was reached by 11.3% and 15.5%, and at week 14 by 23.5% and 23.7%, of patients with UC and CD, respectively (Figure 2A and B). This group identified a low HBI at baseline and hospitalization in the past 12 mo as independent predictors of clinical remission at week 14 in patients with CD.
These data were followed by a longer-term study that included some patients from the previous German induction cohort as well as additional patients with IBD[18] (Table 2). Based on nonresponding imputation analysis, clinical remission was observed in 22% and 19% of patients with UC and CD, respectively, at week 14 (Figure 2A and B). Clinical remission at week 54 was reported by 15/60 (25%) patients with UC and 14/67 (21%) patients with CD. Nonresponse status at week 14 was an indicator of a low likelihood of clinical response and remission at week 54 in patients with both diseases. In addition, the reduction of CRP at week 14 in patients with UC and CD, and of faecal calprotectin in patients with UC, was predictive of clinical remission at week 54.
More recently, a large prospective cohort based on the Swedish National Quality Registry for IBD (SWIBREG), evaluating the data at week 12, 52 and the last follow-up, reported that clinical remission was obtained with vedolizumab after 52 wk in 64% and 60% of patients with UC and CD, respectively, 86% of whom had previously used TNFα inhibitors[19] (Table 2). Elevated CRP at baseline and prior use of anti-TNFα were associated with a higher risk of vedolizumab discontinuation.
The large, Israeli, real-world study in patients with IBD who had high rates of previous anti-TNFα therapy, showed similar efficacy of vedolizumab in patients with UC and CD[20] (Table 2; Figure 2A and B). A retrospective cohort of 50 patients with IBD from the UK demonstrated similar rates of effectiveness compared to the other real-world studies[21] (Table 2; Figure 2A and B).
Very recently, real-world data on the effectiveness of vedolizumab on gut and articular symptoms in 163 patients with IBD were reported by an Italian group[22] (Table 2). Steroid-free remission was observed in 71 (43.6%; UC: 45.6%, CD: 41.7%) and 29 (40.8%; UC: 40.0 %, CD: 41.7%) patients at weeks 10 and 22, respectively. At the same time points, a response on articular symptoms was achieved in 39.5% and 45.4% of patients with IBD who had active spondyloarthritis at baseline. The only factor associated with response on articular manifestations was the coexistence of a concomitant intestinal benefit at both weeks 10 and 22. These data suggest that the improvement of articular symptoms could be mainly related to the intestinal response.
A large real-world cohort in the United States was published by the US VICTORY (Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases) consortium and included 212 patients with CD, 90% of whom were TNFα-antagonist exposed[23] (Table 2). This retrospective study of seven medical centres from across the United States reported rates of clinical remission of 18%, 35%, and 54% at 6, 12 and 18 mo, respectively. Prior TNFα-inhibitor use, severe disease activity, active perianal disease, and smoking history were associated with a lower likelihood of achieving clinical remission. Cumulative rates of mucosal healing and “deep remission” (defined as a combination of clinical remission and mucosal healing) after 12 mo were observed in 63% and 26% of patients with CD, respectively. Patients with previous anti-TNFα treatment and severe disease activity were less likely to obtain mucosal healing.
In another study from the United States, two centres in Boston enrolled 172 patients with IBD, almost all with previous use of TNFα antagonist[24] (Table 2). Similar rates of clinical response, clinical remission, and steroid-free remission at week 14 were reported by patients with UC and CD (Figure 2A and B). Early response at week 6 was a significant predictive factor of week 14 response/remission in patients with UC, with a trend toward significance in those with CD. Elevated CRP (> 8 mg/L) at baseline was associated with a lower likelihood of achieving clinical response/remission in patients with both diseases.
Vivio et al[25] reported data on 102 patients with IBD, of whom 51 were followed prospectively (Table 2). At week 14, 55% of the patients with UC in the prospective cohort achieved clinical remission. Rates of mucosal healing and endoscopic improvement after a median treatment duration of 22 wk were higher in patients with UC (69% and 76%) than in patients with CD (30% and 52%).
The combination of vedolizumab and anti-TNFα drugs (infliximab or adalimumab) in the treatment of IBD was very recently reported by a case series of 6 patients with UC and 4 patients with CD[34]. Before combination therapy, all patients were treated with anti-TNFα, however they still had an active disease even after the optimization of the dosage and/or the infusion interval. At the time of inclusion 4 patients received concomitant immunomodulators and 1 patient received systemic corticosteroid. The patients were prospectively followed for at least 12 mo (median 17 mo) and at the end of the follow-up period all patients achieved clinical remission, and 8 out of 10 could stop the anti-TNFα treatment.
In 2 case reports previously published vedolizumab was successfully used in association with an anti-TNFα drug for the treatment of a patient with chronic refractory pouchitis and axial spondylarthritis and a patient with CD and erythema nodosum[35,36].
These data suggest that combination treatment of vedolizumab and anti-TNFα therapy might represent a therapeutic option in selected patients with IBD, however further larger studies are needed.
Several factors have been evaluated as predictors of response and remission to vedolizumab and a recent review summarized the current data[37]. Overall, patients with less disease activity (by clinical and inflammatory indices), naïve to TNFα inhibitors, and having higher vedolizumab trough levels at induction[38-40] had a greater likelihood of responding to treatment in both disease groups.
Concomitant immunomodulatory treatment was not associated with improved results in the GEMINI 1 and 2 studies[10,11]. However, the interpretation of these data is limited by the small sample size of patients receiving concomitant immunomodulators and their interruption during the maintenance period in both trials. In addition, the studies were not designed to address outcomes in patients on combination immunosuppressive therapy. No consistent benefit of adding an immunomodulatory agent to vedolizumab was observed in some real-world studies and in a small group of patients with IBD[15,20,23,24,41]. These data are in line with the finding that combination therapy did not lead to higher early vedolizumab trough levels[38]. A potential explanation is the low immunogenic profile of vedolizumab, which differs from that of anti-TNFα agents[42,43]. Currently, only a multicentre study and a recent case series showed that the addition of an immunomodulatory agent to vedolizumab was associated with an increased clinical response and remission in patients with CD and UC[26,44]. Further studies are needed to better define this aspect.
Safety data on vedolizumab have been evaluated from four double-blind and two open-label trials in an analysis that included over 2800 vedolizumab-exposed patients with IBD who were treated for up to 5 years[45]. A very good safety profile and minimal immunogenicity were reported.
The risk of progressive multifocal leukoencephalopathy (PML) is a potential safety concern for drugs that block lymphocyte migration. More than 500 cases have occurred in natalizumab-treated patients[46]. However, no cases of PML have been observed during treatment with vedolizumab according to the concept that the gut selectivity of vedolizumab is protective against the development of PML[47].
Vedolizumab is not associated with an increased risk of serious or opportunistic infections, and few patients (< 1%) discontinued therapy because of infection. The most common events were upper respiratory tract infections, which accounted for approximately half of the total infections. Lower respiratory tract, lung infections, and abdominal and enteric infections were reported with similar rates as those in the placebo group. Serious infections including sepsis, Clostridium difficile infections, and tuberculosis occurred very rarely (≤ 0.6% of patients). Independent risk factors for serious infections were younger age (HR = 0.97) and concomitant steroid use (HR = 1.88) in patients with CD, and prior anti-TNFα failure (HR = 1.99) in patients with UC. Concomitant narcotic analgesic use was a risk factor for patients with both CD and UC (HR = 2.72 and HR = 2.68, respectively).
The rate of malignancy was consistent with that generally reported in patients with IBD.
A total of 23 hepatobiliary events were observed among vedolizumab-treated patients, most of which were hepatic steatosis and transaminase increases. Five hepatic events were considered serious and vedolizumab was interrupted. Appropriate treatment resulted in resolution or near resolution of the liver abnormalities.
Infusion-related reactions occurred in ≤ 5% of patients, and < 1% of patients discontinued the infusion or received an incomplete dose. Transient anti-drug antibodies were reported by 4% of subjects enrolled in the GEMINI 1 and GEMINI 2 trials, suggesting that loss of response related to the development of anti-drug antibodies may be a rare event.
Additionally, a post-hoc analyses of the GEMINI 1 and GEMINI 2 studies did not report any significant differences in infections or other adverse events amongst the age groups, confirming a good safety profile in older (> 55 years) patients[48].
No higher rate of adverse events than the one expected with anti-TNFα therapy alone was observed in the small series of patients treated with a combination of vedolizumab and anti-TNFα agents.
The good safety profile of vedolizumab may be related to its mechanism of action, which is characterized by a gut-selectivity, without systemic action.
Cumulative evidence from real-world studies has not pointed out any relevant differences in infectious and noninfectious adverse events compared to those seen in the RCTs[49]. Therefore, postmarketing data have confirmed the favourable safety profile of vedolizumab observed in the GEMINI program.
Vedolizumab represents an interesting new therapeutic option for the treatment of patients with UC and CD that are refractory to either conventional treatments or TNFα inhibitors[50]. The efficacy and safety of vedolizumab in patients with IBD were demonstrated in the pivotal GEMINI studies. However, the stringent and restrictive inclusion and exclusion criteria in the study designs may limit the translation of clinical trial results into patients commonly seen in the clinic. In fact, patients enrolled in RCTs only partially represent the IBD population encountered during routine clinical practice[13].
Real-world studies confirm the effectiveness of vedolizumab in the clinical practice setting and have also evaluated long-term data. Even though the interpretation of the data is limited by significant heterogeneity in the study designs, real-world experience series provide additional relevant evidence[51]. A systematic review and pooled analysis on the effectiveness and safety of real-world studies has recently been published[52].
Mucosal healing is a relevant therapeutic target in patients with IBD because it is associated with a reduction in hospitalization, IBD-related surgery, bowel damage, and risk of colonic dysplasia. There is increasing evidence that achieving mucosal healing can favourably alter the natural course of IBD[53,54]. Therefore, the rates of long-term mucosal healing with vedolizumab reported by Noman, in keeping with the one-year mucosal healing data observed in the GEMINI 1 trial and in the real-world US VICTORY consortium study, appear very promising.
Safety data from all the GEMINI studies showed an overall rate of adverse events similar to that reported in the placebo group. In addition, an increasing amount of real-world data has confirmed the reassuring safety profile of vedolizumab over an extended treatment period.
In conclusion, vedolizumab has demonstrated efficacy and safety in patients who failed TNF-α antagonists, and should therefore be considered a valid second-line induction and maintenance therapy for these patients. In addition, along with other biologic drugs, vedolizumab should be considered as a first-line treatment for steroid-dependent and steroid-refractory patients and for patients not responding or intolerant to immunosuppressant agents, thanks to its favourable safety profile. Although head-to-head prospective trials to compare the safety of biologic drugs are not available, in patients in whom it is preferable to avoid systemic immunosuppression (patients with high risk of opportunistic infections or the elderly[55,56]), vedolizumab may be a safer alternative.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Silva AP, Naito Y, Trifan A S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Orlando A, Armuzzi A, Papi C, Annese V, Ardizzone S, Biancone L, Bortoli A, Castiglione F, D’Incà R, Gionchetti P. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: The use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 2. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 3. | Feagan BG, Lémann M, Befrits R, Connell W, D’Haens G, Ghosh S, Michetti P, Ochsenkühn T, Panaccione R, Schreiber S. Recommendations for the treatment of Crohn’s disease with tumor necrosis factor antagonists: an expert consensus report. Inflamm Bowel Dis. 2012;18:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, Van der Woude J, Baert F, Eliakim R, Katsanos K. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Fiorino G, Correale C, Fries W, Repici A, Malesci A, Danese S. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Cominelli F. Inhibition of leukocyte trafficking in inflammatory bowel disease. N Engl J Med. 2013;369:775-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 10. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 11. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1567] [Article Influence: 130.6] [Reference Citation Analysis (1)] |
| 12. | Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D’Haens G, Ben-Horin S, Xu J, Rosario M. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 536] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 13. | Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002-1007; quiz e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 14. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D’Haens G, Dotan I, Dubinsky M, Feagan B. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1408] [Article Influence: 140.8] [Reference Citation Analysis (115)] |
| 15. | Amiot A, Grimaud JC, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2016;14:1593-1601.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 16. | Amiot A, Serrero M, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46:310-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Baumgart DC, Bokemeyer B, Drabik A, Stallmach A, Schreiber S; Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice--a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Stallmach A, Langbein C, Atreya R, Bruns T, Dignass A, Ende K, Hampe J, Hartmann F, Neurath MF, Maul J. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease - a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44:1199-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, Karling P, Söderman C; SWIBREG Vedolizumab Study Group, Myrelid P, Cao Y, Sjöberg D, Thörn M, Karlén P, Hertervig E, Strid H, Ludvigsson JF, Almer S, Halfvarson J. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand J Gastroenterol. 2017;52:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 20. | Kopylov U, Ron Y, Avni-Biron I, Koslowsky B, Waterman M, Daher S, Ungar B, Yanai H, Maharshak N, Ben-Bassat O. Efficacy and Safety of Vedolizumab for Induction of Remission in Inflammatory Bowel Disease-the Israeli Real-World Experience. Inflamm Bowel Dis. 2017;23:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Samaan MA, Pavlidis P, Johnston E, Warner B, Digby-Bell J, Koumoutsos I, Fong S, Goldberg R, Patel K, Gulati S. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol. 2017;8:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Macaluso FS, Orlando R, Fries W, Scolaro M, Magnano A, Pluchino D, Cappello M, Morreale GC, Siringo S, Privitera AC. The real-world effectiveness of vedolizumab on intestinal and articular outcomes in inflammatory bowel diseases. Dig Liver Dis. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 24. | Shelton E, Allegretti JR, Stevens B, Lucci M, Khalili H, Nguyen DD, Sauk J, Giallourakis C, Garber J, Hamilton MJ. Efficacy of Vedolizumab as Induction Therapy in Refractory IBD Patients: A Multicenter Cohort. Inflamm Bowel Dis. 2015;21:2879-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Vivio EE, Kanuri N, Gilbertsen JJ, Monroe K, Dey N, Chen CH, Gutierrez AM, Ciorba MA. Vedolizumab Effectiveness and Safety Over the First Year of Use in an IBD Clinical Practice. J Crohns Colitis. 2016;10:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Allegretti JR, Barnes EL, Stevens B, Storm M, Ananthakrishnan A, Yajnik V, Korzenik J. Predictors of Clinical Response and Remission at 1 Year Among a Multicenter Cohort of Patients with Inflammatory Bowel Disease Treated with Vedolizumab. Dig Dis Sci. 2017;62:1590-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Loftus EV Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D’Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Vermeire S, Loftus EV Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BE, Danese S, D’Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Crohn’s Disease. J Crohns Colitis. 2017;11:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [PubMed] |
| 30. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 31. | Wang MC, Zhang LY, Han W, Shao Y, Chen M, Ni R, Wang GN, Wei FX, Zhang YW, Xu XD. PRISMA--efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2014;93:e326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [PubMed] |
| 33. | Noman M, Ferrante M, Bisschops R, De Hertogh G, Van den Broeck K, Rans K, Rutgeerts P, Vermeire S, Van Assche G. Vedolizumab Induces Long-term Mucosal Healing in Patients With Crohn’s Disease and Ulcerative Colitis. J Crohns Colitis. 2017;11:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Buer LCT, Høivik ML, Warren DJ, Medhus AW, Moum BA. Combining Anti-TNF-α and Vedolizumab in the Treatment of Inflammatory Bowel Disease: A Case Series. Inflamm Bowel Dis. 2018;24:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Hirten R, Longman RS, Bosworth BP, Steinlauf A, Scherl E. Vedolizumab and Infliximab Combination Therapy in the Treatment of Crohn’s Disease. Am J Gastroenterol. 2015;110:1737-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Bethge J, Meffert S, Ellrichmann M, Conrad C, Nikolaus S, Schreiber S. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4:e000127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Barré A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Yacoub W, Williet N, Pouillon L, Di-Bernado T, De Carvalho Bittencourt M, Nancey S, Lopez A, Paul S, Zallot C, Roblin X. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Williet N, Boschetti G, Fovet M, Di Bernado T, Claudez P, Del Tedesco E, Jarlot C, Rinaldi L, Berger A, Phelip JM. Association Between Low Trough Levels of Vedolizumab During Induction Therapy for Inflammatory Bowel Diseases and Need for Additional Doses Within 6 Months. Clin Gastroenterol Hepatol. 2017;15:1750-1757.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | Rosario M, French JL, Dirks NL, Sankoh S, Parikh A, Yang H, Danese S, Colombel JF, Smyth M, Sandborn WJ. Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn’s Disease. J Crohns Colitis. 2017;11:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 41. | Gouynou C, Peyrin-Biroulet L. Letter: addition of methotrexate neither restores clinical response nor improves the pharmacokinetic profile of vedolizumab-treated patients. Aliment Pharmacol Ther. 2017;46:1019-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2374] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 43. | Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Macaluso FS, Orlando R, Renna S, Sapienza C, Ventimiglia M, Rizzuto G, Cottone M, Orlando A. Letter: the addition of an immunosuppressant in patients with unsatisfactory response to vedolizumab. Aliment Pharmacol Ther. 2018;47:1040-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 46. | Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, Schlain B, Campagnolo D, Belachew S, Ticho B. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 47. | Krupka N, Baumgart DC. Designing biologic selectivity for inflammatory bowel disease--role of vedolizumab. Drug Des Devel Ther. 2014;9:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Yajnik V, Khan N, Dubinsky M, Axler J, James A, Abhyankar B, Lasch K. Efficacy and Safety of Vedolizumab in Ulcerative Colitis and Crohn’s Disease Patients Stratified by Age. Adv Ther. 2017;34:542-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 49. | Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 50. | Armuzzi A, Gionchetti P, Daperno M, Danese S, Orlando A, Lia Scribano M, Vecchi M, Rizzello F; GIVI (Gruppo Italiano su Vedolizumab nelle IBD) Group. Expert consensus paper on the use of Vedolizumab for the management of patients with moderate-to-severe Inflammatory Bowel Disease. Dig Liver Dis. 2016;48:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Salleron J, Danese S, D’Agay L, Peyrin-Biroulet L. Effectiveness Research in Inflammatory Bowel Disease: A Necessity and a Methodological Challenge. J Crohns Colitis. 2016;10:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Engel T, Ungar B, Yung DE, Ben-Horin S, Eliakim R, Kopylov U. Vedolizumab in IBD-Lessons From Real-world Experience; A Systematic Review and Pooled Analysis. J Crohns Colitis. 2018;12:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 53. | Shah SC, Colombel JF, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245-1255.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 54. | Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 55. | Desai A, Zator ZA, de Silva P, Nguyen DD, Korzenik J, Yajnik V, Ananthakrishnan AN. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D’Inca R, Bossa F, Angelucci E, Biancone L, Gionchetti P. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |