Published online May 28, 2018. doi: 10.3748/wjg.v24.i20.2181
Peer-review started: February 28, 2018
First decision: March 15, 2018
Revised: March 21, 2018
Accepted: April 16, 2018
Article in press: April 15, 2018
Published online: May 28, 2018
Processing time: 89 Days and 3.2 Hours
To evaluate indoleamine-2,3-dioxygenase 1/cyclooxygenase 2 (IDO1/COX2) expression as an independent prognostic biomarker for colorectal cancer (CRC) patients.
We retrospectively studied the medical records of 95 patients who received surgical resection from August 2008 to January 2010. All patients were randomly assigned to adjuvant treatment with or without celecoxib groups after surgery. We performed standard immunohistochemistry to assess the expression levels of IDO1/COX2 and evaluated the correlation of IDO1/COX2 with clinicopathological factors and overall survival (OS) outcomes.
The expression of nuclear IDO1 was significantly correlated with body mass index (P < 0.001), and IDO1 expression displayed no association with sex, age, tumor differentiation, T stage, N stage, carcinoembryonic antigen, cancer antigen 19-9, CD3+ and CD8+ tumor infiltrating lymphocytes, and COX2. In univariate analysis, we found that nuclear IDO1 (P = 0.039), nuclear/cytoplasmic IDO1 [hazard ratio (HR) = 2.044, 95% confidence interval (CI): 0.871-4.798, P = 0.039], nuclear IDO1/COX2 (HR = 3.048, 95%CI: 0.868-10.7, P = 0.0049) and cytoplasmic IDO1/COX2 (HR = 2.109, 95%CI: 0.976-4.558, P = 0.022) all yielded significantly poor OS outcomes. Nuclear IDO1 (P = 0.041), nuclear/cytoplasmic IDO1 (HR = 3.023, 95%CI: 0.585-15.61, P = 0.041) and cytoplasmic IDO1/COX2 (HR = 2.740, 95%CI: 0.764-9.831, P = 0.038) have significantly poor OS outcomes for the CRC celecoxib subgroup. In our multivariate Cox model, high coexpression of cytoplasmic IDO1/COX2 was found to be an independent predictor of poor outcome in CRC (HR = 2.218, 95%CI: 1.011-4.48, P = 0.047) and celecoxib subgroup patients (HR = 3.210, 95%CI: 1.074-9.590, P = 0.037).
Our results showed that cytoplasmic IDO1/COX2 coexpression could be used as an independent poor predictor for OS in CRC.
Core tip: It was reported that indoleamine-2,3-dioxygenase 1 (IDO1) is an inhibitory factor that suppresses the T cell response to tumors. In this study, we evaluated IDO1/cyclooxygenase 2 (COX2) expression as an independent prognostic biomarker for colorectal cancer (CRC) patients. In our multivariate Cox model, high coexpression of cytoplasmic IDO1/COX2 was found to be an independent predictor of poor outcome in CRC patients and celecoxib subgroup patients. Our results showed that cytoplasmic IDO1/COX2 coexpression could be used as an independent predictor for poor overall survival in CRC.
- Citation: Ma WJ, Wang X, Yan WT, Zhou ZG, Pan ZZ, Chen G, Zhang RX. Indoleamine-2,3-dioxygenase 1/cyclooxygenase 2 expression prediction for adverse prognosis in colorectal cancer. World J Gastroenterol 2018; 24(20): 2181-2190
- URL: https://www.wjgnet.com/1007-9327/full/v24/i20/2181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i20.2181
Colorectal cancer (CRC) is a leading cause of cancer-related death worldwide. Nearly one million cases of CRC are diagnosed worldwide each year[1,2]. Because of genetic mutations and environmental factors, CRC development is a very complex process and is determined by multistage factors[3,4]. Currently, immunotherapy has become one of the most promising treatments for CRC[5].
Recent studies have demonstrated that the tumor microenvironment plays a vital role in the progression of cancer development - e.g., cancer cells, through expressing inhibitory proteins, such as PD-L1 and CTLA4, create an immunosuppressive microenvironment[6-8]. Clinical trials have shown that combining PD-1/PD-L1 with CTLA4 blockade therapy seems to be a better therapy than single blockade. However, this favorable outcome is achieved in only less than 40% of patients[9]. Other studies have confirmed that the tumor microenvironment has more inhibitory factors, including indoleamine-2,3-dioxygenase 1 (IDO1), and suppresses the T cell response to tumors. IDO1 belongs to a unique class of mammalian heme dioxygenase enzymes and is the first and rate-limiting enzyme in the degradation of the essential amino acid tryptophan, resulting in the accumulation of their metabolites such as kynurenine[9]. T cells sense low tryptophan and high kynurenine via mTORC and GCN2 signaling pathways to initiate an amino acid starvation response, resulting in T cell cycle arrest and cell death, and favoring the differentiation of regulatory T cells; as a result, the immune mediator is escapes in cancer[10].
In humans, IDO1 is usually expressed only in placental endothelial cells and mature dendritic cells. Activating T lymphocytes could express interferon-r in the tumor microenvironment, resulting in inducing IDO1 expression in most tissues and cell types and inhibiting T cell responses to tumor cells[11]. Many human tumors still express IDO1 through PKC and PI3K signaling triggered by PGE2 in the absence of T cell infiltration. Constitutive expression of cyclooxygenase 2 (COX2) by MAPK signaling could induce PGE2 production[11]. Because many tumors harbor oncogenic mutations in these signaling pathways, they could express IDO1 constitutively in the absence of interferon-r. Therefore, IDO1 and COX2 are currently of great interest in cancer research as prognostic and therapeutic biomarkers of tissues and sera.
CRC has demonstrated high heterogeneity in recent years. Hence, biomarkers need to be identified and enabled to stratify the different subgroups. Similar to other tumors, such as endometrial carcinoma and liver and ovarian cancers, the IDO expression levels are correlated with the overall survival (OS) of CRC patients[12-16]. One study showed that IDO1 expression at the invasive front was significantly associated with OS[17]. One report has hypothesized that the nuclear localization of IDO1 promotes the immunosuppression independence of enzyme activity[18]. In CRC, the level of COX2 expression was increased in up to 85 cases but not in the normal colonic epithelium. A selective COX2 inhibitor, celecoxib, could improve chemosensitivity when CRC cells are exposed to the combination with 5-FU and CPT-11[19] and could reduce hand-foot syndrome induced by capecitabine[20]. However, whether IDO1/COX2 coexpression is correlated with OS in CRC patients remains unknown.
In this study, we conducted a retrospective analysis for the potential prognostic importance of the correlation of IDO1 and COX2 in survival outcome prognosis, including their coexpression, cytoplasmic and nuclear localization of IDO1, and tumor-infiltrating lymphocytes (TILs).
All tissues were collected from 95 patients who had undergone surgical resection from August 2008 to January 2010 at the Department of Colorectal Surgery of Sun Yat-sen University (Guangzhou, China). Patients were randomly assigned to adjuvant treatment with XELOX/capecitabine alone combined with or without celecoxib groups after surgery. All patients in the groups received celecoxib 200 mg/m2 twice daily, given for 14 d (day 1 to day 14) of a 3-wk cycle for total of 6-8 cycles[20]. The eligibility criteria were as follows: (1) Stage II/III CRC eligible for adjuvant chemotherapy; (2) all tumor tissue pathological diagnoses confirmed to be CRC by a pathologist. The cases were selected consecutively based on the availability of resection tissues and follow-up data.
Formalin-fixed, paraffin-embedded tumor specimens were cut into 4-μm sections. After baking at 60 °C for 2 h, the samples were deparaffinized in xylene and rehydrated in a series of graded ethanol. Next, the samples were incubated with 3% hydrogen peroxide for 10-15 min to block endogenous peroxidase activity. The sections were microwaved for antigen retrieval in 0.01 mol/L sodium citrate buffer (pH 6.0) for 30 min, and then were pre-incubated in 10% normal goat serum for 30 min to block nonspecific staining. The sections were then incubated with the primary rabbit anti-human IDO1 monoclonal antibody (working dilution, 1:100; Cell Signaling Technology, Danvers, MA, United States), rabbit antihuman COX2 monoclonal antibody (working dilution, 1:200; Beijing Golden Bridge Biotechnology, China), rabbit antihuman CD3 monoclonal (working dilution: 1:50; Beijing Golden Bridge Biotechnology) and mouse antihuman CD8 monoclonal (working dilution, 1:100; Beijing Golden Bridge Biotechnology) overnight at 4 °C. Subsequently, the samples were incubated with secondary antibody (Dako, Glostrup, Denmark) at room temperature for 0.5 h.
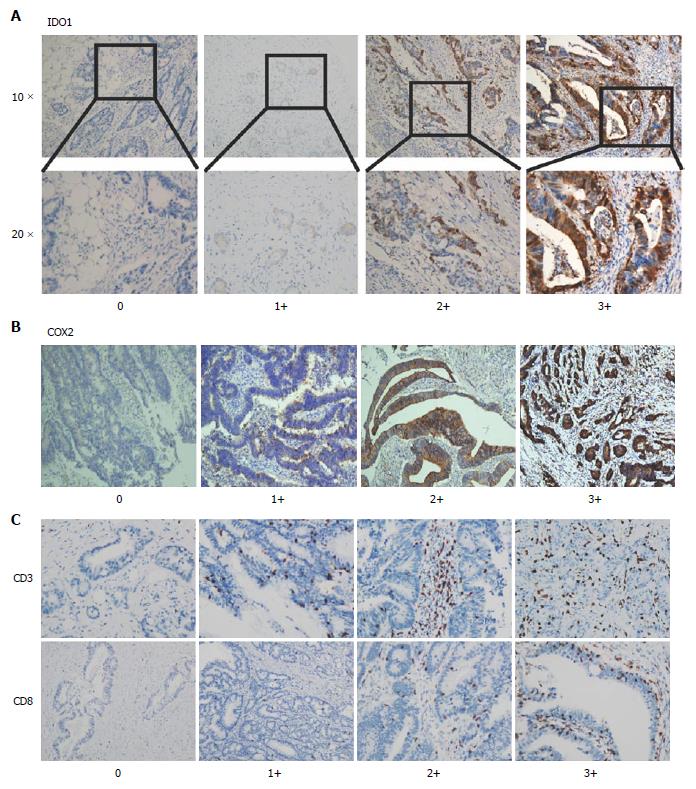
All the stained slides were scored independently by two experienced pathologists who were blinded to the patients’ identity and clinical status. H-scores of dominant staining intensity (0, 1+, 2+ and 3+) and the percentage of positive tumor cells (0 to 100%) of immunostaining were adopted for the expression data analysis. IDO1 expression was classified as high or low based on whether the H-score was above or below the score of 0.1. COX2 expression was considered high if the score was above 0.6 as the median cut-off. T cell infiltration of tumors was assessed by semiquantitative estimation of the density of CD3-positive/CD8-positive (CD3+/CD8+) cells and was scored as follows: 1+: No or sporadic CD3+/CD8+CD3þ cells; 2+: Moderate numbers of CD3+/CD8+ cells; 3+: Abundant occurrence of CD3+/CD8+ cells; and 4+: Highly abundant occurrence of CD3+/CD8+ cells[21].
The last date of follow-up was October 2017. All patients (51 males and 44 females) were followed up every 3 mo in the first 2 years and every 6 mo thereafter. History and physical examination should be given every 3 to 6 mo for 2 years, and then every 6 mo for a total of 5 years. A carcinoembryonic antigen (CEA) test and abdominal and pelvic ultrasound test were recommended at baseline and every 3 to 6 mo for 2 years, then every 6 mo for a total of 5 years. Colonoscopy is recommended at approximately 1 year after resection. Repeat colonoscopy is typically recommended at 3 years, and every 5 years thereafter, unless follow-up colonoscopy indicates advanced adenoma, in which case colonoscopy should be repeated in 1 year. Chest, abdominal and pelvic CT scans were recommended annually for up to 5 years. During the follow-up, 33 patients (34.7%) died of cancer-related causes. Sixty-two patients (65.3%) were still alive at the time of the last follow-up report.
The SPSS software package (version 23.0; IBM Corp, Armonk, NY, United States) and GraphPad Prism (version 7.0; GraphPad Software Inc, La Jolla, CA, United States) were used for statistical analysis. OS was defined as the time from the diagnosis of CRC to death of the patient or last date of follow-up. Chi-square test was used to assess the correlation of the IDO1 status with clinicopathologic characteristics. Survival curves were generated using the Kaplan-Meier method, and differences between curves were assessed by the log-rank test. The Cox multivariate proportional hazards regression model was used to determine the independent risk factors that influence OS. P-values < 0.05 were considered to be statistically significant.
To elucidate the biological significance of IDO1/COX2 in CRC, especially in the CRC celecoxib subgroup, we used immunohistochemical staining to test the expression of IDO1 and COX2 in the selected 95 CRC specimens. The results showed that IDO1 expression is primarily localized in the cytoplasm within the nucleus of tumor cells (Figure 1).
To gain insight into the role of the localization of IDO1 protein in CRC, we correlated cytoplasmic and nuclear IDO1 expression in the study cohort of 95 CRC patients with certain clinical and pathological factors. The expression of nuclear IDO1 was significantly correlated with body mass index (BMI) (P < 0.001); however, cytoplasmic IDO1 showed no relationship with BMI (P = 0.16). We observed no relationship between cytoplasmic and nuclear IDO1 expression and clinical factors such as sex, age, cancer (colon and rectum), tumor differentiation, T stage, N stage, CEA, cancer antigen (CA)19-9, CD3+ and CD8+ TILs, COX2, and celecoxib treatment (Tables 1 and 2).
| Characteristic | Total | Low IDO1 | High IDO1 | P-value |
| Sex | 0.074 | |||
| Male | 52 | 22 (42.3) | 30 (57.7) | |
| Female | 43 | 27 (62.8) | 16 (37.2) | |
| Age in yr | 0.65 | |||
| ≥ 60 | 30 | 17 (56.7) | 13 (43.3) | |
| < 60 | 65 | 32 (49.2) | 33 (50.8) | |
| Cancers | 0.93 | |||
| Colon | 46 | 24 (52.2) | 22 (47.8) | |
| Rectum | 49 | 25 (51.0) | 24 (49.0) | |
| BMI | 0.16 | |||
| ≥ 25 | 20 | 7 (35.0) | 13 (65.0) | |
| < 25 | 75 | 42 (56.0) | 33 (44.0) | |
| Tumor differentiation | 0.47 | |||
| Moderate and poor | 78 | 42 (54.5) | 35 (45.5) | |
| Well | 17 | 7 (41.2) | 10 (58.8) | |
| Colon cancer stage | 0.52 | |||
| 3 | 20 | 12 (60.0) | 8 (40.0) | |
| 2 | 26 | 12 (46.2) | 14 (53.8) | |
| T stage | 0.69 | |||
| 4 | 29 | 14 (48.3) | 15 (51.7) | |
| 2/3 | 17 | 10 (58.8) | 7 (41.2) | |
| N stage | 0.96 | |||
| 1/2 | 20 | 11 (55.0) | 9 (45.0) | |
| 0 | 26 | 13 (50.0) | 13 (50.0) | |
| Rectum cancer stage | 0.67 | |||
| 3 | 24 | 11(45.8) | 13 (54.2) | |
| 2 | 25 | 14 (46.0) | 11 (44.0) | |
| T stage | 0.68 | |||
| 4 | 22 | 12 (55.6) | 10 (45.4) | |
| 2/3 | 27 | 12 (44.4) | 15 (55.6) | |
| N stage | 0.88 | |||
| 1/2 | 24 | 12 (50.0) | 12 (50.0) | |
| 0 | 25 | 13 (52.0) | 12 (48.0) | |
| CEA in ng/mL | 0.45 | |||
| > 5 | 42 | 24 (57.1) | 18 (42.9) | |
| ≤ 5 | 53 | 25 (47.2) | 28 (42.8) | |
| CA19-9 in U/mL | 0.22 | |||
| > 37 | 17 | 6 (35.3) | 11 (64.7) | |
| ≤ 37 | 78 | 43 (55.1) | 35 (44.9) | |
| CD3 TILs | 0.27 | |||
| High | 36 | 22 (61.1) | 14 (38.9) | |
| Low | 59 | 28 (47.5) | 31 (42.5) | |
| CD8 TILs | 0.96 | |||
| High | 22 | 12 (54.5) | 10 (45.5) | |
| Low | 73 | 38 (52.5) | 35 (47.5) | |
| COX2 | 0.92 | |||
| High | 48 | 26 (54.2) | 22 (45.8) | |
| Low | 47 | 24 (51.1) | 23 (48.9) | |
| Treatment group | 0.58 | |||
| Celecoxib | 44 | 25 (56.8) | 19 (43.2) | |
| Non-celecoxib | 51 | 25 (49.0) | 26 (51.0) |
| Characteristic | Total | Low IDO1 | High IDO1 | P-value |
| Sex | 0.70 | |||
| Male | 51 | 39 (76.5) | 12 (33.5) | |
| Female | 44 | 36 (81.8) | 8 (19.2) | |
| Age in yr | 0.92 | |||
| > 60 | 30 | 24 (80.0) | 6 (20.0) | |
| ≤ 60 | 65 | 51 (78.5) | 14 (21.5) | |
| Cancers | 0.52 | |||
| Colon | 46 | 38 (82.6) | 18 (17.4) | |
| Rectum | 49 | 37 (75.5) | 12 (24.5) | |
| BMI | < 0.001 | |||
| > 25 | 20 | 9 (45.0) | 11 (55.0) | |
| ≤ 25 | 75 | 66 (88.0) | 9 (12.0) | |
| Tumor differentiation | 0.87 | |||
| Moderate and poor | 78 | 60 (76.9) | 18 (23.1) | |
| Well | 17 | 14 (82.3) | 3 (17.7) | |
| Conlon cancer stage | 0.98 | |||
| 3 | 20 | 17 (85.0) | 3 (15.0) | |
| 2 | 26 | 21 (80.8) | 5 (19.2) | |
| T stage | 0.71 | |||
| 4 | 29 | 23 (19.3) | 6 (20.7) | |
| 2/3 | 17 | 15 (88.2.) | 2 (11.8) | |
| N stage | 0.98 | |||
| 1/2 | 20 | 16 (80.0) | 4 (20.0) | |
| 0 | 26 | 22 (84.6) | 4 (15.4) | |
| Rectum cancer stage | 0.44 | |||
| 3 | 24 | 17 (70.8) | 7 (29.2) | |
| 2 | 25 | 21 (84.0) | 4 (16.0) | |
| T stage | 0.94 | |||
| 4 | 22 | 16 (72.7) | 6 (27.3) | |
| 2/3 | 27 | 21 (77.8) | 6 (22.2) | |
| N stage | 0.28 | |||
| 1/2 | 24 | 16 (66.7) | 8 (33.3) | |
| 0 | 25 | 21(84.0) | 4 (16.0) | |
| CEA in ng/mL | 0.37 | |||
| > 5 | 42 | 35 (83.3) | 7 (16.7) | |
| ≤ 5 | 53 | 39 (73.6) | 14 (26.4) | |
| CA19-9 in U/mL | 0.78 | |||
| > 37 | 81 | 41 (50.6) | 40 (49.4) | |
| ≤ 37 | 17 | 8 (47.1) | 9 (52.9) | |
| CD3 TILs | 0.96 | |||
| High | 36 | 28 (77.8) | 8 (22.2) | |
| Low | 59 | 47 (79.7) | 12 (20.3) | |
| CD8 TILs | 0.26 | |||
| High | 22 | 15 (68.2) | 7 (31.8) | |
| Low | 73 | 60 (82.2) | 13 (17.8) | |
| COX2 | 0.84 | |||
| High | 48 | 38 (79.2) | 10 (20.8) | |
| Low | 47 | 37 (78.7) | 10 (21.3) | |
| Treatment group | 0.16 | |||
| Celecoxib | 44 | 38 (86.4) | 6 (13.6) | |
| Non-celecoxib | 51 | 37 (72.5) | 14 (27.5) |
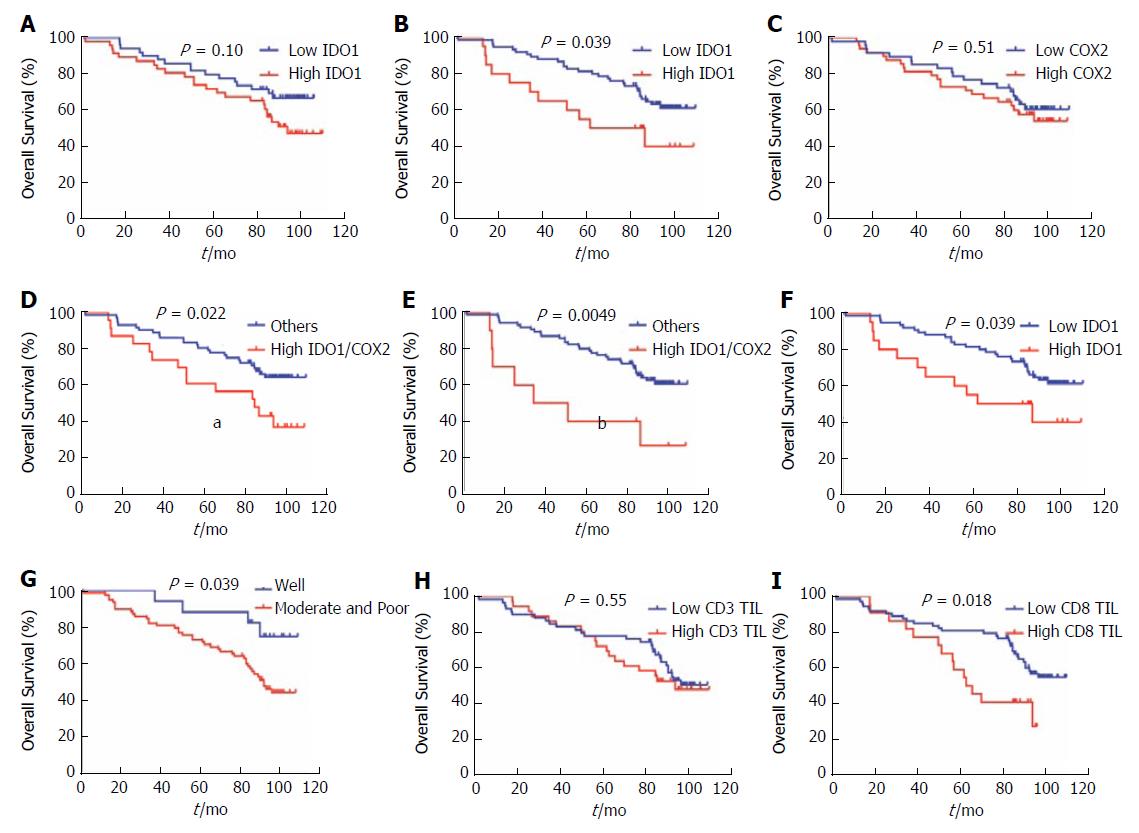
We analyzed the correlation between IDO1 and traditional clinicopathologic parameters with patients’ outcomes by univariate analysis. We also performed analyses to determine whether IDO1 and COX2 expression and localization represent potential independent predictors for the OS outcome in CRC patients. We observed that cytoplasmic IDO1 and COX2 expression could not predict OS outcomes in our univariate analysis (cytoplasmic IDO1: P = 0.10; COX2: P = 0.51). However, nuclear IDO1 (P = 0.039), nuclear/cytoplasmic IDO1 (hazard ratio (HR) = 2.044, 95% confidence interval (CI): 0.871-4.798, P = 0.039), nuclear IDO1/COX2 (HR = 3.048, 95%CI: 0.868-10.7, P = 0.0049), cytoplasmic IDO1/COX2 (HR = 2.109, 95%CI: 0.976-4.558, P = 0.022), tumor differentiation (HR = 2.798, 95%CI: 1.373-5.702, P = 0.039), CEA (HR = 2.137, 95%CI: 1.141-4.004, P = 0.025), and CD8 TILs (HR = 2.096, 95%CI: 0.975-4.504, P = 0.018) (Table 3) yielded significantly poor OS outcomes in CRC patients (Figure 2B-G, Supplementary Figure 1E) but not with other clinicopathologic parameters such as sex, age, BMI, T stage, N stage, CA19-9 and CD3+ TILs, including whether celecoxib was used or not (Figure 2A, 2C, 2H, Supplementary Figure 1A-D, 1F-J).
| HR | 95%CI | P value | |
| Sex, male vs female | 0.750 | 0.399-1.411 | 0.37 |
| Age in yr, ≤ 60 vs > 60 | 0.899 | 0.472-1.714 | 0.74 |
| Cancer, colon vs rectum | 1.279 | 0.712-2.296 | 0.41 |
| BMI, > 25 vs ≤ 25 | 1.579 | 0.697-3.579 | 0.21 |
| Tumor differentiation, moderate and poor vs well | 2.798 | 1.373-5.702 | 0.039 |
| Stage, 3 vs 2 | 1.003 | 0.534-1.882 | 0.99 |
| T stage, T4 vs T2/3 | 1.418 | 0.755-2.664 | 0.27 |
| N stage, N1/2 vs N0 | 1.005 | 0.536-1.887 | 0.99 |
| CEA in ng/mL, > 5 vs ≤ 5 | 2.137 | 1.141-4.004 | 0.025 |
| CA19-9 in U/mL, > 37 vs ≤ 37 | 1.262 | 0.547-2.911 | 0.56 |
| CD3 TILs, high vs low | 1.195 | 0.649-2.198 | 0.55 |
| CD8 TILs, high vs low | 2.096 | 0.975-4.504 | 0.018 |
| Nuclear IDO1, high vs low | 2.044 | 0.871-4.798 | 0.039 |
| Cytoplasmic IDO1, high vs low | 1.690 | 0.901-3.173 | 0.10 |
| Nuclear and cytoplasmic IDO1, high vs low | 2.044 | 0.871-4.798 | 0.039 |
| COX2, high vs low | 1.235 | 0.659-2.314 | 0.51 |
| Nuclear IDO1/COX2, IV vs I/II/III | 3.048 | 0.868-10.7 | 0.0049 |
| Cytoplasmic IDO1/COX2, IV vs I/II/III | 2.109 | 0.976-4.558 | 0.022 |
| Treatment group, celecoxib vs non-celecoxib | 0.943 | 0.489-1.826 | 0.86 |
We also performed multivariate Cox modeling to analyze whether IDO1/COX2 represent potential independent predictors for the OS outcome in CRC patients. Combined cytoplasmic IDO1/COX2 coexpression analysis yielded a stronger predictor index, with HR = 2.218 (95%CI: 1.011-4.48, P = 0.047) in the IDO1High/COX2High group, and tumor differentiation was significantly correlated with OS (HR = 3.473, 95%CI: 1.201-10.046, P = 0.022) (Table 4) but not nuclear IDO1, cytoplasmic IDO1, nor combined nuclear IDO1/COX2 expression. Our results revealed that cytoplasmic IDO1/COX2 coexpression and tumor differentiation were independent predictors for poor OS in CRC.
| HR | 95%CI | P-value | |
| Cytoplasmic IDO1 and COX2, IV vs I/II/III | 2.218 | 1.011-4.48 | 0.047 |
| Tumor differentiation, poor and moderate vs well | 3.473 | 1.201-10.046 | 0.022 |
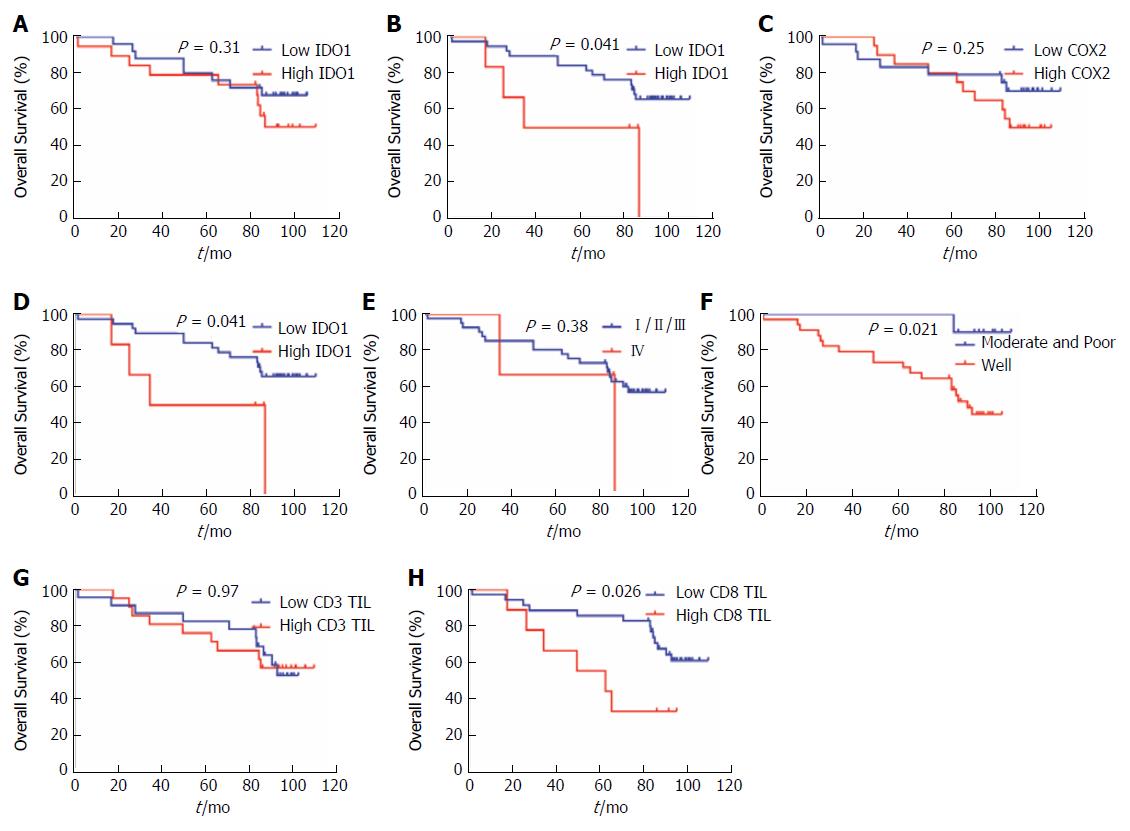
We also performed analyses to determine whether IDO1 and COX2 expression and localization represent potential independent predictors for OS outcome in CRC patients. We observed that cytoplasmic IDO1 and COX2 expression could not predict OS outcomes in univariate analysis (cytoplasmic IDO1: P = 0.31; COX2: P = 0.25). However, nuclear IDO1 (P = 0.041), nuclear/cytoplasmic IDO1 (HR = 3.023, 95%CI: 0.585-15.61, P = 0.041), cytoplasmic IDO1/COX2 (HR = 2.740, 95%CI: 0.764-9.831, P = 0.038) (Table 5), tumor differentiation (HR = 7.396, 95%CI: 2.749-19.90, P = 0.021) and CD8 TILs (HR = 2.821, 95%CI: 0.774-10.29, P = 0.026) have significantly poor OS outcomes for the CRC celecoxib subgroup (Figure 3B, 3D, 3F, 3H and 3I) but not with other clinicopathologic parameters such as sex, age, BMI, T stage, N stage, CEA, CA19-9 and CD3+ TILs (Figure 3A, 3C, 3E and 3G, Supplementary Figure 2A-I).
| HR | 95%CI | P value | |
| Sex, male vs female | 0.854 | 0.329-2.219 | 0.74 |
| Age in yr, ≤ 60 vs > 60 | 1.249 | 0.432-3.609 | 0.70 |
| Cancer, colon vs rectum | 1.034 | 0.420-2.543 | 0.94 |
| BMI, > 25 vs ≤ 25 | 1.328 | 0.351-5.020 | 0.71 |
| Tumor differentiation, moderate and poor vs well | 7.396 | 2.749-19.90 | 0.021 |
| Stage, 3 vs 2 | 1.075 | 0.415-2.782 | 0.88 |
| T stage, T4 vs T2/3 | 1.389 | 0.537-3.596 | 0.50 |
| N stage, N1/2 vs N0 | 1.075 | 0.415-2.782 | 0.88 |
| CEA in ng/mL, > 5 vs ≤ 5 | 1.934 | 0.743-5.033 | 0.21 |
| CA19-9 in m/L, > 37 vs ≤ 37 | 1.551 | 0.431-5.575 | 0.43 |
| CD3 TILs, high vs low | 1.02 | 0.414-2.510 | 0.97 |
| CD8 TILs, high vs low | 2.821 | 0.774-10.29 | 0.026 |
| Nuclear IDO1, high vs low | 3.023 | 0.585-15.61 | 0.041 |
| Cytoplasmic IDO1, high vs low | 1.623 | 0.617-4.267 | 0.31 |
| Nuclear and cytoplasmic IDO1, high vs low | 3.023 | 0.585-15.61 | 0.041 |
| COX2, high vs low | 1.746 | 0.672-4.541 | 0.25 |
| Nuclear IDO1/COX2, IV vs I/II/III | 1.885 | 0.279-12.76 | 0.38 |
| Cytoplasmic IDO1/COX2, IV vs I/II/III | 2.740 | 0.764-9.831 | 0.038 |
We further performed the multivariate Cox modeling to analyze whether IDO1/COX2 represents potential independent predictors for OS outcome in the CRC celecoxib subgroup. Combined cytoplasmic IDO1/COX2 coexpression analysis yielded a stronger predictor index, with HR = 3.210 (95%CI: 1.074-9.590, P = 0.037) in the IDO1High/COX2High group, and tumor differentiation was significantly correlated with OS (HR = 11.962, 95%CI: 1.526-23.787, P = 0.018) (Table 6) but not nuclear IDO1, cytoplasmic IDO1, nor combined nuclear IDO1/COX2 expression. Our results revealed that cytoplasmic IDO1/COX2 coexpression and tumor differentiation were independent poor predictors of OS in the CRC celecoxib subgroup.
| HR | 95%CI | P-value | |
| Cytoplasmic IDO1 and COX2, IV vs I/II/III | 3.210 | 1.074-9.590 | 0.037 |
| Tumor differentiation, poor and moderate vs well | 11.962 | 1.526-23.787 | 0.018 |
Current immunotherapy has been achieving very effective and promising results, especially for stage IV disease. However, more than 50% of these patients who need more new therapies will progress with resistance to immunotherapy[22]. IDO1 is associated with T cell apoptosis through depleting tryptophan in the tumor microenvironment. Therefore, IDO1 inhibitors have emerged as new options for cancer therapy. However, a recent study suggested the alternative hypothesis that nuclear IDO1 promotes immunosuppression instead of enzyme activity[18]. In previous studies, high IDO expression in CRC has been found to be associated with the presence of metastatic disease and outcome and a reduction in CD3-positive TILs, revealing the important role in therapeutic blockade for this disease[12,17]. In up to 85% of CRC patients, COX2 is highly expressed but not in normal colonic epithelium. Celecoxib is a COX2 inhibitor used in the treatment regimen for CRC; previous studies have demonstrated celecoxib in combination with chemotherapy to overcome resistance in therapy-refractory cancer cells in vitro and in vivo[19]. However, clinical studies have not been clarified to show the role of celecoxib in CRC patients and its potential prognostic importance.
In the present study, we evaluated CRC patients treated with or without celecoxib. We found no significant relationship with IDO1 or COX2 expression and OS in patients treated with or without celecoxib. However, our discovery revealed that cytoplasmic IDO1 and COX2 were correlated with OS in patients treated with or without celecoxib. Additionally, our data further found that nuclear IDO1 and COX2 were not correlated with OS in patients of either group. However, one recent study showed that nuclear IDO1 plays a more important role in CRC instead of enzyme activity. From our data, nuclear IDO1 could not be an independent prognostic factor for CRC patients. Some other unknown factors in the nucleus might combine to nuclear IDO1, possibly influencing the OS of CRC patients. These patients in our study have not been treated with IDO1 inhibitors. Therefore, whether nuclear expression affects IDO1 inhibitors is unclear.
Constitutive IDO1 expression is dependent on an autocrine loop of PGE2 production through activating the PI3K and PKC pathways and subsequent activation of IDO1 transcription by factors such as ETV4. PGE2 production mediates the expression of COX2. However, in our study, we found that IDO1 or COX2 expression was not correlated with OS. Three explanations are possible. First, CRC patients were treated with celecoxib only for no more than 6 mo. COX2 might still influence the expression of IDO1, which would negatively regulate effector T cells. Second, another signaling pathway might activate IDO1 expression in CRC patients. Third, these patients were treated with celecoxib but not combined with IDO1 inhibitors.
There are some limitations in our current study. This study was a retrospective study, with its intrinsic associated limitations. Second, although our cohort size consists of well-annotated celecoxib groups, its number is still modest. Third, to minimize bias and immunohistochemistry methodological limitations, we have herein adopted rigorous standardized assay methods in our study. All immunohistochemistry scores were affirmed by two blinded, well-trained clinical pathologists working independently. Furthermore, a larger clinical sample cohort size would be valuable to validate our results, and more chemotherapy-resistant patients need to be considered.
The results of the current study demonstrate that the coexpression of cytoplasmic IDO1 and COX2 plays a key role in survival prognosis for CRC patients; IDO1 or COX2, nuclear IDO1 and COX2 alone may not serve as a feasible biomarker for prognostic prediction. Therefore, localization of IDO1 and COX2 may serve as a better biomarker to predict CRC patient OS.
Colorectal cancer (CRC) is a leading cause of cancer-related death worldwide. Because of genetic mutations and environmental factors, CRC development is a very complex process and is determined by multistage factors. Currently, immunotherapy has become one of the most promising treatments for CRC. However, whether indoleamine-2,3-dioxygenase 1/cyclooxygenase 2 (IDO1/COX2) coexpression is correlated with overall survival (OS) in CRC patients remains unknown.
CRC has demonstrated high heterogeneity in recent years. Recent studies have demonstrated that IDO1 can suppress the T cell response to tumors. A selective COX2 inhibitor, celecoxib, could improve chemosensitivity when CRC cells are exposed to the combination of 5-FU and CPT-11 and could reduce hand-foot syndrome induced by capecitabine. In this study, we conducted a retrospective analysis for the potential prognostic importance of the correlation of IDO1 and COX2 in survival outcome prognosis, including their coexpression, cytoplasmic and nuclear localization of IDO1, and tumor-infiltrating lymphocytes.
This study aimed to clarify the potential significance of IDO1/COX2 as a prognostic biomarker in CRC in vitro.
Immunohistochemical staining of IDO1 and COX2 was performed in a clinical cohort consisting of 96 CRC cases. Expression of IDO1 and COX2 was correlated with clinicopathological indicators and the clinical outcome of CRC patients.
In the CRC group, combined cytoplasmic IDO1/COX2 coexpression analysis yielded a stronger predictor index, with hazard ratio (HR) = 2.218 (95% confidence interval (CI): 1.011-4.48, P = 0.047) in the IDO1High/COX2High group, and tumor differentiation was significantly correlated with OS (HR = 3.473, 95%CI: 1.201-10.046, P = 0.022) but not nuclear IDO1, cytoplasmic IDO1, nor combined nuclear IDO1/COX2 expression. Our results revealed that cytoplasmic IDO1/COX2 coexpression and tumor differentiation were independent predictors for poor OS in CRC.
In the CRC celecoxib subgroup, combined cytoplasmic IDO1/COX2 coexpression analysis yielded a stronger predictor index, with HR = 3.210 (95%CI: 1.074-9.590, P = 0.037) in the IDO1High/COX2High group, and tumor differentiation was significantly correlated with OS (HR = 11.962, 95%CI: 1.526-23.787, P = 0.018) but not nuclear IDO1, cytoplasmic IDO1, nor combined nuclear IDO1/COX2 expression.
The results of the current study demonstrate that the coexpression of cytoplasmic IDO1 and COX2 plays a key role in survival prognosis in CRC patients.
IDO1 could be a novel therapeutic target for human CRC, especially as a biotarget of immunotherapy.
We would like to thank Dr. Zhi-Tao Xiao and Dr. Yang Zhao for collecting the clinical data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Awad Z, Mayol J, Takamatsu S S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2071] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 2. | Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Kornblihtt AR. Epigenetics at the base of alternative splicing changes that promote colorectal cancer. J Clin Invest. 2017;127:3281-3283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Brenner H, Hoffmeister M. [Colorectal cancer screening: evidence and implementation]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Singh PP, Sharma PK, Krishnan G, Lockhart AC. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep (Oxf). 2015;3:289-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384-3391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 1135] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 7. | Deng C, Li Z, Guo S, Chen P, Chen X, Zhou Q, Chen J, Yu X, Wu X, Ma W. Tumor PD-L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology. 2016;6:e1269047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Wang X, Huang S, Zhang Y, Zhu L, Wu X. The application and mechanism of PD pathway blockade for cancer therapy. Postgrad Med J. 2018;94:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6274] [Article Influence: 482.6] [Reference Citation Analysis (0)] |
| 10. | Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen E, Van den Eynde BJ. Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunol Res. 2017;5:695-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1403] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 12. | Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 13. | Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu XJ, Pan ZZ, Wan DS, Zeng YX, Zhang XS. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Ando T, Ito H, Hatano Y, Tomita H, Kuno T, Saito K. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci. 2015;106:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, Huang W, Li JJ, Song HF, Xia JC. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, Van Maerken T, Salmon I, Cuvelier CA, Demetter P. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Gritsina GA, Zhai L, Ladomersky ER, Qian J, Lauing KL, Bui TM, Rivetta CV, Horbinski CM, Wainwright DA. Nuclear localization of immunosuppressive IDO1 in cancer: a paradigm-shift to the tryptophan depletion theory. Faseb J. 2017;31. |
| 19. | Rahman M, Selvarajan K, Hasan MR, Chan AP, Jin C, Kim J, Chan SK, Le ND, Kim YB, Tai IT. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers in vivo. Neoplasia. 2012;14:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Zhang RX, Wu XJ, Lu SX, Pan ZZ, Wan DS, Chen G. The effect of COX-2 inhibitor on capecitabine-induced hand-foot syndrome in patients with stage II/III colorectal cancer: a phase II randomized prospective study. J Cancer Res Clin Oncol. 2011;137:953-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegård J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Wang M, Yin B, Wang HY, Wang RF. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |