Published online Jun 7, 2018. doi: 10.3748/wjg.v24.i21.2279
Peer-review started: January 19, 2018
First decision: February 3, 2018
Revised: March 28, 2018
Accepted: April 26, 2018
Article in press: April 26, 2018
Published online: June 7, 2018
Processing time: 136 Days and 1.2 Hours
To examine the correlation between magnetic resonance imaging (MRI) and endoscopic index of severity (CDEIS) in patients with Crohn’s disease (CD).
This was a retrospective study of 104 patients with CD that were treated at the Ruijin Hospital between March 2015 and May 2016. Among them, 61 patients with active CD were evaluated before/after treatment. MRI and endoscopy were performed within 7 d. CDEIS was evaluated. MRI parameters included MaRIA scores, total relative contrast enhancement (tRCE), arterial RCE (aRCE), portal RCE (pRCE), delay phase RCE (dRCE), and apparent diffusion coefficient. The correlation and concordance between multiple MRI findings and CDEIS changes before and after CD treatment were examined.
Among the 104 patients, 61 patients were classified as active CD and 43 patients as inactive CD. Gender, age, disease duration, and disease location were not significantly different between the two groups (all P > 0.05). CRP levels were higher in the active group than in the inactive group (25.12 ± 4.12 vs 5.14 ± 0.98 mg/L, P < 0.001). Before treatment, the correlations between CDEIS and MaRIAs in all patients were r = 0.772 for tRCE, r = 0.754 for aRCE, r = 0.738 for pRCE, and r = 0.712 for dRCE (all MaRIAs, P < 0.001), followed by MRI single indexes. Among the active CD patients, 44 cases were remitted to inactive CD after treatment. The correlations between CDEIS and MaRIAs were r = 0.712 for aRCE, r = 0.705 for tRCE, r = 0.685 for pRCE, and r = 0.634 for dRCE (all MaRIAs, P < 0.001).
Arterial MaRIA should be an indicator for CD follow-up and dynamic assessment. CD treatment assessment was not completely concordant between CDEIS and MRI.
Core tip: Magnetic resonance imaging (MRI) is accurate in evaluating Crohn’s disease (CD) activity and treatment efficacy, but endoscopy (CD endoscopic index of severity) is still the first choice. There are few available data about the concordance between MRI and endoscopy findings before and after treatment. This study provides evidence that MRI indicators are the most sensitive when the disease progresses.
- Citation: Zhu NY, Zhao XS, Miao F. Magnetic resonance imaging and Crohn’s disease endoscopic index of severity: Correlations and concordance. World J Gastroenterol 2018; 24(21): 2279-2290
- URL: https://www.wjgnet.com/1007-9327/full/v24/i21/2279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i21.2279
Crohn’s disease (CD) is an inflammatory bowel disease that may involve the entire gastrointestinal tract[1]. The morbidity of CD has increased in recent years[2]. CD is characterized by segmental and transmural inflammation with nearly 70% involvement of the small bowel, particularly the terminal ileum[3,4]. Since CD can easily recur, accurate and comprehensive evaluation and follow-up are essential to design an individualized treatment program[5].
Magnetic resonance imaging (MRI) of bowels can not only display eventual lesions in the bowel mucosa and sub-mucosa, but also show mesenteric vessel changes and complications. MRI is an important method in the non-invasive diagnosis of CD[6-10]. The Crohn’s Disease Endoscopic Index of Severity (CDEIS)[11] is calculated based on endoscopy findings and can only show mucosal ulcers and stenosis. It is generally used to assess CD activity and the accuracy of MRI indicators. Nevertheless, there are few available data about the strength of the association between MRI and CDEIS for the evaluation of CD before and after treatment.
It is currently uncertain whether MRI abnormalities are concordant with changes in CDEIS and whether MRI is only a supplementary/accessory assessment method to endoscopy or could be substituted to endoscopy during follow-up. Of course, MRI is a non-invasive examination, improving the patients’ quality of life and compliance to follow-up. Tielbeek et al[12] showed that MRI is fairly reproducible but had a moderate agreement with CDEIS; nevertheless, they did not examine the two examinations during follow-up or before/after treatment. Similar results were observed by Rimola et al[6,13], but again without follow-up or treatment efficacy assessment.
Therefore, the aim of the present study was to examine the correlation and concordance between multiple MRI findings and CDEIS changes before and after CD treatment. The results could improve our understanding of CD and provide non-invasive modalities for examining the efficacy of treatments.
This was a retrospective study of 104 patients with CD and treated at the Ruijin Hospital between March 2015 and May 2016. The project was approved by the ethics committee of the Ruijin Hospital. The need for individual consent was waived because of the retrospective nature of the study.
All patients diagnosed with CD during the study period were included. The exclusion criteria were: (1) Poor MR image quality that could not be used for diagnosis and measurements; or (2) incomplete clinical data. The diagnosis of CD was based on the criteria from the World Health Organization (WHO)[14]. These criteria are: (1) Non-contiguous/segmental lesions visible by imaging, endoscopy, and/or the resected specimen; (2) manifesting as paving stones/longitudinal ulcer visible by imaging, endoscopy, and/or the resected specimen; (3) inflammatory lesions of the entire wall based on clinical manifestations and/or resected specimen showing abdominal masses, and stenosis visible by imaging and endoscopy; (4) histopathological manifestations of non-cheese-like granuloma; (5) cleft/fistula visible by imaging, endoscopy, and/or the resected specimen; and (6) anal lesions visible by clinical manifestations and/or biopsy/resected specimen. The diagnosis of CD is made in the presence of: (1) Criteria 1+2+3 and any one of 4, 5, or 6; or (2) criterion 4 and any two of 1, 2, or 3[14].
Endoscopic and MRI examinations were performed within 7 days. The disease course ranged from 1 to 5 years in all patients.
A first MRI and endoscopy were performed in the 104 included patients. According to the CDEIS score[11] before treatment, the patients were classified as active CD (CDEIS > 6) or inactive CD (CDEIS ≤ 6). A second MRI and endoscopy were conducted in 61 active CD patients after 24-26 wk of medical therapy with glucocorticoids, infliximab (IFX), or adalimumab (ADA).
Intestinal preparation was performed routinely the night before endoscopy. Double balloon enteroscopy was performed using an oral intubation depth of about 220 cm and a mean anal intubation depth of 120 cm. Colonoscopy was performed by pushing the endoscope from the anus to the distal ileum. All endoscopic examinations were performed by the same two gastroenterologists.
CDEIS was determined as previously reported[11]: CDEIS = (12 × the number of bowel segments with deep ulcers + 6 × the number of bowel segments with superficial ulcers + affected bowel surfaces with no ulcer + ulcerated surface) ÷ the total number of affected segments + 3 × the number of ulcerated stenosis + 3 × the number of stenosis with no ulcer.
All patients were instructed to fast overnight prior to the MRI examination. The patients were requested to take polyethylene glycol electrolyte powder at 8 PM the day before MRI. Isotonic mannitol solution (2.5%; 2000 mL) was prepared by adding 250 mL of hyperosmotic mannitol solution (0.05 kg of mannitol, concentration of 20%) to 1750 mL of water. Each patient was given three to four 500-mL glasses of isotonic mannitol solution (total, 1500-2000 mL) to optimize the distention of the small bowel. Each glass was given within 10 min. The first glass was given at 40-45 min before MRI. All patients completed bowel preparation before MRI.
All MRI examinations were performed using a 1.5 T MRI unit (GE Signa, HDxt, GE Healthcare, Waukesha, WI, United States). Patients were placed in the supine position with an abdomen coil. MRI was performed with the following sequences: (1) Transverse fast imaging employing steady-state acquisition (FIESTA): Echo time/repetition time (TE/TR) 1.34/3.559 ms, slice thick/gap 5/1 mm, flip angle 55, bandwidth 125, number of excitation (NEX) 1.0, frequency (Freq) 224, field of view (FOV) 40 × 40 cm; (2) coronal T2 Weight Single-Shot Fast Spin Echo (T2WSSFSE): TE/TR 74.56/1800 ms, slice thick/gap 5/1 mm, bandwidth 31.25, Freq 288, FOV 40 cm × 40 cm; (3) coronal FIESTA: TE/TR 1.364/3.285 ms, slice thick/gap 5/1 mm, flip angle 55, bandwidth 125; (4) transverse diffusion weight imaging (DWI): b values were 0, 600 s/mm2, TE/TR 67.5/1800 ms, slice thick/gap 5/1 mm, Freq 128, NEX 2.0; and (5) coronal Liver Acquisition with Volume Acceleration (LAVA) dynamic enhanced scan: TE/TR 1.452/3.12 ms, slice thickness/gap 4-4.4/1 mm, flip angle 12, bandwidth 125, Freq 288, FOV 40 cm × 40 cm; contrast agent, Magnevist 0.2 mL/kg, injection rate of 2 mL/s, enhanced scan point of 20, 50, and 90 s after contrast agent injection.
All MR images were independently reviewed by two experienced gastrointestinal radiologists who were blinded to the CDEIS results. Since the CDEIS represents the worst segment seen during endoscopy, the radiologists selected the worst segment on MRI for analysis. In the present study, each lesion observed during MRI could be matched to the endoscopy findings.
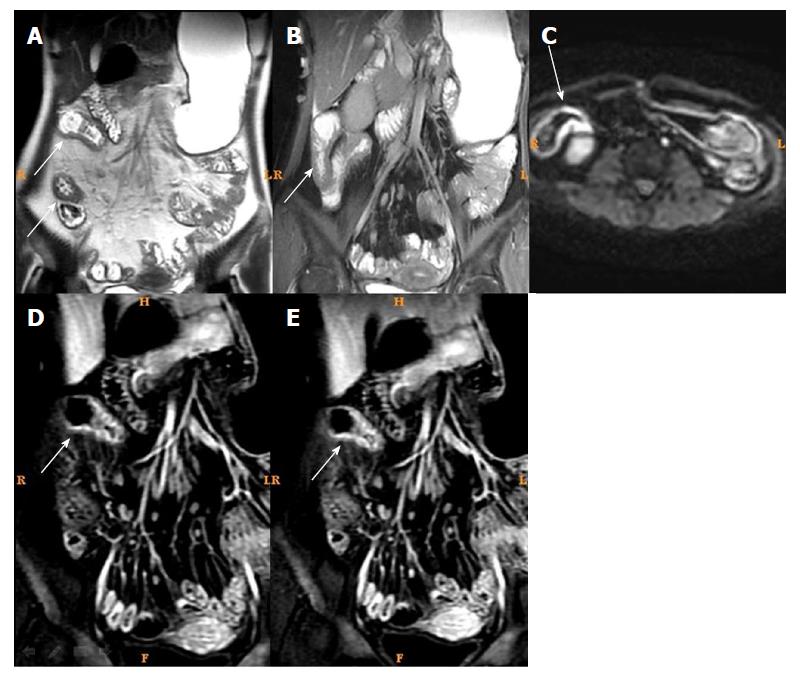
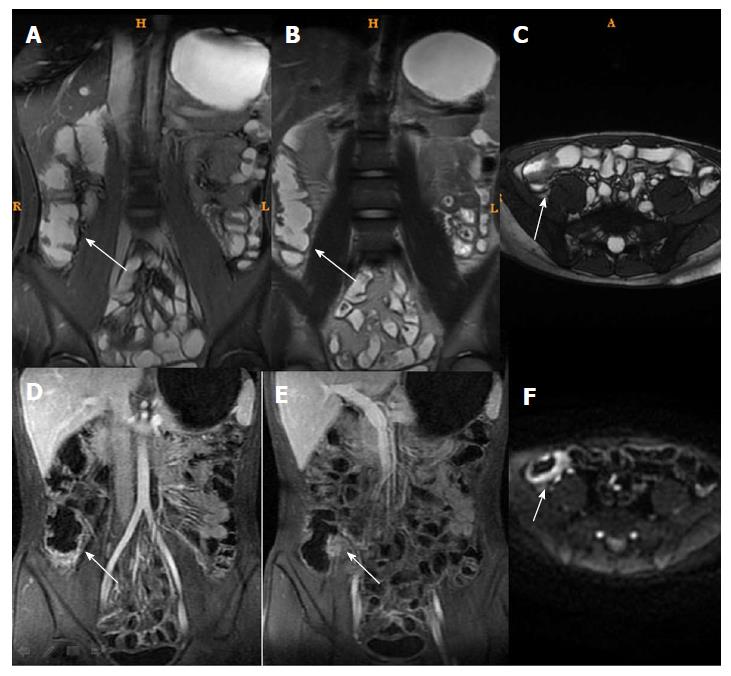
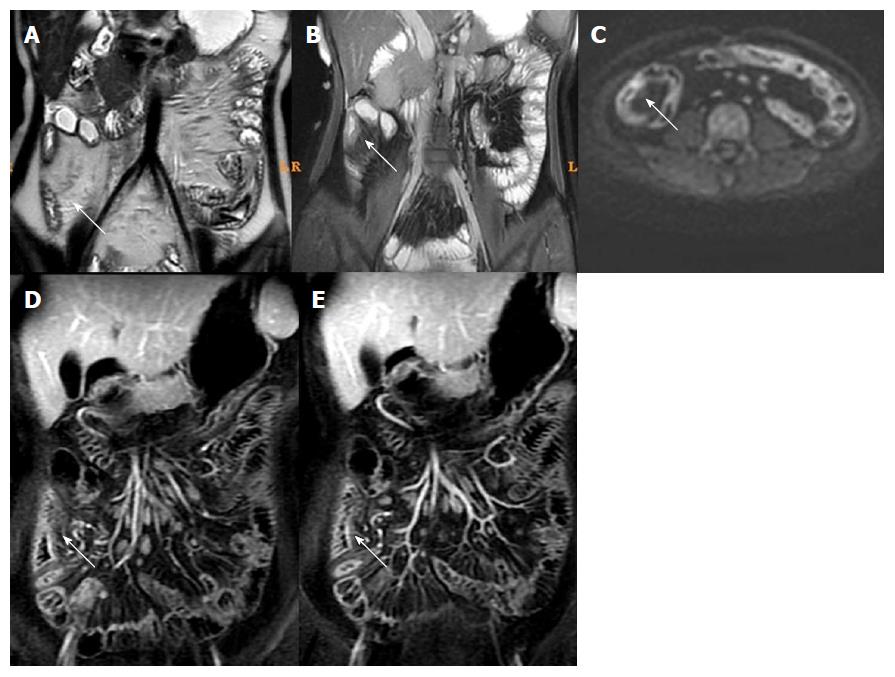
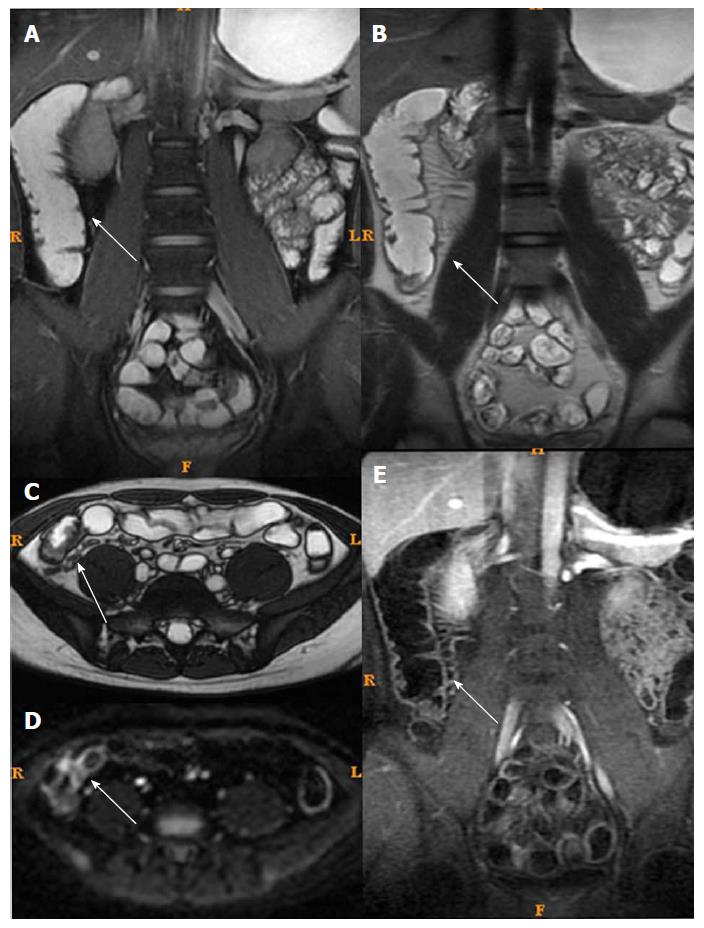
T2WI can show the intestinal wall thickening, serosal edema (T2WI high signal), and mucosal defects suggesting ulcers[6-11,15]. For each individual, bowel thickness was measured using the T2WI sequence. Wall edema[6-11,15] (hyperintensity on T2WI of bowel wall relative to the signal of the psoas muscle), ulcer in mucosa[6] (deep depression in the mucosal surface of a thickened segment), and reactive lymph nodes (enlarged > 1 cm) were observed in T2WI. LAVA dynamic enhanced sequence was used to evaluate[4,16]: (1) Wall enhancement pattern: layer stratified enhancement or non-layer stratified enhancement; (2) changes in morphology including shortened mesenteric border, pseudodiverticulum, and stenosis; and (3) perienteric exudation, wall edema, ulcer in mucosa, reactive lymph nodes, perienteric exudation, morphological changes, and layer stratified enhancement, each defined as present or absent.
For patients in the active phase, regions of interest (ROIs) of < 0.5-cm2 were placed on the mucous layer of the lesion segment. In active CD, the mucous layer can be seen clearly due to edema in the sub-mucous layer. For inactive CD, the ROI was placed on the whole bowel wall since the mucous and sub-mucous layers cannot be differentiated. According to a study by Semelka et al[17], quantitative measurement of ROIs of wall signal intensity (WSI) was conducted before and after intravenous contrast administration. Relative contrast enhancement (RCE) was calculated according to: RCE = (WSIpost-enhancement - WSIpre-enhancement)/(WSIpre-enhancement) × 100 × SDnoisepre-enhancement/SDnoise post-enhancement), where SDnoise pre-enhancement is the average of three standard deviations (SDs) of the signal intensity measured outside of the body before enhancement, and SDnoise post-enhancement presents the same noise after enhancement.
DWI can be used to measure the movement of water molecules in living bodies. In the presence of acute inflammation, the edema, exudation of intestinal wall tissue, and elevated inflammatory cytokine levels limit the movement of the water molecules in tissues and cells (i.e., the diffusion is limited). Hence, the DWI signals increase while apparent diffusion coefficient (ADC) values decrease. Those values are reversed when inflammation improves[6-11,15]. In DWI sequences, ROIs of ADC placed on the bowel wall of CD lesions were measured using the Functool Software, and the average values were obtained. A simplified Magnetic Resonance Index of Activity (MaRIA) was calculated for each segment using the formula 1.5 × wall thickness (mm) + 0.02 × RCE + 5 × edema + 10 × ulceration.
Artery enhancement sequence on T1W1 shows the blood supply of the intestine. aRCE is the enhancement rate during arterial phase and represents the degree of blood supply. pRCE is the blood supply during the portal phase. dRCE is the blood supply during the period of delay. In the presence of acute inflammation, the enhancement rates of the various phases are elevated. If the peak value of the enhancement curve is delayed, the inflammation is likely to be improved or chronic[6-11,15]. The average RCE (total RCE, tRCE; arterial phase RCE, aRCE; portal phase RCE, pRCE; delay phase RCE, dRCE) and ADC values of the lesions in each patient were obtained. ∆tRCE, ∆aRCE, ∆pRCE, ∆dRCE, ∆ADC, ∆MaRIA, ∆thickness, and ∆CDEIS were defined as ∆CDEIS = (indicators after treatment-indicators before treatment)/indicators before treatment.
If the lesions were improved after medical treatment of CD, the following MRI manifestations could be seen: (1) T2WI showed that the thickening of the intestinal wall was alleviated, edema was alleviated or had disappeared, and mucosal ulcers were healed; (2) dynamic T1W1 enhancement sequence showed that the enhancement of the lesion segment had weakened, and the intestinal wall was no longer stratified; (3) the exudation surrounding the intestines was reduced or had disappeared, and the enlarged lymph nodes surrounding the intestines had shrunk; and (4) DWI sequence showed that the signals of the diseased segment were reduced and ADC values were increased.
Statistical analysis was performed using SPSS 19.0 for Windows (IBM, Armonk, NY, United States). Categorical variables (intestinal wall edema, ulcer in mucosa, reactive lymph nodes, perienteric exudation, wall enhancement pattern, and morphological changes) were analyzed using the Spearman correlation. Continuous variables (bowel thickness, tRCE, aRCE, pRCE, dRCE, ADC values, MaRIA) were expressed as mean ± standard deviation, and the Pearson correlation analysis was performed. ∆MRI indicators were analyzed with ∆CDEIS using the Pearson correlation. An inter-observer agreement evaluation between the two radiologists was performed using the kappa statistics. Two-sided P-values < 0.05 were considered statistically significant.
Table 1 presents the characteristics of the patients. Among the 104 patients, 61 patients (male/female, 36/25; mean age, 27.5 ± 11.4 years) were classified as having active CD (CDEIS > 6) and 43 patients (male/female, 24/19; mean age, 24.4 ± 8.0 years) as having inactive CD (CDEIS ≤ 6). Gender, age, disease duration, and disease location were not significantly different between the two groups (all P > 0.05). C-reactive protein (CRP) levels were higher in the active group than in the inactive group (P < 0.001).
| All n = 104 | Active n = 61 | Inactive n = 43 | P value | |
| Gender (M/F) | 59/45 | 36/25 | 23/20 | 0.230 |
| Age | 31.37 ± 9.56 | 27.5 ± 11.4 | 34.44 ± 5.37 | 0.650 |
| Disease duration | 3.5 | 3.9 | 3.3 | 0.550 |
| Disease location | ||||
| Rectum | 0 | 0 | 0 | |
| Sigmoid/left colon | 4 | 2 | 2 | |
| Transverse colon | 14 | 7 | 7 | |
| Right colon | 16 | 10 | 6 | |
| Ileum | 70 | 42 | 28 | |
| Treatment regimen | ||||
| Glucocorticoid | 23 | 23 | 0 | |
| IFX | 18 | 18 | 0 | |
| ADA | 20 | 20 | 0 | |
| Edema | 61 | 61 | 0 | < 0.001 |
| Reactive lymph nodes | 25 | 16 | 9 | 0.311 |
| Mucosal ulcer | 49 | 38 | 11 | < 0.001 |
| Enhancement pattern | 61 | 61 | 0 | 0.006 |
| Morphological changes | 31 | 12 | 19 | 0.023 |
| Perienteric exudation | 38 | 38 | 0 | < 0.001 |
| CRP (mg/L) | 18.34 ± 8.45 | 25.12 ± 4.12 | 5.14 ± 0.98 | < 0.001 |
| ADC (mm2/s) | 1.87 ± 0.471 | 1.598 ± 0.383 | 1.949 ± 0.431 | 0.001 |
| Thickness (mm) | 7.89 ± 3.23 | 9.23 ± 3.36 | 6.75 ± 2.49 | 0.001 |
| tRCE (%) | 78.34 ± 45.34 | 92.153 ± 101.34 | 40.592 ± 11.019 | 0.017 |
| aRCE (%) | 124.45 ± 61.11 | 181.46 ± 97.80 | 92.63 ± 45.48 | < 0.001 |
| pRCE (%) | 254.21 ± 198.22 | 321.90 ± 231.03 | 201.32 ± 124.66 | 0.020 |
| dRCE (%) | 377.15 ± 223.21 | 466.18 ± 260.08 | 271.91 ± 209.66 | 0.002 |
| MaRIA | ||||
| tRCE | 20.37 ± 3.42 | 26.18 ± 5.02 | 6.44 ± 1.03 | < 0.001 |
| aRCE | 18.88 ± 4.11 | 28.40 ± 4.84 | 6.43 ± 2.74 | < 0.001 |
| pRCE | 26.32 ± 2.89 | 35.09 ± 4.64 | 6.94 ± 2.58 | < 0.001 |
| dRCE | 19.26 ± 3.21 | 36.81 ± 5.11 | 7.25 ± 2.32 | 0.001 |
| CDEIS | 8.15 ± 4.03 | 10.57 ± 3.02 | 3.46 ± 1.23 | 0.001 |
An inter-observer agreement evaluation between the two radiologists was performed using kappa statistics, which showed a high correlation (0.936) when considering all parameters. Therefore, the average results of the two radiologists were used for evaluation.
Table 1 presents the MRI findings before treatments. Higher proportions of patients in the active group showed edema, mucosal ulcer, enhancement pattern, morphological changes, and perienteric exudation than in the inactive group (all P < 0.05).
On MRI and compared with the inactive group, the active group showed lower ADC (P = 0.001) and higher thickness, tRCE, aRCE, pRCE, dRCE, and MaRIA (all P < 0.05). At endoscopy, the active group showed higher CDEIS scores than the inactive group (P = 0.001) (Table 1).
MRI quantitative parameters (ADC value, bowel thickness, tRCE, aRCE, pRCE, dRCE, and MaRIA scores) were significantly correlated with CDEIS. The highest correlation was found between MaRIA and CDEIS with coefficients of r = 0.772 for tRCE, r = 0.754 for aRCE, r = 0.738 for pRCE, and r = 0.712 for dRCE, followed by tRCE, aRCE, pRCE, dRCE, bowel thickness, and ADC value (r = 0.661, 0.634, 0.518, 0.507, 0.356, and -0.276, respectively) (Table 1).
In the active CD group, CDEIS was significantly correlated with MaRIAs, tRCE, aRCE, pRCE, dRCE, bowel thickness, and ADC, with coefficients of r = 0.789, 0.767, 0.745, 0.718, 0.726, 0.548, 0.54, 0.459, 0.311, and -0.207, respectively (Table 2). On the other hand, in the inactive CD group, MaRIA (for tRCE), and tRCE were positively correlated with CDEIS (r = 0.746 and 0.718, respectively) (Table 2, and Figures 1 and 2).
| All n = 104 | Active group n = 61 | Inactive group n = 43 | ||||
| r | P value | r | P value | r | P value | |
| ADC | -0.276 | 0.012 | -0.207 | 0.016 | -0.202 | 0.356 |
| Thickness | 0.356 | 0.001 | 0.311 | 0.002 | 0.952 | 0.013 |
| tRCE | 0.661 | < 0.001 | 0.726 | < 0.001 | 0.718 | < 0.001 |
| aRCE | 0.634 | < 0.001 | 0.548 | < 0.001 | 0.238 | 0.274 |
| pRCE | 0.519 | < 0.001 | 0.540 | < 0.001 | 0.921 | 0.022 |
| dRCE | 0.507 | < 0.001 | 0.459 | < 0.001 | 0.022 | 0.920 |
| MaRIA | ||||||
| tRCE | 0.772 | < 0.001 | 0.789 | < 0.001 | 0.746 | < 0.001 |
| aRCE | 0.754 | < 0.001 | 0.767 | < 0.001 | 0.334 | 0.288 |
| pRCE | 0.738 | < 0.001 | 0.745 | < 0.001 | 0.230 | 0.471 |
| dRCE | 0.712 | < 0.001 | 0.718 | < 0.001 | 0.280 | 0.378 |
| CDEIS | 8.15 ± 4.03 | 10.57 ± 3.02 | 3.46 ± 1.23 | |||
All 61 patients in the active group underwent MRI and endoscopy examinations after medical treatment. The correlation coefficients between CDEIS and MaRIAs were r = 0.771 for MaRIA of aRCE, r = 0.755 for MaRIA of dRCE, r = 0.740 for MaRIA of pRCE, and r = 0.736 for MaRIA of tRCE, which were all higher than that between CDEIS and single MRI parameters. Among single MRI indicators, the highest correlation was found for aRCE. The same correlation order was found between ∆MaRIAs and ∆CDEIS as that between MaRIAs and CDEIS. For single ∆MRI indicators, the correlation was in the order of ∆aRCE > ∆ADC > ∆pRCE > ∆dRCE > ∆tRCE, with r = 0.593, -0.545, 0.529, 0.512, and 0.467, respectively (Table 3). No correlation was observed between CDEIS and bowel thickness (Table 3).
| CDEIS | ΔCDEIS | ||||
| r | P value | r | P value | ||
| ADC | -0.467 | < 0.001 | ΔADC | -0.545 | 0.001 |
| Thickness | 0.242 | 0.201 | Δthickness | 0.407 | 0.148 |
| tRCE | 0.548 | 0.002 | ΔtRCE | 0.467 | 0.018 |
| aRCE | 0.619 | < 0.001 | ΔaRCE | 0.593 | 0.002 |
| pRCE | 0.493 | 0.008 | ΔpRCE | 0.529 | 0.004 |
| dRCE | 0.490 | 0.015 | ΔdRCE | 0.512 | 0.003 |
| MaRIA | ΔMaRIA | ||||
| tRCE | 0.736 | < 0.001 | tRCE | 0.724 | < 0.001 |
| aRCE | 0.771 | < 0.001 | aRCE | 0.781 | < 0.001 |
| pRCE | 0.740 | < 0.001 | pRCE | 0.724 | < 0.001 |
| dRCE | 0.755 | < 0.001 | dRCE | 0.760 | < 0.001 |
After treatment, 17 of the 61 patients remained with active CD. Table 4 presents the characteristics of these patients. Gender, age, disease duration, disease location, and CRP levels were similar between the two groups. The inactive group showed better clinical and MRI performances than the active group after treatments (all P < 0.05). In those 17 patients, no statistical correlation was found between endoscopy score and MRI indicators. The remaining 44 patients remitted into inactive CD. The correlations between CDEIS and MRI parameters in these 44 cases were in the order of MaRIA for aRCE > MaRIA for tRCE > MaRIA for pRCE > MaRIA for dRCE > aRCE > ADC value > tRCE > pRCE > dRCE, with r = 0,712, 0.705, 0.685, 0.634, 0.697, -0.516, 0.420, 0.350, and 0.341, respectively (Table 5).
| Remained active (n = 17) | Improved to inactive (n = 44) | P value | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| Gender (M/F) | 10/7 | 26/18 | 0.332 | ||
| Age | 30.4 ± 5.5 | 26.7 ± 10.1 | 0.563 | ||
| Disease duration | 1-5 | 1-5 | |||
| Disease location | 0.916 | ||||
| Sigmoid/left colon | 1 | 1 | |||
| Transverse colon | 3 | 4 | |||
| Right colon | 4 | 6 | |||
| Ileum | 14 | 28 | |||
| Treatment regimen | 0.292 | ||||
| Glucocorticoids | 8 | 15 | |||
| Infliximab | 6 | 12 | |||
| Adalimumab | 3 | 17 | |||
| CRP | 28.01 ± 5.22 | 20.91 ± 5.45 | 0.054 | ||
| CDEIS | 12.23 ± 5.12 | 10.47 ± 3.43 | 10.12 ± 2.11 | 3.11 ± 0.21 | 0.001 |
| CRP | 28.01 ± 5.215 | 15.12 ± 4.32 | 20.91 ± 5.45 | 5.84 ± 0.743 | 0.002 |
| ADC | 1.52 ± 0.12 | 1.44 ± 0.34 | 1.59 ± 0.17 | 1.73 ± 0.2 | 0.001 |
| Thickness | 9.12 ± 1.21 | 7.66 ± 1.41 | 8.2 ± 2.22 | 5.42 ± 1.32 | 0.012 |
| tRCE | 89.14 ± 13.33 | 69.49 ± 12.11 | 82.11 ± 12.47 | 45.32 ± 4.53 | 0.021 |
| aRCE | 179.03 ± 20.66 | 166.16 ± 22.44 | 181.14 ± 34.1 | 89.76 ± 12.71 | 0.001 |
| pRCE | 330.02 ± 67.12 | 285.27 ± 57.71 | 301.32 ± 54.12 | 199.23 ± 23.2 | 0.001 |
| dRCE | 453.29 ± 54.05 | 385.5 ± 45.32 | 440.18 ± 33.09 | 257.22 ± 44.13 | 0.001 |
| MaRIA | |||||
| tREC | 35.17 ± 5.66 | 30.12 ± 3.12 | 26.56 ± 2.90 | 6.23 ± 1.11 | < 0.001 |
| aRCE | 28.22 ± 6.76 | 19.12 ± 4.09 | 29.47 ± 5.22 | 6.48 ± 1.38 | < 0.001 |
| pRCE | 37.79 ± 5.59 | 29.21 ± 4.21 | 36.28 ± 4.72 | 7.11 ± 1.74 | < 0.001 |
| dRCE | 36.09 ± 8.12 | 25.2 ± 5.77 | 27.08 ± 5.79 | 7.22 ± 1.59 | < 0.001 |
| ESR | 24.186 ± 3.210 | 18.28 ± 3.38 | 21.49 ± 3.33 | 3.184 ± 0.568 | < 0.001 |
| Remained active n = 17 | Improved to inactive n = 44 | |||
| r | P value | r | P value | |
| ADC | -0.219 | 0.518 | -0.516 | 0.002 |
| Thickness | 0.105 | 0.758 | 0.170 | 0.568 |
| tRCE | 0.356 | 0.203 | 0.420 | 0.005 |
| aRCE | 0.376 | 0.255 | 0.697 | 0.002 |
| pRCE | 0.305 | 0.113 | 0.350 | 0.010 |
| dRCE | 0.381 | 0.134 | 0.341 | 0.015 |
| MaRIA | ||||
| tRCE | 0.268 | 0.400 | 0.705 | < 0.001 |
| aRCE | 0.268 | 0.399 | 0.712 | < 0.001 |
| pRCE | 0.306 | 0.334 | 0.685 | < 0.001 |
| dRCE | 0.309 | 0.329 | 0.634 | < 0.001 |
Among MRI qualitative indicators, statistical analysis could not be done for mucosal ulcer because of its low frequency (16/61). Edema in the submucosa and perienteric exudation were decreased (61/61 and 18/18) after treatment. In addition, the enhancement pattern of the bowel wall in inactive CD patients changed to non-stratified enhancement (44/61), whereas it remained stratified enhancement in active CD patients (17/61) (Figures 3 and 4).
MRI is fairly reproducible but shows only a moderate agreement with CDEIS[6,12,13]. Furthermore, the concordance of the two examinations during follow-up or before/after treatment remains uncertain. Therefore, this study aimed to examine the correlation and concordance among multiple MRI findings and CDEIS changes before and after CD treatment. The results strongly suggest that MRI artery phase-enhanced indexes were the most sensitive indicators, especially arterial MaRIA, for CD follow-up and dynamic assessment of the therapeutic effects. CD treatment assessment was not completely concordant between CDEIS and MRI.
The clinical course of CD usually presents an acute-remission-recur cycle. Therefore, regular monitoring and follow-up are needed. The assessment methods for the diagnosis and follow-up include clinical manifestations, endoscopy, histopathology, computed tomography (CT), and MRI[18]. In clinical practice, there is often a low correlation between clinical symptoms and bowel inflammatory activity. Clinical symptoms may be unrelated to endoscopy and imaging findings[19,20]. Endoscopy and histopathology exams are the first choice for the diagnosis of CD[1,18]. Nevertheless, these approaches are invasive and ill-suited for regular monitoring and follow-up. Therefore, MRI is probably one of the most appropriate methods for the long-term evaluation and monitoring of CD.
An early study on the efficacy of CD treatment was reported using MRI enhanced index and bowel thickness[21]. Other MRI evaluations, such as mucosal ulcer and ADC value, were used in recent studies[22]. Some studies[23-27] focused on the accuracy of MRI indicators for the evaluation of CD and the response to medical therapy. One study reported that changes in CD clinical activity were significantly correlated with changes in MRI activity score[28]. Bowel wall thickening, mesenteric lymphadenopathy, and fat wrapping with vascular proliferation were the MRI parameters that changed significantly after induction and maintenance treatment in responders[28]. The changes in MRI activity score were mostly pronounced during the first 3 months of treatment compared with long-term treatments (weeks 52-54)[28]. In the present study, both MRI scores (MaRIA) and single MRI indicators (ADC, tRCE, aRCE, pRCE, dRCE, and bowel thickness) were evaluated. After treatment, MaRIA scores, ADC, tRCE, aRCE, pRCE, dRCE, ∆ADC, ∆tRCE, ∆aRCE, ∆pRCE, and ∆dRCE remained correlated with CDEIS, but bowel thickness was not, possibly because CD is a chronic and recurrent disease. Both edema and chronic fibrosis can be found in thickened bowel segment. After effective medical therapy, inflammation may be improved and edema may have regressed, but fibrous adipose tissue hyperplasia may be present or become more serious. This may weaken the correlation between bowel thickness and CDEIS. Secondly, compared with other studies, the evaluation timing after treatment was different. Therefore, the decision when to make the MRI evaluation is still an issue. Various MRI assessment timings may produce different results in treatment effect. Thirdly, our sample size was limited. Nevertheless, a recent study supports the use of MaRIA for the evaluation of CD[29].
In the present study, higher correlations were found for MaRIA scores than that for single MRI indicators. Among them, the MaRIA score of aRCE showed the best correlation after treatment. Among single MRI indicators, the best correlation was found between aRCE and CDEIS. Possible reasons that MRI artery phase enhanced indexes were the most sensitive for efficacy assessment after CD treatment may be decreased blood supply to mucosal ulcer and improved inflammation.
Nevertheless, each correlation coefficient of enhanced indexes was decreased compared with those before treatment in 61 patients with CD. In general, MRI findings, as a treatment evaluation method, were not completely matched with CDEIS, especially after 24-26 wk of effective treatment. Grouped by treatment effect, good correlation between MRI and CDEIS results was found in active CD patients who experienced remission but not among those who remained with active CD after treatment. This finding may also reflect that time has an impact on the changes between MRI and CDEIS.
DWI has recently been shown to be an appropriate tool for the follow-up of CD[30,31]. In the present study, the correlation between ADC values and CDEIS after treatment was increased compared with that before treatment, especially ∆ADC. Though ADC value was proved to be a reliable independent indicator for the evaluation of CD and with a similar value to that of enhancement indicators in previous studies[9,22], the present study showed that it was more valuable and reliable to follow-up the change of ADC values for dynamic monitoring. It had a good value reflecting CD prognosis during periods rather than at specific time point of the disease.
Among qualitative indicators, because of strict requirement for bowel distension, no advantage was shown for MRI detecting mucosal ulcer compared with endoscopy. Other MRI indicators, such as edema, exudation, and enhancement pattern, were sensitive and matched the CDEIS changes before and after treatment. Because these are subjective indicators and may vary among observers, they seem to be less accurate and dependable indicators compared with RCE and ADC values. Nevertheless, a study showed that endoscopy and MRI were concordant, even without bowel preparation[32]. Additional studies are warranted on this point.
The present study is not without limitations. The sample size was small and from a single center. In addition, the retrospective nature of the study prevented the study of parameters that were not routinely collected. Thirdly, all treated patients were grouped together, but different treatments might have different impact on MRI findings. Finally, MRI T2W1 and T1W1 dynamic enhancement sequences can show intestinal fistula but, in the present study, the frequency of fistula was low. Therefore, reliable statistical analyses could not be performed. Additional studies are necessary to improve upon these results.
In conclusion, MRI indicators were correlated with CDEIS, but such correlation was decreased in patients with active CD that became inactive after treatment. CD treatment assessment was not completely concordant between CDEIS and MRI. MRI artery phase enhanced indexes seemed to be the most sensitive indicators, especially MaRIA score of aRCE. MaRIA scores were better than single MRI indicators for CD follow-up and dynamic assessment of therapeutic effects.
Crohn’s disease (CD) is an inflammatory bowel disease that may involve the entire gastrointestinal tract. CD easily recurs, and accurate and comprehensive evaluation and follow-up are essential to design an individualized treatment program. Crohn’s Disease Endoscopic Index of Severity (CDEIS) is generally used to assess CD activity. However, it is currently uncertain whether MRI abnormalities are concordant with changes in CDEIS. In addition, whether MRI is only a supplementary/accessory assessment method to endoscopy or could substitute endoscopy during follow-up remains unclear.
The clinical symptoms of CD may be unrelated to endoscopy and imaging findings. Endoscopy and histopathology are the first methods of choice for the diagnosis of CD. Nevertheless, these approaches are invasive and ill-suited for regular monitoring and follow-up. Therefore, MRI is probably one of the most appropriate methods for long-term evaluation and monitoring of CD.
We hypothesized that CDEIS changes correlated with MaRIA scores as well as individual MRI parameters before and after CD treatment. The present study aimed to help us to understand the pathological changes of CD and provide non-invasive modalities for examining therapeutic effects.
One hundred and four patients with CD were analyzed retrospectively. Among them, 61 and 43 patients were considered to have active CD (CDEIS > 6) and inactive CD (CDEIS ≤ 6), respectively. MaRIA scores as well as individual MRI parameters, including total relative contrast enhancement (tRCE), arterial RCE (aRCE), portal RCE (pRCE), delay phase RCE (dRCE), and apparent diffusion coefficient (ADC), were evaluated. Correlation and concordance between multiple MRI findings and CDEIS were examined.
In the present study, we found that CDEIS had correlations with MaRIAs at baseline in all patients, including tRCE, aRCE, pRCE, dRCE (all MaRIAs, P < 0.001), followed by single MRI indexes. Among the 61 active CD patients, 44 cases were remitted to inactive CD after treatment. In the 44 patients who achieved remission, correlations between CDEIS and all MaRIAs remained after treatment. However, the values of the correlation coefficient (r) were decreased. The most significant correlations were found between MaRIAs for aRCE and CDEIS.
MRI indicators had correlations with CDEIS in patients with active CD before treatment. However the correlations were decreased in patients with active CD that became inactive after treatment. The assessment was not completely concordant between CDEIS and MRI in patient with CD before and after treatment. The MaRIA score of aRCE seemed to be an important indicator. For dynamic assessment of therapeutic effects, MaRIA scores were better than single MRI indicators.
Endoscopic results were not completely consistent with MR data among CD patients. The most sensitive indicators in evaluating efficacy by MR were relevant indicators during the MR enhanced arterial phase. The most appropriate timing for performing MR evaluation and monitoring disease conditions after treatment of CD should be explored in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheifetz AS, Gassler N, Kreisel W S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465-83; quiz 464, 484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1560] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 3. | Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn’s disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30:367-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Ippolito D, Invernizzi F, Galimberti S, Panelli MR, Sironi S. MR enterography with polyethylene glycol as oral contrast medium in the follow-up of patients with Crohn disease: comparison with CT enterography. Abdom Imaging. 2010;35:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Rimola J, Ordás I, Rodriguez S, García-Bosch O, Aceituno M, Llach J, Ayuso C, Ricart E, Panés J. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Macarini L, Stoppino LP, Centola A, Muscarella S, Fortunato F, Coppolino F, Della Valle N, Ierardi V, Milillo P, Vinci R. Assessment of activity of Crohn’s disease of the ileum and large bowel: proposal for a new multiparameter MR enterography score. Radiol Med. 2013;118:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, Naganuma M, Araki A, Watanabe M. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014;147:334-342.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, Roelofs JJ, Ponsioen CY, Vos FM, Stoker J. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Feng Q, Ma Z, Zhang S, Wu J. Usefulness of diffusion tensor imaging for the differentiation between low-fat angiomyolipoma and clear cell carcinoma of the kidney. Springerplus. 2016;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983-989. [PubMed] |
| 12. | Tielbeek JA, Makanyanga JC, Bipat S, Pendsé DA, Nio CY, Vos FM, Taylor SA, Stoker J. Grading Crohn disease activity with MRI: interobserver variability of MRI features, MRI scoring of severity, and correlation with Crohn disease endoscopic index of severity. AJR Am J Roentgenol. 2013;201:1220-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, Pellisé M, Ayuso C, Ricart E, Donoso L. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 514] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 14. | Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 15. | Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, Cohen R, Windsor A, Obichere A, Hansmann A. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Rodriguez P, Mendez R, Matute F, Hernandez P, Mendoza JL. Imaging Crohn disease: MR enterography. J Comput Assist Tomogr. 2014;38:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Semelka RC, Shoenut JP, Silverman R, Kroeker MA, Yaffe CS, Micflikier AB. Bowel disease: prospective comparison of CT and 1.5-T pre- and postcontrast MR imaging with T1-weighted fat-suppressed and breath-hold FLASH sequences. J Magn Reson Imaging. 1991;1:625-632. [PubMed] |
| 18. | Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: what does it mean? World J Gastroenterol. 2013;19:7552-7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Madsen SM, Thomsen HS, Schlichting P, Dorph S, Munkholm P. Evaluation of treatment response in active Crohn’s disease by low-field magnetic resonance imaging. Abdom Imaging. 1999;24:232-239. [PubMed] |
| 22. | Bhatnagar G, Dikaios N, Prezzi D, Vega R, Halligan S, Taylor SA. Changes in dynamic contrast-enhanced pharmacokinetic and diffusion-weighted imaging parameters reflect response to anti-TNF therapy in Crohn’s disease. Br J Radiol. 2015;88:20150547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Tielbeek JA, Löwenberg M, Bipat S, Horsthuis K, Ponsioen CY, D’Haens GR, Stoker J. Serial magnetic resonance imaging for monitoring medical therapy effects in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Stoppino LP, Della Valle N, Rizzi S, Cleopazzo E, Centola A, Iamele D, Bristogiannis C, Stoppino G, Vinci R, Macarini L. Magnetic resonance enterography changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn’s disease: correlation with SES-CD and clinical-biological markers. BMC Med Imaging. 2016;16:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Van Assche G, Herrmann KA, Louis E, Everett SM, Colombel JF, Rahier JF, Vanbeckevoort D, Meunier P, Tolan D, Ernst O. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal Crohn’s disease. J Crohns Colitis. 2013;7:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, Arévalo JA, Aduna M, Andreu M, Radosevic A. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 27. | Eder P, Michalak M, Katulska K, Lykowska-Szuber L, Krela-Kazmierczak I, Stawczyk-Eder K, Klimczak K, Szymczak A, Linke K. Magnetic resonance enterographic predictors of one-year outcome in ileal and ileocolonic Crohn’s disease treated with anti-tumor necrosis factor antibodies. Sci Rep. 2015;5:10223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Eder P, Katulska K, Krela-Kaźmierczak I, Stawczyk-Eder K, Klimczak K, Szymczak A, Linke K, Łykowska-Szuber L. The influence of anti-TNF therapy on the magnetic resonance enterographic parameters of Crohn’s disease activity. Abdom Imaging. 2015;40:2210-2218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Rimola J, Alvarez-Cofiño A, Pérez-Jeldres T, Ayuso C, Alfaro I, Rodríguez S, Ricart E, Ordás I, Panés J. Comparison of three magnetic resonance enterography indices for grading activity in Crohn’s disease. J Gastroenterol. 2017;52:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Klang E, Kopylov U, Eliakim R, Rozendorn N, Yablecovitch D, Lahat A, Ben-Horin S, Amitai MM. Diffusion-weighted imaging in quiescent Crohn’s disease: correlation with inflammatory biomarkers and video capsule endoscopy. Clin Radiol. 2017;72:798.e7-798.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Kim JS, Jang HY, Park SH, Kim KJ, Han K, Yang SK, Ye BD, Park SH, Lee JS, Kim HJ. MR Enterography Assessment of Bowel Inflammation Severity in Crohn Disease Using the MR Index of Activity Score: Modifying Roles of DWI and Effects of Contrast Phases. AJR Am J Roentgenol. 2017;208:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Dinter DJ, Chakraborty A, Brade J, Back W, Neff KW, Singer MV, Böcker U. Endoscopy and magnetic resonance imaging in patients with Crohn’s disease: a retrospective single-centre comparative study. Scand J Gastroenterol. 2008;43:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |