Published online Jun 7, 2018. doi: 10.3748/wjg.v24.i21.2291
Peer-review started: March 27, 2018
First decision: April 11, 2018
Revised: April 27, 2018
Accepted: May 11, 2018
Article in press: May 11, 2018
Published online: June 7, 2018
Processing time: 69 Days and 20.1 Hours
To investigate the effect of dietary fiber on symptoms and esophageal function testing parameters in non-erosive gastroesophageal reflux disease (GERD) (NERD) patients.
Thirty-six NERD patients with low (< 20 g/d) dietary fiber intake were enrolled in the study. They were examined with the use of symptom questionnaire (GERD-Q), high-resolution esophageal manometry, 24-h esophageal pH-impedance examinations, and food frequency questionnaire before and after 10 d of usual diet supplemented by psyllium 5.0 g TID. Complete data of 30 patients were available to the final analysis. The obtained results were analyzed with the use of non-parametric statistics (Wilcoxon matched pairs test).
The number of patients experiencing heartburn was less (93.3% at baseline vs 40% at the end of the study, P < 0.001) and the GERD-Q score decreased (mean ± SD: 10.9 ± 1.7 vs 6.0 ± 2.3, P < 0.001) after the treatment period. Minimal resting lower esophageal sphincter (LES) pressure increased from 5.41 ± 10.1 to 11.3 ± 9.4 mmHg (P = 0.023), but no change in residual LES pressure and mean resting pressure was found. Total number of gastroesophageal refluxes (GER) decreased from 67.9 ± 17.7 to 42.4 ± 13.5 (P < 0.001) predominantly by acid and weak acid types of GERs. No significant change in mean esophageal pH and % of time pH < 4 was registered. Maximal reflux time decreased from 10.6 ± 12.0 min to 5.3 ± 3.7 min (P < 0.05).
Fiber-enriched diet led to a significant increase of minimal lower esophageal sphincter resting pressure, a decrease of number of gastroesophageal refluxes, and a decrease of heartburn frequency per week in NERD.
Core tip: Low dietary fiber intake is associated with decreased stomach and gut motility and delayed gastric emptying, which may contribute to the risk of gastroesophageal reflux and gastroesophageal reflux disease (GERD) symptom frequency. The ability of dietary fibers to bind nitric oxide contained in food may diminish its negative effect on lower esophageal sphincter pressure. Our study is the first prospective trial demonstrating that increasing dietary fiber intake results in an increase of minimal esophageal resting pressure, a decrease in the number of gastroesophageal refluxes, and a decrease in heartburn episodes per week in patients with non-erosive GERD.
- Citation: Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol 2018; 24(21): 2291-2299
- URL: https://www.wjgnet.com/1007-9327/full/v24/i21/2291.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i21.2291
Gastroesophageal reflux disease (GERD) is a common condition that is characterized by reflux of gastric content into the esophagus and is associated with symptom-related quality of life decrease and numerous complications[1-4]. Impaired gastroesophageal motility with an increased number of transient lower esophageal sphincter relaxations (TLESR), acidification of the esophagus, and low esophageal clearance are considered to be the most important factors in the pathogenesis of GERD[1,5-7]. Current treatment of GERD includes lifestyle modification, antisecretory drug use, and anti-reflux surgery[6-9]. While healing of reflux esophagitis requires profound suppression of gastric acid secretion and long-term use of maintenance treatment with proton pump inhibitors (PPIs), patients with non-erosive GERD (NERD) may also benefit from other treatment options, like lifestyle or diet modification[10]. Dietary fiber supplementation may be one of the nutrients used for usual diet modification in GERD patients. It was shown that decreased stomach and gut motility, prolonged period of gastric content evacuation, and gastric over-distension associated with low dietary fiber intake and low fiber consumption may play a crucial role in formation of hiatal hernia, which negatively interferes with anti-reflux barrier[11-13]. Increased intragastric pressure and decreased motility are also established risk factors of gastroesophageal reflux[14-22]. The beneficial effect of dietary fiber on esophageal motility in GERD patients is also assumed to be mediated through its ability to bind nitric oxide contained in food and diminish its negative influence on lower esophageal sphincter (LES) pressure[23,24]. It was demonstrated that some of the dietary fibers may affect not only the rate of gastric emptying but also decrease gastric acidity, making the number of gastroesophageal refluxes lower and reducing their damaging capacity[25].
There is no direct evidence to date on the positive influence of dietary fiber on GERD. Therefore, the aim of the present study was to evaluate the effect of dietary fiber on the presence of gastroesophageal reflux, esophageal acidity, lower esophageal sphincter pressure, and clinical manifestations of non-erosive gastroesophageal reflux disease in patients with low dietary fiber intake.
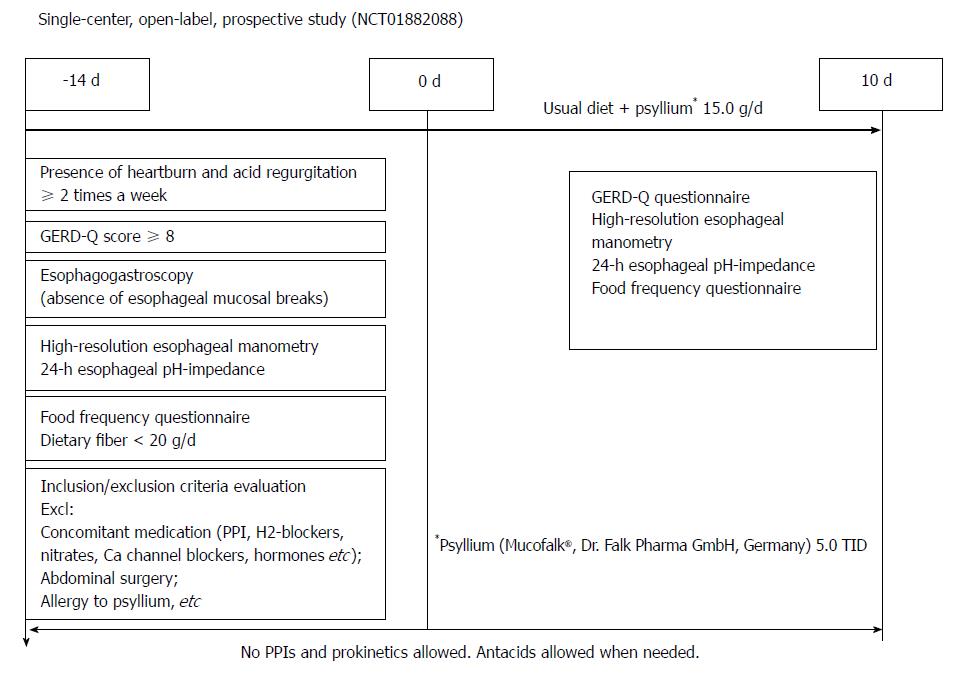
This single-center open-label prospective study was performed from 2012 to 2016 at the Department of Gastroenterology and Hepatology of Federal Research Center of Nutrition and Biotechnology according to Good Clinical Practice guidelines, the Declaration of Helsinki (1996). Study design, protocol and patients’ informed consent form were approved by the Institute of Nutrition IRB (Moscow, Russia). This study was registered on the ClinicalTrials.gov website (NCT01882088).
Enrollment criteria were: willingness to participate in the study (signed informed consent); clinical diagnosis of NERD; dietary fiber deficiency confirmed by validated dietary questionnaire; and pathological gastroesophageal reflux by 24-h esophageal pH-impedance. Exclusion criteria were as follows: antisecretory (PPI, H2-histamine receptor blockers) drug use 14 d before day 0, concomitant medications including nitrates, beta-adrenergic blocking agents, calcium channels blockers, and any of the hormones (topic steroids for less than 14 d before enrollment were allowed), inability to perform any of the diagnostic procedures required by the study protocol, allergy to psyllium, previous abdominal surgery, and general condition of the patient not allowing to participate in the study by the opinion of the investigator.
Step 1: The presence of GERD symptoms, their severity and frequency were evaluated by certified gastroenterologists. The primary selection criteria for GERD patients was the presence of heartburn and acid regurgitation for at least 2 times a week. These symptoms were verified with a language-specific version of the international GERD-Q questionnaire[26]. Symptom score of 8 points or higher was considered a positive for the presence of GERD. All patients included in the study had a history of heartburn for more than 6 mo and a previous response to acid suppressive therapy (either PPIs or H2-hystamine receptor blockers). We did our best to exclude other reasons that would mask the disease (i.e., excluded the use of medication known to affect esophageal motility and sensing; excluded functional heartburn, etc.).
Step 2: Endoscopy studies were performed using Exera II CV-180 panendoscope (Olympus Ltd, Osaka, Japan). Absence of esophageal erosions and positive results on the GERD-Q questionnaire were necessary to proceed with further examination.
High-resolution esophageal manometry (HRM). HRM studies were performed using a solid-state 36 channel 10Fr catheter (UniTip, Unisensor AG, Portsmouth, NH, United States) inserted transnasally from the pharynx to the stomach after fasting. After the patients were allowed time to adapt to the catheter placement, they were usually given 10 liquid swallows of 5 mL water. Standard software was used to analyze the obtained results (Solar GI, MMS, Enschede, the Netherlands)[27-30]. Mean and minimal resting pressure of lower esophageal sphincter pressure at rest and after 10 swallows of water, residual pressure and percent of relaxation, and their change after the course of treatment were recorded. Any type of achalasia or signs of major motility disorders by Chicago classification v 3.0[30] were exclusion criteria.
Step 3: Twenty-four h esophageal pH-impedance. Twenty-four h esophageal pH-impedance studies were made with the use of Ohmega equipment (MMS, Enschede, the Netherlands) and 2pH-6 impedance channels catheters (UniTip). The studies were performed by the standard technique[31-33]. Catheters were inserted transnasally and located with esophageal pH electrode 5 cm above the upper border of the lower esophageal sphincter, as defined by high resolution manometry. Patients were instructed to press the event marker button on the pH data logger to mark their meal times (then excluded from the analysis), body posture, symptom occurrence, and drug intake. These events together with time of onset were also marked by the patients into the paper diary to exclude mistakes. Patients were encouraged to maintain their normal daily activities throughout the measurement and to continue their regular diet. Manual review of the tracings was performed by experienced operators. Reflux episodes were defined as a decrease from baseline of more than 50% impedance moving from the distal to the proximal extent.
Step 4: Dietary intake of energy and macro- and micronutrients were determined using a validated PC-based Food Frequency Questionnaire (FFQ-1.0, Institute of Nutrition, Moscow, Russia). Dietary fiber intake deficiency was established when daily fiber intake was less than 20 g/d.
If the presence of NERD by endoscopy and GERD-Q questionnaire and low dietary fiber intake were confirmed, eligible subjects were examined with the use of high-resolution esophageal manometry and 24-h esophageal pH-impedance. Presence of pathological gastroesophageal reflux by esophageal pH-impedance studies, positive symptom index, symptom association probability, and symptom sensitivity indexes were necessary to proceed to the dietary intervention phase.
Patients were provided with psyllium (Mucofalk®, Dr. Falk PharmaGmbH, Germany) in sachets by 5.0 g and were instructed to use it three times a day (15 g per day that is an equivalent of 12.5 g of soluble dietary fiber). Psyllium was used in accordance with the manufacturer’s recommendations: the content of the sachet was mixed with at least 150 mL of water, and the resulting suspension was taken as soon as possible, followed by an additional drink of liquid (1 cup). Besides psyllium supplementation, patients were advised to follow their usual diet. Formal interview on compliance with the study drug was performed at the end of the study and the number of used and unused sachets brought by the patient was counted.
No PPIs, H2-hystamine receptors blockers, or prokinetics were allowed during the study. Antacid use was allowed when needed. It was recommended to use hydrotalcit 0.5 g (Rutacid, KRKA, Slovenia) no more than four times a day after meal. Patients were instructed to chew the tablet and then swallow it. Patients were asked to note the presence of heartburn, acid regurgitation and stool frequency during the treatment period.
Design of the study is shown on the Figure 1. Repeated 24-h esophageal pH-impedance, high resolution esophageal manometry, GERD-Q, and food frequency questionnaires were performed on the 10th day of treatment (end of treatment).
Main studied outcomes were GERD symptom presence during last 7 d, changes in the total GERD-Q score, number of reflux episodes (GER), their acidity and duration; lower esophageal sphincter (LES) mean resting pressure, minimal LES resting pressure, residual LES pressure, and percent of relaxation.
The obtained data were analyzed using standard software (Statistica 10, StatSoft Inc., United States). Wilcoxon matched pairs test of non-parametric module was used to assess changes of the studied parameters after the course of fiber supplementation in comparison to baseline. A P value of 0.05 was considered statistically significant.
Sample size calculation and power analysis. No similar studies were found in the literature to acquire data on the effect of psyllium on GERD symptoms and esophageal HRM and pH-impedance. We hypothesized that the main effect of the intervention would be a decrease in heartburn frequency. Our previous studies showed that GERD-Q score in the NERD patients group was (mean ± SD) 10.0 ± 1.5. To calculate sample size, we assumed that psyllium supplementation decrease GERD-Q score to 'normal' values (i.e., less than 8) and choose a value of 7. Sample size calculation was performed using 1-Way ANOVA[34]. Effect size calculation was performed for every comparison. Value of size effect less than 0.2 indicated a small effect, 0.5 indicated a medium-sized effect, and 0.8 or higher indicated a large-size effect.
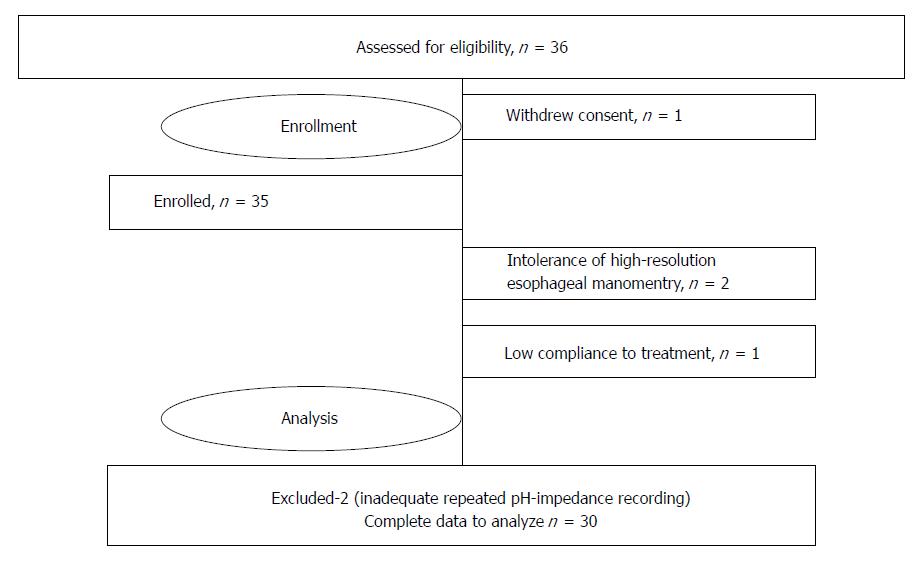
Thirty-six patients were enrolled in the study, and complete data from 30 were included in the final analysis (Table 1). One patient withdrew informed consent before day 0, and another patient was excluded due to non-compliance. One of the enrolled patients could not tolerate esophageal manometry, and in one case, there was no possibility to place the catheter due to narrow nasal passages and a deviated nasal septum. Migration of the pH-impedance probe was found in two patients during repeated examination. The data of mentioned these six patients were excluded from the final analysis (Figure 2).
| Population characteristics | Result |
| Total subjects in the study, n | 30 |
| Male/Female, n | 18/12 |
| Ethnic characteristic | Non-Hispanic Caucasians 100% |
| Age, yr, mean ± SD | 34.7 ± 9.3 |
| BMI, kg/m2, mean ± SD | 26.7 ± 6.9 |
| Weight, kg, mean ± SD | 82.5 ± 17.9 |
| Waist/hip ratio, mean ± SD | 0.91 ± 0.08 |
| Smoking, yes, n (%) | 6 (20) |
| Alcohol use, yes, n (%) | 14 (46.7) |
| Alcohol, g/d, mean ± SD | 1.1 ± 1.7 |
| Dietary fiber intake, g/d, mean ± SD | 6.0 ± 2.3 |
| Hiatal hernia | |
| Presence, n (%) | 16 (53.3) |
| Size, cm, mean ± SD | 0.9 ± 0.5 |
| Esophageal motility disorders per Chicago 3.0 | |
| Ineffective esophageal motility, n (%) | 14 (46.7) |
| Fragmented peristalsis, n (%) | 9 (30.0) |
| Normal, n (%) | 7 (23.3) |
| Mean stool frequency per week, mean ± SD | 7.0 ± 2.0 |
Complete resolution of heartburn (i.e., absence of the symptom during 7 consecutive days) was found in 18 of the 30 participants (60%) at the end-point (P = 0.0004) (Table 2). GERD-Q score decrease from (mean ± SD) 10.9 ± 1.7 at the baseline to 6.0 ± 2.3 at the end of treatment period (P < 0.001) (Table 2).
| Baseline | EOT | P value | |
| Symptoms’ characteristics | |||
| Presence of heartburn during 7 d, % of patients | 93.3 | 40 | 0.000438 |
| GERD-Q score, mean ± SD | 10.9 ± 1.7 | 6.0 ± 2.3 | 0.000003 |
| High resolution esophageal manometry (lower esophageal sphincter function) | |||
| At rest, mean ± SD | |||
| Mean resting pressure, mmHg | 22.0 ± 9.4 | 26.5 ± 11.3 | 0.37 |
| Minimal resting pressure, mmHg | 5.41 ± 10.1 | 11.3 ± 9.4 | 0.023 |
| Average, after 10 swallows of water, mean ± SD | |||
| Mean resting pressure, mmHg | 20.5 ± 9.5 | 22.0 ± 10.3 | 0.11 |
| Minimal resting pressure, mmHg | 14.1 ± 8.0 | 14.9 ± 6.4 | 0.008 |
| Residual pressure, mmHg | 7.5 ± 6.1 | 7.0 ± 5.4 | 0.94 |
| % Relaxation | 49.7 ± 15.0 | 51.3 ± 19 | 0.3 |
| Esophageal 24-hrs pH-impedance, mean ± SD | |||
| Number of refluxes | 67.9 ± 17.7 | 42.4 ± 13.5 | 0.000002 |
| Number of acid refluxes | 43.2 ± 14.7 | 30.3 ± 15.3 | 0.002415 |
| Number of weak acid refluxes | 23.9 ± 11.7 | 11.3 ± 8.27 | 0.000016 |
| Number of non-acid refluxes | 0.7 ± 1.1 | 0.6 ± 1.7 | 0.34 |
| Mean pH | 5.9 ± 0.8 | 5.7 ± 0.9 | 0.06 |
| % time pH < 4 | 5.6 ± 4.8 | 5.5 ± 7.57 | 0.20 |
| Maximal reflux time, min | 10.6 ± 12 | 5.3 ± 3.7 | 0.017 |
| Number of high gastroesophageal refluxes (17 cm above LES), mean ± SD | 23.1 ± 9.2 | 12.2 ± 6.6 | 0.000004 |
| Gastric acid exposure | |||
| Mean pH, mean ± SD | 1.2 ± 0.29 | 1.3 ± 0.36 | 0.35 |
Mean lower esophageal sphincter resting pressure increased, but it did not reach statistical significance (mean ± SD: 22.6 ± 9.4 mm Hg vs 25.6 ± 11.8 mm Hg; P = 0.47). In the majority of patients, minimal resting pressure at rest as well as during functional tests with 10 water swallows was significantly decreased by the end of the study compared to the baseline (Table 2). No influence of the treatment on residual pressure and proportion of relaxation were found during the study.
The number of all but non-acid GERs significantly decreased (Table 2), resulting in a significant shortening of maximal reflux time (mean ± SD, 10.6 ± 12.0 at baseline to 5.3 ± 3.7 minutes at the end of treatment, P = 0.017). However, no significant changes in the mean esophageal pH and proportion of time with pH < 4 in the lower esophagus were found during the study.
Dietary fiber supplementation was well tolerated. No serious adverse event was registered during the study. Because of the primary indication of psyllium (laxative), significant increase in bowel movements was expected, but it was not necessary to withdraw treatment due to severe diarrhea (stool frequency per week, mean ± SD 7 ± 2 at baseline vs 8 ± 3 at the end of the treatment period, P = 0.00002).
Antacid use was registered in two out of 30 patients, and the number of taken tablets did not exceed the allowed maximum per day.
In this open-label prospective study, we demonstrated for the first time that intake of dietary fibers increases LES minimal resting pressure and decreases the number of acid, weakly-acid, and total refluxes. It was associated also with twice as low frequency of heartburn and GERD-Q score in patients with NERD. The effect of dietary interventions on the symptoms of gastroesophageal reflux disease is poorly studied. Available data are based predominantly on epidemiological studies. In the HUNT study in a Swedish population there was a negative correlation between coffee intake and reflux symptoms, with an approximate 40% decrease in risk among people who drank more than seven cups of coffee per day compared to those who drank less than one cup (OR = 0.6; 95%CI: 0.4-0.7)[35]. Also, a moderate and dose-dependent association between increasing frequency of meals of salted fish or meat and reflux symptoms was observed (P value for linear trend = 0.0007). The risk of reflux among people who ate salted food three times per week or more was higher by 50% compared with those who never ate salted food (OR = 1.5; 95%CI: 1.2-1.8). With increasing dietary fiber content in the predominantly consumed bread type (HUNT 2; cross sectional data), the risk of reflux significantly decreased (P value for linear trend, 0.0001). People who preferred to eat bread with 7% dry weight of dietary fibers or more had an approximately halved risk of having reflux symptoms compared with those who predominantly ate white, low fiber (1%-2%) content bread (OR = 0.5; 95%CI: 0.4-0.7)[35].
In a cross-sectional study by El-Serag et al[36], a non-significant trend for higher total caloric (energy) intake and lower fiber intake among persons with GERD symptoms was found. There was a dose-response relationship between GERD symptoms and total energy (calories per day) (P = 0.06), saturated fat (P = 0.04), cholesterol (P = 0.03), and fat servings (P = 0.06) intake. Specifically, saturated fat intake was positively associated with an increased risk of GERD symptoms. The authors noted that dietary fiber intake remained inversely associated with the risk of GERD symptoms in fully adjusted models, while associations between the other nutrients and GERD symptoms were not altered in direction or magnitude of the effect after adjusting for BMI, energy consumption, or demographics[36]. Surprisingly, despite solid epidemiological evidence of the possible protective effects of dietary fibers on GERD symptoms and risks of esophageal adenocarcinoma development[24,37,38], interventional studies supporting the effect of diet modification on esophageal function are still scarce, and we did not find any in which dietary fiber was used.
Assessment of nutritional factors affecting the presence of GERD symptoms showed that low dietary fiber intake is one of the typical features of GERD patients’ diet[39,40]. Inverse medium strength correlation was found between dietary fiber intake and the presence of GERD (Spearman rank R = -0.26, P < 0.05)[40].
The significant influence of dietary fiber on esophageal motility and especially LES function in NERD patients was found in our study (Table 2). The function of LES after different meals was also studied by Sun et al[41] in eight GERD patients during the 2 h after a standard and fatty test meal. Increase in TLESRs was found after any test meal, but a decrease in resting pressure of LES was found only after the fatty meal, which was also associated with increased numbers of reflux episodes and percent of time with pH < 4. It was concluded that the combination of a decrease in LES pressure and TLESR is a major event that resulted in more severe and prolonged refluxes in GERD patients. These data correspond with the results of our study. Significant increase in minimal resting pressure of LES was found in our patients after treatment with dietary fibers, but there were no changes in percent of LES relaxation. Therefore, at least one component of anti-reflux barrier (LES pressure) was partly restored and, accordingly, the number of refluxes of all types has to be decreased, which was shown in our study (Table 2).
In this study, increased intake of dietary fiber significantly impacted the total number of refluxes and especially acid refluxes, according to the results of 24-h esophageal pH-impedance. The effect of different diets on esophageal acid exposure was assessed in a few studies. In one cross-over study, it was shown that esophageal acid exposure was greater during the high-calorie than low-calorie diet (mean, 8.6% ± 2.0% vs 5.2% ± 1.4% time pH < 4/24 h; P < .01). No difference was observed between the high-fat and low-fat diets [mean, 8.6% ± 2.0% vs 8.2% ± 1.6% time pH < 4/24 h; P = non-significant (NS)]. In contrast, the frequency of reflux symptoms was not affected by calorie density (median, 6; range, 2-12 vs median, 8; range, 2-13; P = NS) but was increased by high-fat content (median, 11; range, 5-18 vs median, 6; range, 2-12; P < 0.05)[42]. The effect of carbohydrate quote reduction (to < 20 g of carbohydrates a day) on esophageal acid exposure and symptoms of GERD within 1 week (3 to 6 d) was assessed in a prospective study. After the start of intervention, the percentage of time with pH < 4 decreased from 5.1% to 2.5% (P = 0.022), and Johnson-DeMeester score significantly reduced (mean ± SE of 34.7 ± 10.1 before the diet vs 14.0 ± 3.7 after initiating the diet; P = 0.023)[43]. The mean GERD Symptom Assessment Scale-Distress Subscale (GSAS-ds) score decreased from 1.28 to 0.72 (P = 0.0004), and, specifically, the severity score of the symptom “heartburn or burning pain inside the chest or breast bone” improved from 1.88 ± 0.23 prior to the diet to 0.88 ± 0.23 following initiation of the diet (P = 0.019). Unfortunately, only eight subjects of the same sex were enrolled in the study, and no data regarding the actual diet and amount of dietary fiber were provided by the authors.
The dose of the dietary fiber used in the study was chosen based on the on-label information, ethical considerations, safety reasons, and the need for dose standardization. The enrolled patients had very low basal dietary fiber intake (approximately 6.0 g/d, Table 1), therefore, supplementation with 12.5 g of soluble fiber a day during the study drew near the recommended daily allowance, according to national Russian guidelines (20 g/d)[44]. This dose of dietary fiber was far from that dose recommended in the United States (14 g/1000 kcal/d, using the energy guideline of 2000 kcal/d for women and 2600 kcal/d for men, the recommended daily dietary fiber intake is 28 g/d for women and 36 g/d for men)[45]. This difference may partly explain why the mean lower esophageal sphincter resting pressure increase did not reach statistical significance (Table 2). The effects of dietary fiber on GERD symptoms seen in epidemiological studies were dose-dependent, i.e. higher dose of consumed dietary fiber was associated with a lower risk of heartburn[35]. Efficacy and safety of higher dietary fiber doses in GERD patients need to be studied in a specially designed dose escalating trial. Since the recommended daily allowances differ around the world, it seems reasonable to confirm the obtained results in countries with different dietary habits.
Psyllium was chosen for the dietary intervention because the mentioned preparation is the only dietary fiber approved as a drug in Russia. The amount of dietary fiber in the drug is controlled, in contrast to food supplements were the quantity of psyllium may somewhat differ. According to the aim of the study, we needed to guarantee the amount of dietary fiber consumed to ensure the validity of the results.
The means of supplementation may also play an important role. A healthy diet is more readily accepted by patients than regular intake of drugs or food supplements[46,47]. However, in that case, the actual amount of the fiber consumed is more difficult to control.
The performed study has a number of limitations that were predictable at the phase of planning. For example, no previous data on the influence of psyllium on esophageal motility were available. Therefore, it was not possible to estimate the sample size necessary to achieve statistically significant results on the mean lower esophageal sphincter resting or residual pressure. We suppose that the results obtained here may help to plan further studies. Another limitation is the absence of a placebo-control. Due to the nature of psyllium and its preparation, it is almost impossible to produce a comparator of similar viscosity, solubility in water, and taste. Our study did, however, provide additional data for evidence-based modification of NERD-patient diet.
In conclusion, a fiber-enriched diet led to a significant increase of minimal lower esophageal sphincter resting pressure and a decrease of the number of gastroesophageal refluxes and frequency of heartburn per week in NERD patients with low dietary fiber intake. Psyllium 5.0 g TID was well tolerated by non-erosive GERD patients with low dietary fiber intake. Larger and placebo controlled studies are needed to confirm the obtained results.
Frequency of heartburn is negatively correlated with the amount of dietary fiber consumed according to epidemiological studies. Low dietary fiber intake is associated with decreased stomach and gut motility and delayed gastric emptying, which may contribute to the risk of gastroesophageal reflux. The ability of dietary fibers to bind nitric oxide contained in food may diminish its negative effect onto low esophageal sphincter pressure, but it has not been clinically proven yet. This is the first prospective trial demonstrating that an increase of dietary fiber consumed results in a significant increase of minimal esophageal resting pressure a decrease of the number of gastroesophageal refluxes and frequency of heartburn per week in patients with non-erosive gastroesophageal reflux disease (GERD) (NERD).
Reflux disease symptoms are associated with low consumption of dietary fiber, according to epidemiological studies. However, no studies were available to date that evaluated the effect of dietary fibers on esophageal motility and reflux pattern and there were no interventional studies demonstrating the effect of dietary fibers on GERD symptoms. For the first time, we showed that additional daily consumption of 12.5 g of soluble dietary fiber is associated with an increase in minimal lower esophageal sphincter resting pressure and a decrease in the number of gastroesophageal refluxes and frequency of heartburn per week in NERD.
The main objective of the study was to evaluate the effect of increased dietary fiber consumption on the number of gastroesophageal refluxes, esophageal acidity, the lower esophageal sphincter pressure, and clinical manifestations of NERD in patients with low dietary fiber intake.
The study was conducted as a pilot single-center prospective trial with very strict inclusion criteria aimed to support the diagnosis and to exclude other reasons able to affect esophageal motility and NERD symptoms. Change in GERD-Q questionnaire score, lower esophageal sphincter function by high resolution esophageal manometry, number of different types of gastroesophageal refluxes, and acid exposure time were assessed before and after patient diet modification (increased intake of dietary fiber). Data were analyzed using non-parametric statistics.
Our study is the first prospective trial demonstrating that increasing the amount of dietary fiber consumed results in an increase of minimal esophageal resting pressure and a decrease of the number of gastroesophageal refluxes and frequency of heartburn per week in patients with non-erosive GERD. Diet modification with additional psyllium (5.0 g TID) was well tolerated by non-erosive GERD patients with low dietary fiber intake.
Our results are consistent with epidemiological studies that found an inverse correlation between the amount of dietary fibers consumed and symptoms of GERD. We demonstrated that diet modification with an addition of 12.5 of soluble fiber a day led to a decrease of GERD symptom frequency, an increase in lower esophageal sphincter resting pressure, and a decrease in the number of gastroesophageal refluxes. These findings are promising and suggest that nutritional interventions may be effective in GERD management.
Well-planned trials are needed to examine further novel potential mechanisms of nutritional support for patients with esophageal disorders. Moreover, multicenter, placebo-controlled, dose-escalating trials are necessary to confirm our results, to establish the dose necessary to reach the optimal effect on esophageal motility and NERD symptoms, and to evaluate the effect of different types of dietary fibers.
Authors acknowledge Dr. Falk PharmaGmbH, Germany, Russian office, for non-financial support of the study by providing study drug (Mucofalk®) to conduct the study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): D
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Contini S, Shimatani T, Skrypnyk IN, Yücel O S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-20; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2454] [Article Influence: 129.2] [Reference Citation Analysis (2)] |
| 2. | Ronkainen J, Aro P, Storskrubb T, Lind T, Bolling-Sternevald E, Junghard O, Talley NJ, Agreus L. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population--the Kalixanda study. Aliment Pharmacol Ther. 2006;23:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Wahlqvist P, Reilly MC, Barkun A. Systematic review: the impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther. 2006;24:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Mason J, Hungin AP. Review article: gastro-oesophageal reflux disease--the health economic implications. Aliment Pharmacol Ther. 2005;22 Suppl 1:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Armstrong D, Marshall JK, Chiba N, Enns R, Fallone CA, Fass R, Hollingworth R, Hunt RH, Kahrilas PJ, Mayrand S. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol. 2005;19:15-35. [PubMed] |
| 6. | Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391, 1391.e1-1391.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 394] [Article Influence: 23.2] [Reference Citation Analysis (35)] |
| 7. | An evidence-based appraisal of reflux disease management--the Genval Workshop Report. Gut. 1999;44 Suppl 2:S1-S16. [PubMed] |
| 8. | Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392-1413, 1413.e1-1413.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1120] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 10. | Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 342] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779-784. [PubMed] |
| 13. | Kahrilas PJ, Shi G, Manka M, Joehl RJ. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology. 2000;118:688-695. [PubMed] |
| 14. | Penagini R, Carmagnola S, Cantù P, Allocca M, Bianchi PA. Mechanoreceptors of the proximal stomach: Role in triggering transient lower esophageal sphincter relaxation. Gastroenterology. 2004;126:49-56. [PubMed] |
| 15. | Emerenziani S, Sifrim D. Gastroesophageal reflux and gastric emptying, revisited. Curr Gastroenterol Rep. 2005;7:190-195. [PubMed] |
| 16. | Nilsson M, Lagergren J. The relation between body mass and gastro-oesophageal reflux. Best Pract Res Clin Gastroenterol. 2004;18:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999;94:2840-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Stene-Larsen G, Weberg R, Frøyshov Larsen I, Bjørtuft O, Hoel B, Berstad A. Relationship of overweight to hiatus hernia and reflux oesophagitis. Scand J Gastroenterol. 1988;23:427-432. [PubMed] |
| 19. | de Vries DR, van Herwaarden MA, Smout AJ, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | El-Serag HB, Tran T, Richardson P, Ergun G. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol. 2006;41:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211. [PubMed] |
| 23. | Karamanolis G, Tack J. Nutrition and motility disorders. Best Pract Res Clin Gastroenterol. 2006;20:485-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Terry P, Lagergren J, Ye W, Wolk A, Nyrén O. Inverse association between intake of cereal fiber and risk of gastric cardia cancer. Gastroenterology. 2001;120:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Harju E. Guar gum benefits duodenal ulcer patients by decreasing gastric acidity and rate of emptying of gastric contents 60 to 120 minutes postprandially. Am Surg. 1984;50:668-672. [PubMed] |
| 26. | Kaybysheva VO, Kucheryavy YuA, Trukhmanov AS. Results of multicenter observation study on application of international questionnaire GerdQ for diagnostics of gastroesophageal reflux disease. Russian J Gastroenterol Hepatol Coloproctol. 2013;5:15-23. |
| 27. | Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677-G684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988-G997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1451] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 31. | Sifrim D, Silny J, Holloway RH, Janssens JJ. Patterns of gas and liquid reflux during transient lower oesophageal sphincter relaxation: a study using intraluminal electrical impedance. Gut. 1999;44:47-54. [PubMed] |
| 32. | Sifrim D, Holloway R, Silny J, Xin Z, Tack J, Lerut A, Janssens J. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology. 2001;120:1588-1598. [PubMed] |
| 33. | Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024-1031. [PubMed] |
| 34. | Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2198] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 35. | Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut. 2004;53:1730-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 233] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut. 2005;54:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Ness-Jensen E, Hveem K, El-Serag H, Lagergren J. Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2016;14:175-82.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 38. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] |
| 39. | Konovalova M, Morozov S, Isakov V. Nutritional pattern of Russian gastroesophageal reflux disease patients. United European Gastroenterol J. 2015;3:A291. |
| 40. | Morozov S, Konovalova M, Isakov V. Reflux type and number are related to nutritional patterns in GERD patients. United European Gastroenterol J. 2015;3:A292. |
| 41. | Sun XH, Ke MY, Wang ZF, Liu XH. [Effects of two test-meals on transient lower esophageal sphincter relaxation in patients with gastroesophageal reflux disease and mechanism of gastroesophageal reflux]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:628-633. [PubMed] |
| 42. | Fox M, Barr C, Nolan S, Lomer M, Anggiansah A, Wong T. The effects of dietary fat and calorie density on esophageal acid exposure and reflux symptoms. Clin Gastroenterol Hepatol. 2007;5:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Austin GL, Thiny MT, Westman EC, Yancy WS Jr, Shaheen NJ. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci. 2006;51:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Norms of physiological needs for energy and nutrients for different population groups of the Russian Federation. Guidelines: M: Federal center of hygiene and epidemiology of Rospotrebnadzor, 2009. . |
| 45. | US Department of Agriculture (USDA), US Department of Health and Human Services. Dietary Guidelines for Americans. Washington, DC: USDA 2005; . |
| 46. | American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1724] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 47. | Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD. Nutrition principles and recommendations in diabetes. Diabetes Care. 2004;27 Suppl 1:S36-S46. [PubMed] |