Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.266
Peer-review started: October 27, 2017
First decision: November 14, 2017
Revised: November 18, 2017
Accepted: December 5, 2017
Article in press: December 5, 2017
Published online: January 14, 2018
Processing time: 80 Days and 0.6 Hours
To investigate predictive and prognostic value of serum alpha-fetoprotein (AFP) level and its dynamic changes in patients with advanced gastric cancer with elevated serum AFP (AFPAGC).
One hundred and five patients with AFPAGC were enrolled in the study, and all of them underwent at least one cycle of systemic chemotherapy at our institute and had serum AFP ≥ 20 ng/mL at diagnosis or recurrence. Clinicopathologic features, serum AFP level at diagnosis and changes during treatment, first-line chemotherapy regimens, efficacy and toxicity, and survival information were collected. A Person’s χ2 or Fisher’s exact test was used to measure the differences between variables. Survival prognostic factors were investigated using the Kaplan-Meier method and Cox regression.
Median serum AFP level was 161.7 ng/mL (range, 22.9-2557110 ng/mL). Objective response rates (ORR) was significantly lower in the AFP ≥ 160 ng/mL group than in the AFP < 160 ng/mL group (30.4% vs 68.3%, P < 0.001). ORR to doublet regimens was significantly lower in the AFP ≥ 160 ng/mL group, whereas ORR to triplet regimens was similar between the two groups. Liver metastasis rate was significantly higher in the AFP ≥ 160 ng/mL group than in the AFP < 160 ng/mL (69.8% vs 50.0%, P < 0.001). Overall survival (OS) in the two cohorts did not show any significant difference (P = 0.712). Dynamic changes of AFP were consistent with response to chemotherapy, and median OS of patients with a serum AFP decline ≥ 50% and those with a serum AFP decline < 50% was 17.5 m and 10.0 m, respectively (P = 0.003). Hepatic (P = 0.005), peritoneal (P < 0.001), non-regional lymph node metastasis (P < 0.001), and portal vein tumor thrombus (PVTT) (P = 0.042) were identified as independent prognostic factors for AFPAGC.
Real-time examination of AFP has great predictive and prognostic value for managing AFPAGC. For those with markedly elevated AFP, triplet regimens may be a better choice.
Core tip: Alpha-fetoprotein (AFP)-producing gastric cancer is a rare and aggressive subtype of gastric cancer, characterized by frequent liver metastasis and poor prognosis. We measured AFP and its changes over time during treatment, which revealed that AFP, as a biomarker of advanced gastric cancer with elevated serum AFP (AFPAGC), is significantly associated with response to chemotherapy. The decline in AFP after chemotherapy was found to be related to good prognosis for AFPAGC. We finally attempted to find an optimal treatment regimen for AFPAGC, which suggests that for those with markedly elevated AFP, triplet regimens may be a better choice.
- Citation: Wang YK, Shen L, Jiao X, Zhang XT. Predictive and prognostic value of serum AFP level and its dynamic changes in advanced gastric cancer patients with elevated serum AFP. World J Gastroenterol 2018; 24(2): 266-273
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/266.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.266
Gastric cancer (GC) remains the second leading cause of cancer-related death worldwide. Alpha-fetoprotein (AFP)-producing GC (AFPGC) is rare, accounting for 2.3%-7.1% of all GCs[1]. In 1970, Bourreille’s group first reported a case of AFPGC, and its pathological specimen was immunohistochemically positive for AFP[2]. Previous work suggests that AFPGC is associated with a poor prognosis due to frequent liver metastasis[3,4]. However, most studies of AFPGC were based on surgically resected samples and many patients with advanced GC with elevated serum AFP (AFPAGC) have already missed an opportunity for surgical resection[5], with palliative chemotherapy having been a mainstay treatment. AFP is the most representative biomarker for AFPAGC, but how it is involved in the development and progression of AFPAGC remains known. On the other hand, due to the rarity of this special form of cancer, there is limited data in the literature about its optimal treatment.
In the present study, we studied whether AFP can be used to predict prognosis, and measured its dynamic changes over time during treatment, with an aim to find an optimal treatment regimen for AFPAGC and identify prognostic factors for this subtype of advanced GC.
From 2006 to 2016, 2047 patients were diagnosed with advanced gastric adenocarcinoma at our institute. Subjects were enrolled if they were diagnosed with primary gastric adenocarcinoma; had no chance for surgery at diagnosis or had relapsed after radical resection (relapse types included anastomotic recurrence and distant metastasis); underwent at least one cycle of systemic chemotherapy at our institute (total number of chemotherapy cycles ranged from one to seven, with a median number of cycles of four in this study); and had serum AFP ≥ 20 ng/mL at diagnosis or recurrence. The exclusion criteria were concomitant liver diseases, such as hepatitis, cirrhosis, fatty liver, or alcoholic liver, and concomitant second or multiple primary tumors. We chose 105 patients and measured pre-treatment serum AFP using radioimmunoassay (normal range: < 7 ng/mL).
We collected data including age, gender, primary lesion site, histological type, Lauren classification, human epidermal growth factor receptor-2 (HER2) status, metastasis site, serum AFP level at diagnosis and changes during treatment, first-line chemotherapy regimen, efficacy and toxicity, local treatment for liver metastasis, and survival information.
All patients were regularly followed from the date of first hospitalization at our center. Laboratory examinations were performed every 1 or 2 wk, and enhanced computed tomography or magnetic resonance imaging was performed to evaluate therapeutic efficacy every 6 wk during chemotherapy. Objective response rate (ORR) was evaluated using RECIST version 1.0 (before 2009) and RECIST version 1.1, and adverse reactions were recorded. Overall survival (OS) was defined as the time from diagnosis to death from any cause or last follow-up.
A Person’s χ2 test was used to measure the differences among variables, and a Fisher’s exact test was used when the sample size was less than five. To identify prognostic factors for APFAGC, survival durations were calculated using the Kaplan-Meier method and Cox regression. For all tests, a P-value < 0.05 was considered significant. SPSS software (version 21.0; SPSS, Chicago, IL, United States) was used for analyses. GraphPad Prism 6 (GraphPad Software, Inc, La Jolla, CA, United States) was used for graphing.
A total of 105 AFPAGC patients were evaluated. They ranged in age from 27 to 78 years, with a median age of 59 years. Most of the patients were diagnosed with locally advanced or metastatic GC at the initial diagnosis. Only eight patients who had recurrent disease after radical gastrectomy were involved in this study, including three cases with non-regional lymph node metastasis, six cases with liver metastasis, one case with peritoneal metastasis, and one case with anastomotic recurrence. Median serum AFP level was 161.7 ng/mL (range, 22.9-2557110 ng/mL). Nearly two-thirds (64.5%) of the patients had serum AFP < 500 ng/mL at the time of diagnosis. With regard to immunohistochemical staining (IHC) for AFP, IHC results were available in only 14 patients, of whom eight were AFP positive.
As for the primary lesion site, 41 (39.8%) tumors were located at the gastroesophogeal junction (GEJ). In histological examination, 29.4% and 64.7% patients were identified as well-differentiated and poorly differentiated adenocarcinoma, respectively. Notably, six (5.9%) patients were diagnosed with hepatoid adenocarcinoma, which was defined as a special subtype of primary gastric adenocarcinoma characterized histologically by “hepatocellular carcinoma (HCC) like differentiation”[6]. Besides, 45 (57.0%) patients had intestinal type based on the Lauren classification, and 20 (24.4%) patients HER2 positive.
As expected, 60.0% of patients were detected with liver metastasis, while only 15.4% with peritoneal dissemination. Also, portal vein tumor thrombus (PVTT) in AFPAGC had an occurrence rate of 12.4% in this study. The clinicopathological features of AFPAGC are detailed in Table 1.
| Variable | AFPAGC(n = 105) | Median OS (mo) | P value |
| Sex | |||
| Male | 82 (78.1) | 15.0 | 0.144 |
| Female | 23 (21.9) | 11.3 | |
| Age (yr) | |||
| ≥ 60 | 49 (46.7) | 15.0 | 0.189 |
| < 60 | 56 (53.3) | 12.0 | |
| Serum AFP level (ng/mL) | |||
| ≥ 500 | 37 (35.2) | 13.0 | 0.806 |
| < 500 | 68 (64.5) | 14.6 | |
| Primary lesion site | |||
| EGJ | 41 (39.8) | 15.0 | 0.245 |
| Non-EGJ | 62 (60.2) | 12.0 | |
| Differentiation degree | |||
| Well | 30 (29.4) | 15.4 | 0.496 |
| Poor | 66 (64.7) | 12.9 | |
| HAS | 6 (5.9) | 4.5 | |
| Lauren classification | |||
| Intestinal | 45 (57.0) | 15.4 | 0.352 |
| Non-intestinal | 34 (43.0) | 14.6 | |
| HER2 status | |||
| Positive | 20 (24.4) | 17.5 | 0.583 |
| Negative | 62 (75.6) | 14.6 | |
| LM | |||
| Present | 63 (60.0) | 12.0 | 0.048a |
| Absent | 42 (40.0) | 16.7 | |
| Peritoneal metastasis | |||
| Present | 16 (15.4) | 6.17 | 0.001a |
| Absent | 88 (84.6) | 15.2 | |
| Non-regional LNM | |||
| Present | 56 (53.3) | 11.0 | 0.042a |
| Absent | 49 (46.7) | 17.9 | |
| Other hematogenous metastasis | |||
| Present | 27 (25.7) | 10.5 | 0.004a |
| Absent | 78 (74.3) | 17.5 | |
| PVTT | |||
| Present | 13 (12.4) | 8.3 | 0.011a |
| Absent | 92 (87.6) | 15.0 | |
| First-line regimen | |||
| Doublet regimen | 89 (88.1) | 14.6 | 0.850 |
| Triplet regimen | 12 (11.9) | 15.1 | |
| Evaluation | |||
| PR | 42 (48.3) | 17.6 | 0.007a |
| SD + PD | 45 (51.7) | 11.1 | |
| AFP decline degree | |||
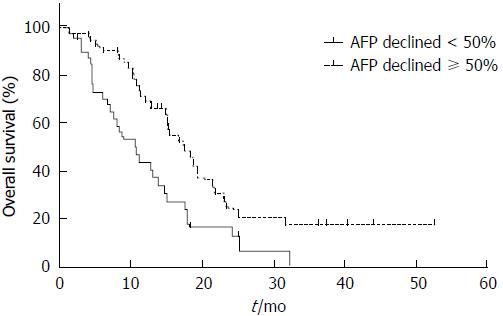
| ≥ 50% | 49 (55.7) | 17.5 | 0.003a |
| < 50% | 39 (44.3) | 10.0 | |
| Local treatment for LM | |||
| Yes | 19 (18.1) | 17.9 | 0.215 |
| No | 86 (81.9) | 12.9 |
In the treatment of inoperable locally advanced and/or metastatic (stage IV) GC, doublet combinations of platinum and fluoropyrimidines were frequently used, and most of triplet regimes were given to those who had potential opportunity for surgery and good performance status in this study. Among the original 105 patients who received first-line systemic chemotherapy, 87 (82.9%) were evaluable for their response. The majority (66.7%, n = 58) received platinum-based doublet regimens, including oxaliplatin + capecitabine in 36 patients, oxaliplatin + S-1 in 7, cisplatin + capecitabine in 12, cisplatin + S-1 in 1, oxaliplatin + 5-FU in 1 , and cisplatin + 5-FU in 1. Seventeen (19.5%) patients received taxane-based doublet regimens, including paclitaxel + capecitabine in 11 patients, paclitaxel + S-1 in 4, paclitaxel + 5-FU in 1, and docetaxel + capecitabine in 1. Eleven (12.6%) patients received triplet regimens, including POS (paclitaxel + oxaplatin + S-1) in 6 patients, DCF (docetaxel + cisplatin + 5-FU) in 4, and PCF (paclitaxel + cisplatin + 5-FU) in 1. In addition, 12 of 20 HER2 positive patients received anti-HER2 therapies, including trastuzumab in 11 patients and lapatinib in 1.
Overall ORR to first-line chemotherapy was 48.3%. ORR to platinum-based doublet regimens was similar to that to triplet regimens (53.4% vs 54.5%), but much higher than that to taxane-based doublet regimens (29.4%). The differences between either of them did not reach statistical significance (Table 2).
| Regimen | Platinum-based doublet regimen(n = 58) | Taxane-based doublet regimen(n = 17) | Triplet regimen(n =11) | P value |
| Overall population | 0.201 | |||
| PR | 31 (53.4) | 5 (29.4) | 6 (54.5) | |
| SD + PD | 27 (46.6) | 12 (76.4) | 5 (45.5) | |
| AFP ≥ 160 ng/mL | 0.067 | |||
| PR | 10 (32.3) | 0 (0.0) | 4 (57.1) | |
| SD + PD | 21 (67.7) | 7 (100.0) | 3 (42.9) | |
| AFP < 160 ng/mL | 0.193 | |||
| PR | 21 (77.8) | 5 (50.0) | 2 (50.0) | |
| SD + PD | 6 (22.2) | 5 (50.0) | 2 (50.0) |
As for toxicity, there were totally 22 (21.0%) patients who suffered severe (≥ grade 3) adverse events (AEs) during first-line systemic chemotherapy, with most frequently occurring severe AEs being bone marrow suppression (13.3%), hand foot syndrome (4.8%), and digestive tract reaction (3.8%). Notably, patients who received triplet regimens had a significantly higher rate of severe AEs (58.3% vs 15.3-23.5%, P = 0.004) (Table 3).
| Regimen | Platinum-based doublet regimen(n = 72) | Taxane-based doublet regimen(n = 17) | Triplet regimen(n =12) | P value |
| ≥ G3 AEs | 11 (15.3) | 4 (23.5) | 7 (58.3) | 0.004 |
With regard to second-line chemotherapy, treatment data were available in 57 patients in this study. Thirty-two patients received second-line systemic chemotherapy, regimens mainly involved taxanes alone or combined with fluorouracil drugs. Nine patients had no chance for second-line treatment due to bad performance status. Moreover, 16 patients received local treatment instead of systemic chemotherapy due to progression after first-line treatment, including transarterial chemoembolization in 11 patients, radiotherapy in 2, ablation in 1, and pleural or intraperitoneal perfusion chemotherapy in 2.
Serum AFP level at diagnosis ranged from 22.9 to 2557110 ng/mL, with a median value of 161.7 ng/mL. As AFP is considered the most representative marker for AFPAGC, we next investigated the association between AFP level and response to chemotherapy, occurrence of liver metastasis, and survival. We chose the median value 160 ng/mL as a cutoff value.
The χ2 test showed that overall ORR in the AFP ≥ 160 ng/mL group was significantly lower than that of the AFP < 160 ng/mL group (30.4% vs 68.3%, P < 0.001). Furthermore, ORR to doublet regimens was significantly lower in the AFP ≥ 160 ng/mL group, whereas ORR to triplet regimens was similar between the two groups (Table 4).
| Variable | AFP ≥ 160 ng/mL | AFP < 160 ng/mL | P value |
| Overall ORR | |||
| PR | 14 (30.4) | 28 (68.3) | < 0.001a |
| SD + PD | 32 (69.6) | 13 (31.7) | |
| ORR to doublet regimens | |||
| PR | 10 (26.3) | 26 (70.3) | < 0.001a |
| SD + PD | 28 (73.7) | 11 (30.7) | |
| ORR to triplet regimens | |||
| PR | 4 (57.1) | 2 (50.0) | 0.652 |
| SD + PD | 3 (42.9) | 2 (50.0) | |
| Liver metastasis rate | 69.8% | 50.0% | 0.030a |
| Median OS | 13.0 mo | 14.8 mo | 0.712 |
The ROC curve analysis for the predictive value of serum AFP is shown in Figure 1. The sensitivity and specificity were 71.1% and 69.0%, respectively, at a cut-off value of 164.8 ng/mL.
In addition, we found that liver metastasis rate was significantly higher in the AFP ≥ 160 ng/mL group than in the AFP < 160 ng/mL group (69.8% vs 50.0%, P < 0.001), although OS did not show any significant difference (P = 0.712, Table 4). We measured serum AFP levels in 81 patients at the time of evaluation, and the patients were sub-classified into two cohorts according to the decline degree of AFP: (1) ≥ 50%(n = 47); and (2) < 50% (including those who had elevated AFP after chemotherapy) (n = 34). A significant correlation was observed between AFP decline degree and response to chemotherapy (72.3% vs 14.7%, P < 0.001, Table 5).
| Variable | AFP decline ≥ 50% | AFP decline < 50% | P value |
| Response | |||
| PR | 34 (72.3) | 5 (14.7) | <0.001 |
| SD + PD | 13 (27.7) | 29 (85.3) |
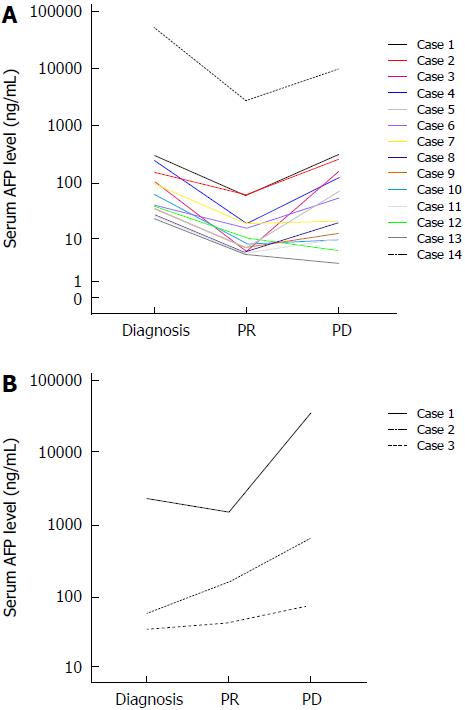
Among the 39 patients who achieved partial response (PR), serum AFP levels were exactly measured in 17 patients until the time of progression. Among them, serum AFP declined by ≥ 50% in 14 patients when evaluated as PR, but re-elevated back to the pre-treatment levels when progressed. No re-evaluation of serum AFP levels was also observed in several patients (Figure 2A). By contrast, serum AFP level did not decline that much in three patients when evaluated as PR, and with the tumor progressed, AFP levels of all these patients elevated markedly, even much higher than the pre-treatment levels (Figure 2B).
The 1-year survival for AFPAGC patients was 41.9% and median OS was 13.9 mo. Potential prognosis-related factors including sex, age, primary tumor features, serum AFP level, liver and extrahepatic metastasis, treatment, and response were examined. Univariate analysis showed that metastasis status (liver metastasis, peritoneal metastasis, non-regional lymph node metastasis, and other hematogenous metastasis), PVTT, response to chemotherapy, and serum AFP decline degree were associated with prognosis (Table 1, Figures 3 and 4). Multivariate analysis showed that hepatic (P = 0.005), peritoneal (P < 0.001), and non-regional lymph node metastasis (P < 0.001), and PVTT (P = 0.042) were independent prognostic factors (Table 6).
| Factor | HR | 95%CI | P value |
| LM (present) | 2.809 | 1.363-5.788 | 0.005a |
| PM (present) | 4.243 | 2.026-8.883 | < 0.001a |
| Non-regional LNM (present) | 3.743 | 1.928-7.268 | < 0.001a |
| Other hematogenous metastasis (present) | 1.479 | 0.692-3.161 | 0.312 |
| PVTT (present) | 2.341 | 1.030-5.320 | 0.048a |
| Response (SD + PD) | 1.92 | 0.953-3.867 | 0.068 |
| AFP decline degree (< 50%) | 1.876 | 0.980-3.589 | 0.057 |
We found that monitoring serum AFP over time had predictive and prognostic value in the management of AFPAGC. AFP is a fetal serum protein produced by fetal and yolk sac cells and its level rapidly decreases after birth. Although AFP is a well-known tumor marker for hepatocellular carcinoma and yolk sac tumors[7], it is also elevated in various extrahepatic tumors, including gastrointestinal tract tumors, as well as pancreatic, gallbladder, lung, and bladder cancers[8].
The definition of AFPGC varies across studies, and elevation of serum AFP or immunohistochemical staining for AFP is often used[1,3,9,10]. As a rare subgroup of GC, AFPGC was reported to be more aggressive than that without AFP production, and to have more liver metastasis, even after radical D2 gastrectomy[5]. Therefore, systemic chemotherapy is a first-line approach for treatment of this special subtype of GC.
Previous studies focused on AFPGC after radical gastrectomy, and few data exist about optimal treatment for AFPAGC. Thus, we studied the treatment, therapeutic response, and outcomes of AFPAGC, and elucidated the predictive and prognostic value of serum AFP in the management of this special cancer. Considering many factors that can cause a mild increase in AFP, such as liver metastasis sites, we selected a threshold of AFP ≥ 20 ng/mL as the inclusion criterion in this study.
Our study revealed that AFP, a biomarker of AFPAGC, was associated with response to chemotherapy. ORR in the AFP ≥ 160 ng/mL group was significantly lower than that of the AFP < 160 ng/mL group (30.4% vs 68.3%, P < 0.001). This may be partially explained by the fact that AFP-producing gastric cell lines were resistant to many drugs[11]. We have known that AFP is not only a product of tumor, but also contributes to tumor aggression as well as regulation of hepatocellular growth and tumorigenesis[12]. Similar to that in HCC[13], AFP also has a crucial role in the proliferation, apoptosis, and angiogenesis of AFPGC cells[14]. Therefore, we speculate that AFP may play a significant role in primary drug resistance in AFPAGC and this warrants more study.
Besides the serum AFP level at diagnosis, we measured the dynamic changes of AFP over time after treatment, and this helped us to predict the efficacy of treatment and early relapse. It is noteworthy to mention that serum AFP does not always increase after tumor recurrence due to high heterogeneity of AFPAGC[15-17], which has been reported in a previous study[18]. Although serum AFP itself cannot be definitely associated with survival, we found AFP decline was significantly associated with prognosis, suggesting the need of real-time assay of AFP during management of AFPAGC. Furthermore, monitoring AFP changes after first-line chemotherapy may suggest tumor behavior and assist with subsequent treatment choices.
To explore optimal treatment regimens for AFPAGC, we analyzed ORR and toxicity of different regimens, and found that platinum-based doublet regimens and triplet regimens had similar ORR in AFPAGC. In the treatment of inoperable locally advanced and/or metastatic (stage IV) GC, doublet combinations of platinum and fluropyrimidines are often used, with an ORR of 52.2%-58.7%[19]. However, triplet regimens are not routinely used in China and Japan[20]. ORR to doublet regimens was significantly lower for subjects with markedly elevated serum AFP in the present study (26.3% vs 56.1%), so triplet regimens may be better for this subgroup compared with doublet regimens, despite frequent ≥ grade 3 adverse events (58.3%). Due to extremely aggressive biological behavior, more aggressive therapy using triplet regimens may be considered for those with high serum AFP level. Optimizing triplet regimens also needs further study.
Targeted therapy may also offer a key to striding over primary drug resistance to some extent. Next-generation sequencing has been applied to GC and the Cancer Genome Altas (TCGA) Research Network defined four major genomic subtypes of GC: Epstein-Barr (EBV)-infected tumors; microsatellite instability (MSI) tumors; genomically stable (GS) tumors; and chromosomally unstable (CIN) tumors[21]. EBV-infected and MSI tumors were identified as potential candidates for immune checkpoint inhibitors[22]. Recent studies suggest that most TCGA tumors with elevated AFP expression were categorized as CIN subtypes, characterized by frequent amplifications of receptor tyrosine kinases, many of which are amenable to blockade by agents in current use or in development[23]. Recurrent amplification of the gene encoding ligand vascular endothelial growth factor A was notable given the activity of the vascular endothelial growth factor-receptor 2 (VEGF-R2) targeted antibody ramucirumab in GC[24,25]. Due to increased VEGF expression and rich neovascularization in AFPGC, which is consistent with high incidence of PVTT (12.4% in our study), anti-angiogenic therapy is thought to be effective. In a case report of a patient with chemotherapy-resistant recurrent AFPGC, after six doses of ramucirumab, metastatic lymph nodes were centrally necrotic, and serum AFP decreased from 1280 to 225 ng/mL[26]. What’s more, apatinib, a small molecular tyrosine kinase inhibitor targeting VEGF-R2, is also anti-angiogenic. Another case report of targeted therapy with apatinib in a patient with advanced AFPGC showed that PFS was achieved in 5 mo[27]. Similarly, in our present study, we also found that serum AFP in one patient decreased from 2000 ng/mL to 400 ng/mL with apatinib. Also, multi-target tyrosine kinase inhibitors, including sorafenib, which was approved for first-line treatment of HCC, were reported to be effective for this type of GC[28], indicating a correlation between the carcinogenesis of AFPGC and HCC.
Therefore, we suspect that anti-angiogenic drugs and multi-target tyrosine kinase inhibitors may have great potential for treating this aggressive subtype of GC, and AFP production may predict response. Thus, combined chemotherapy and molecular targeted treatment should be studied. Overall, AFPAGC is associated with a relatively poor prognosis, and it is a heterogeneous cancer with different clinical outcomes, biological behaviors, and genetic alterations. However, not all AFPAGC patients have a prognosis as poor as we previously thought.
In conclusion, real-time examination of AFP has great predictive and prognostic value in managing AFPAGC. High AFP is associated with poor response to chemotherapy, and AFP decline after chemotherapy is considered related to good prognosis in AFPAGC. Liver metastasis, peritoneal metastasis, non-regional lymph node metastasis, and PVTT are independent prognostic factors for this special cancer. For those with markedly elevated serum AFP, triplet regimens may be a better choice.
Alpha-fetoprotein (AFP)-producing gastric cancer (AFPGC) is a special subgroup of gastric cancer (GC), and there are robust data confirming the poor prognosis for this population, especially for those with resected disease. However, due to aggressive biological behavior and high frequency of liver metastasis, most AFPGC patients were considered as inoperable at the initial diagnosis and there is limited data in the literature about management of AFP- producing advanced GC.
As the precise underlying mechanism of AFPGC remains to be elucidated, the optimal treatment approach requires further consideration, especially for advanced gastric cancer with elevated serum AFP (AFPAGC). Therefore, we performed this study to seek better management regimen for AFPAGC, with an aim to improve the prognosis of this special aggressive cancer.
The main objectives of this study were: (1) to elucidate predictive and prognostic value of serum AFP level and its dynamic changes during management of AFPAGC; and (2) to discover optimal treatment modality for AFPAGC. This would also allow risk stratification for patients with gastric cancer in future clinical trials.
Patient data in this study were obtained by reviewing electronic medical charts. Statistical analyses were performed with SPSS 21.0 software. A Person’s χ2 test was used to measure the differences among variables. To identify prognostic factors for APFAGC, survival durations were calculated using the Kaplan-Meier method and Cox regression.
Our results revealed that for AFPAGC, serum AFP level was associated with liver metastasis rate and response to chemotherapy. Serum AFP decline degree was associated with response to chemotherapy and survival. Furthermore, we investigated optimal chemotherapy regimen for this special population, which revealed that for those with marked AFP elevation, triplet regimens could offer a better objective response rates (ORR) than doublet regimens, but the toxicity is a problem that remains to be solved. Finally, hepatic (P = 0.005), peritoneal (P < 0.001), non-regional lymph node metastasis (P < 0.001), and PVTT (P = 0.042) were identified as independent prognostic factors for AFPAGC.
This is the first study to elucidate the great predictive and prognostic value of real-time examination of serum AFP in managing AFPAGC. We also suggest that for GCs with markedly elevated AFP, triplet regimens may be a better choice. This would also allow risk stratification for patients with gastric cancer in future clinical trials.
Since AFPAGC is rare, for which large prospective clinical trials are not feasible, it is very significant to summarize clinical experience retrospectively. Although our results showed that triplet regimens may offer a better ORR, there remains controversy regarding the utility of triplet regimens due to their toxicity. Therefore, it will be necessary to optimize triplet regimens and find new therapeutic targets in future studies. Next generation sequencing may bring us new insight in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kanat O, Kimura A S- Editor: Chen K L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Wang D, Li C, Xu Y, Xing Y, Qu L, Guo Y, Zhang Y, Sun X, Suo J. Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp Pathol. 2015;8:6345-6355. [PubMed] |
| 2. | Alpert E, Pinn VW, Isselbacher KJ. Alpha-fetoprotein in a patient with gastric carcinoma metastatic to the liver. N Engl J Med. 1971;285:1058-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Hirajima S, Komatsu S, Ichikawa D, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H, Otsuji E. Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol. 2013;19:6055-6061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95-101. [PubMed] |
| 6. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 7. | El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer. 2010;46:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Lin HJ, Hsieh YH, Fang WL, Huang KH, Li AF. Clinical manifestations in patients with alpha-fetoprotein-producing gastric cancer. Curr Oncol. 2014;21:e394-e399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Reim D, Choi YS, Yoon HM, Park B, Eom BW, Kook MC, Ryu KW, Choi IJ, Joo J, Kim YW. Alpha-fetoprotein is a significant prognostic factor for gastric cancer: Results from a propensity score matching analysis after curative resection. Eur J Surg Oncol. 2017;43:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Chang YC, Nagasue N, Kohno H, Ohiwa K, Yamanoi A, Nakamura T. Xenotransplantation of alpha-fetoprotein-producing gastric cancers into nude mice. Characteristics and responses to chemotherapy. Cancer. 1992;69:872-877. [PubMed] |
| 12. | Li M, Li H, Li C, Wang S, Jiang W, Liu Z, Zhou S, Liu X, McNutt MA, Li G. Alpha-fetoprotein: a new member of intracellular signal molecules in regulation of the PI3K/AKT signaling in human hepatoma cell lines. Int J Cancer. 2011;128:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Sauzay C, Petit A, Bourgeois A-M, Barbare J-C, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clinica Chimica Acta. 2016;463:39-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol. 1999;94:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | He L, Ye F, Qu L, Wang D, Cui M, Wei C, Xing Y, Lee P, Suo J, Zhang DY. Protein profiling of alpha-fetoprotein producing gastric adenocarcinoma. Oncotarget. 2016;7:28448-28459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654-661. [PubMed] |
| 17. | Eom BW, Jung SY, Yoon H, Kook MC, Ryu KW, Lee JH, Kim YW. Gastric choriocarcinoma admixed with an alpha-fetoprotein-producing adenocarcinoma and separated adenocarcinoma. World J Gastroenterol. 2009;15:5106-5108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Tomiyama K, Takahashi M, Fujii T, Kunisue H, Kanaya Y, Maruyama S, Yokoyama N, Shimizu N, Soda M. A rare case of recurrent alpha-fetoprotein-producing gastric cancer without re-elevation of serum AFP. J Int Med Res. 2006;34:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 20. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 21. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4859] [Article Influence: 441.7] [Reference Citation Analysis (2)] |
| 22. | Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, Sun W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol. 2016;40:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Arora K, Bal M, Shih A, Moy A, Zukerberg L, Brown I, Liu X, Kelly P, Oliva E, Mullen J. Fetal-type gastrointestinal adenocarcinoma: a morphologically distinct entity with unfavourable prognosis. J Clin Pathol. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1576] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 25. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1769] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 26. | Arakawa Y, Tamura M, Aiba K, Morikawa K, Aizawa D, Ikegami M, Yuda M, Nishikawa K. Significant response to ramucirumab monotherapy in chemotherapy-resistant recurrent alpha-fetoprotein-producing gastric cancer: A case report. Oncol Lett. 2017;14:3039-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Zhu XR, Zhu ML, Wang Q, Xue WJ, Wang YW, Wang RF, Chen SY, Zheng LZ. A case report of targeted therapy with apatinib in a patient with advanced gastric cancer and high serum level of alpha-fetoprotein. Medicine (Baltimore). 2016;95:e4610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Fang YU, Wang L, Yang N, Gong X, Zhang YU, Qin S. Successful multimodal therapy for an α-fetoprotein-producing gastric cancer patient with simultaneous liver metastases. Oncol Lett. 2015;10:3021-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |