Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.237
Peer-review started: October 1, 2017
First decision: October 25, 2017
Revised: November 6, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: January 14, 2018
Processing time: 105 Days and 21.8 Hours
To explore the effectiveness for treating liver fibrosis by combined transplantation of bone marrow-derived endothelial progenitor cells (BM-EPCs) and bone marrow-derived hepatocyte stem cells (BDHSCs) from the liver fibrosis environment.
The liver fibrosis rat models were induced with carbon tetrachloride injections for 6 wk. BM-EPCs from rats with liver fibrosis were obtained by different rates of adherence and culture induction. BDHSCs from rats with liver fibrosis were isolated by magnetic bead cell sorting. Tracing analysis was conducted by labeling EPCs with PKH26 in vitro to show EPC location in the liver. Finally, BM-EPCs and/or BDHSCs transplantation into rats with liver fibrosis were performed to evaluate the effectiveness of BM-EPCs and/or BDHSCs on liver fibrosis.
Normal functional BM-EPCs from liver fibrosis rats were successfully obtained. The co-expression level of CD133 and VEGFR2 was 63.9% ± 2.15%. Transplanted BM-EPCs were located primarily in/near hepatic sinusoids. The combined transplantation of BM-EPCs and BDHSCs promoted hepatic neovascularization, liver regeneration and liver function, and decreased collagen formation and liver fibrosis degree. The VEGF levels were increased in the BM-EPCs (707.10 ± 54.32) and BM-EPCs/BDHSCs group (615.42 ± 42.96), compared with those in the model group and BDHSCs group (P < 0.05). Combination of BM-EPCs/BDHSCs transplantation induced maximal up-regulation of PCNA protein and HGF mRNA levels. The levels of alanine aminotransferase (AST), aspartate aminotransferase, total bilirubin (TBIL), prothrombin time (PT) and activated partial thromboplastin time in the BM-EPCs/BDHSCs group were significantly improved, to be equivalent to normal levels (P > 0.05) compared with those in the BDHSC (AST, TBIL and PT, P < 0.05) and BM-EPCs (TBIL and PT, P < 0.05) groups. Transplantation of BM-EPCs/BDHSCs combination significantly reduced the degree of liver fibrosis (staging score of 1.75 ± 0.25 vs BDHSCs 2.88 ± 0.23 or BM-EPCs 2.75 ± 0.16, P < 0.05).
The combined transplantation exhibited maximal therapeutic effect compared to that of transplantation of BM-EPCs or BDHSCs alone. Combined transplantation of autogenous BM-EPCs and BDHSCs may represent a promising strategy for the treatment of liver fibrosis, which would eventually prevent cirrhosis and liver cancer.
Core tip: In addition to liver regeneration, hepatic neovascularization and endothelial/sinusoidal remodeling are critical factors for the treatment of liver fibrosis. Bone marrow-derived endothelial progenitor cells (BM-EPCs) have been shown to play a role in hepatic angiogenesis and to be beneficial in liver fibrosis. However, BM-EPCs are all derived from healthy individuals in recent studies. Here, we evaluated the feasibility of obtaining normal BM-EPCs from rats with liver fibrosis, and the effectiveness of combined transplantation of BM-EPCs and bone marrow-derived hepatocyte stem cells for treating liver fibrosis in order to achieve the dual effects of hepatic neovascularization and liver regeneration.
- Citation: Lan L, Liu R, Qin LY, Cheng P, Liu BW, Zhang BY, Ding SZ, Li XL. Transplantation of bone marrow-derived endothelial progenitor cells and hepatocyte stem cells from liver fibrosis rats ameliorates liver fibrosis. World J Gastroenterol 2018; 24(2): 237-247
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.237
Liver fibrosis is the scarring process involved in the response of liver to injury. Most hepatocellular carcinoma, which is among the leading causes of cancer death worldwide, develops in the context of severe liver fibrosis and cirrhosis. Liver fibrosis is a necessary stage for cirrhosis and liver cancer. Bone marrow stem cells (BMSCs) have been recently used to treat liver fibrosis; whereas, BMSCs transplantation is not an effective treatment for liver fibrosis, due to the histopathological characteristics of liver fibrosis including deposition of excessive extracellular matrix, remodeling of abnormal vascular and sinusoid constriction[1-6]. Low levels of engraftment and proliferation of exogenous cells in the liver parenchyma[4,7], inhibition of endogenous liver regeneration[4] and lack of a role in promoting hepatic neovascularization are the potential major drawbacks of BMSC transplantation therapy[8].
Endothelial progenitor cells (EPCs) have been shown to play a function in angiogenesis and vasculogenesis[9]. EPCs have been used for the treatment of liver fibrosis/cirrhosis in rats[10-15]. Previous studies demonstrated that transplantation of bone marrow-derived EPCs (BM-EPCs) have a potential role in hepatic neovascularization, endothelial/sinusoidal remodeling and increased hepatic blood flow[10,13]. However, BM-EPCs were all derived from healthy rats in these studies. In the present study, we sought to evaluate the feasibility of clinical application of autogenous EPCs therapy by using normal BM-EPCs obtained from liver fibrosis rats.
Furthermore, loss and destruction of functional hepatocytes is another feature of liver fibrosis[16]. BMSCs have been shown to restore and regenerate hepatic tissue[17]. Our previous study demonstrated that β2m-/Thy-1+ bone marrow-derived hepatocyte stem cells (BDHSCs) derived from the pathoenvironment of liver injury in rats, which could be hepatocyte progenitors[18], promoted hepatocyte proliferation and liver regeneration in liver fibrosis rats[19]. Thus, a combined transplantation of BM-EPCs and BDHSCs may be more beneficial to liver fibrosis than transplantation of BM-EPCs or BDHSCs alone.
In the present study, we successfully obtained normal EPCs in vitro from bone marrow in liver fibrosis rats and evaluated the effectiveness of combined transplantation of BM-EPCs and BDHSCs in vivo for the treatment of liver fibrosis.
Wistar rats (male, 8 wk, 250-300 g) (Animal Experiment Center of Henan Province, China) were housed in a standard animal laboratory. Animal studies were approved by the Animal Ethics Committee of Zhengzhou University and were in compliance with the Chinese National Regulations on the Use of Experimental Animals.
The isolation and culture of BM-EPCs and BDHSCs were performed as described by Smadja et al[20] and Schatteman et al[21]. Wistar rats were subcutaneously injected with a 2:3 solution of carbon tetrachloride (CCl4) and olive oil at a dose of 3 mL/kg body weight (double doses for the first time) twice per week for 6 wk to induce liver fibrosis. Bone marrow cells of rats with liver fibrosis were obtained by flushing femurs and humerus with DMEM/F12 medium (Gibco, New York, NY, United States). Bone marrow mononuclear cells (BMMCs) were then isolated by density gradient centrifugation with Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, United States) from bone marrow cells. After washing with red blood cell lysis buffer, BMMCs were seeded into culture flasks in DMEM/F12 medium supplemented with 10% fetal bovine serum (Gibco). After 24 h, the plastic-adherent cells were removed, and the nonadherent cells were collected, washed and replated into fibronectin-coated (10 μg/mL; BD Biosciences, San Jose, CA, United States) culture flasks with the inducing medium containing DMEM/F12 medium supplemented with 10% fetal bovine serum, 20 ng/mL vascular endothelial growth factor (VEGF), 5 ng/mL basic fibroblast growth factor, 5 ng/mL epidermal growth factor (EGF) and 10 ng/mL insulin-like growth factor-1 (Peprotech, New Jersey, NJ, United States) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). β2m-/Thy-1+ BDHSCs of rats with liver fibrosis were selected and purified by magnetic bead cell sorting as previously reported[18-22].
After 10 d of culture under inducing conditions, 2 × 106 BM-EPCs were incubated with the FcR blocking reagent (Miltenyi Biotec Inc., Auburn, CA, United States) and fluorescein isothiocyanate (FITC)-conjugated rabbit anti-rat CD133 antibody (BD Biosciences) and rabbit anti-rat vascular endothelial growth factor receptor 2 (VEGFR2) antibody (BD Biosciences) for 30 min at 4 °C, respectively. Then, cells were incubated with phycoerythrin (PE)-conjugated goat anti-rabbit secondary antibody (BD Biosciences) for 30 min at 4 °C. The phenotypic expression of BM-EPCs was analyzed by flow cytometry (FACS Scan flow cytometer; BD). BMMCs (2 × 106) cultured without induction for 10 d were used as a control.
BM-EPCs induced for 10 d were incubated with the inducing medium with 1,1’-dioctadecyl-3,3,3’,3’-tetramethyl indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (DiI-acLDL) (Molecular Probes, Eugene, OR, United States) for 4 h at 37 °C in the dark. The cells were fixed with 4% paraformaldehyde for 20 min and incubated with the inducing medium with FITC-conjugated ulex europaeus agglutinin I (FITC-UEA-I) (Sigma-Aldrich) for 1 h at 37 °C in the dark. The cells were photographed under a fluorescence microscope.
For the analysis of capillary tube formation, Matrigel (BD Biosciences, Heidelberg, Germany) was added to the wells of a pre-cooling 24-well plate at 0 °C, and allowed to solidify at 37 °C for 40 min. After 10 d of culture induction, BM-EPCs with the inducing medium were added into the wells (8 × 105 cells/well). Capillary tube formation on Matrigel was observed under an optical microscope after 24 h of incubation.
BM-EPCs induced for 10 d were seeded in a 96-well plate and divided into 3 groups: (1) the blank control group, containing only culture medium; (2) the experimental group, containing BM-EPCs from rats with liver fibrosis; and (3) the control group, containing BM-EPCs of normal rats. The cells were cultured in the incubator with 37 °C, 5% CO2. 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-sulfophenyl)-2H-tetrazole monosodium salt (WST-8) (Neuron Science and Technology Development Co., Ltd., Beijing, China) was added to the plate (10 μL/well, once/24 h) for 10 d. The values of optical density (OD) at 490 nm were detected by a microplate reader and the cytokinetics of BM-EPCs were analyzed.
BM-EPCs were labeled with PKH26 (Sigma-Aldrich), according to the manufacturer’s instructions. PKH26-labeled BM-EPCs were transfused into rats via the tail vein after the rats were treated with CCl4 for 3 and 5 wk. After 6 wk of modeling, the liver tissues were excised, and frozen sections were prepared. PKH26-positive cells were examined by using a fluorescence microscope. The liver sections of CCl4-treated rats transfused with PBS served as a negative control.
Liver fibrosis rat models were induced with CCl4 as described above. The same volume of olive oil was used as a control. Forty rats were randomly divided into five groups (8 rats/group) as follows: (1) the negative group, rats treated with olive oil were transfused with PBS after 3, 4 and 5 wk of modeling; (2) the positive control group, rats treated with CCl4 were transfused with PBS after 3, 4 and 5 wk; (3) the BDHSCs group, rats treated with CCl4 were transfused with PBS containing BDHSCs after 3, 4 and 5 wk of CCl4 induction; (4) the BM-EPCs group, rats treated with CCl4 were transfused with PBS containing BM-EPCs after 3, 4 and 5 wk; and (5) the BM-EPCs/BDHSCs group, rats treated with CCl4 were transfused with PBS containing BDHSCs after 4 wk of induction, and BM-EPCs after 3 and 5 wk. The transfusion after 4 wk of induction was 2 × 105 cells via the branch of portal vein, and transfusion after 3 or 5 wk was 2 × 106 cells via the tail vein. Rats were sacrificed after 6 wk of CCl4 induction. The blood samples were collected from celiac artery for immediate biochemical detection. The liver tissues were stored at -80 °C or fixed in 10% formaldehyde for future analysis.
The liver tissues fixed with formaldehyde were embedded in paraffin, and then stained with Masson’s trichrome collagen staining. The stages of liver fibrosis were graded by the semiquantitative staging scores as follows: 0, no collagen fibers; 1, slight fibrosis, collagen fibers located in the central liver lobule; 2, moderate fibrosis, widened central collagen fibers; 3, severe fibrosis, collagen fibers extended to the edge of liver lobule; 4, liver cirrhosis, pseudolobuli formation[23].
The liver tissue paraffin-embedded sections were blocked with 0.3% H2O2 in methanol for endogenous peroxidase activity, incubated with mouse anti-rat VEGF or PCNA monoclonal antibody (Santa Cruz Biotechnology, Dallas, TX, United States) and peroxidase-conjugated rabbit anti-mouse IgG (Santa Cruz Biotechnology). The sections were stained with diaminobenzidine and counterstained with hematoxylin. Quantification of PCNA expression was carried out by measuring the integrated optical density (IOD) of positive staining area using the ImagePro plus image analysis software. Three random areas were selected per slide from two slides per sample.
The wet liver tissues (100 mg per sample) were homogenized in 1 mL PBS in the presence of 1% protease inhibitors (Sigma-Aldrich). The supernatant fractions of liver homogenates were used to measure the VEGF levels according to the manufacturer’s instructions using Rat VEGF Quantikine Enzyme-linked immunosorbent assay (ELISA) Kits (R&D Systems, Minneapolis, MN, United States).
The extracted total RNA of liver tissues was reverse transcribed into cDNA using ExScriptTM RT reagent Kit (Takara, Kusatsu, Japan). Real-time quantitative polymerase chain reaction (PCR) was performed using SYBR green II (Takara) on a Light Cycler (Roche Diagnostics GmbH, Penzberg, Germany). The forward and reverse primers were as follows: HGF (NM_017017, 122 bp), 5′-TTTCCCGTTGTGAAGGAGAT-3′ and 5′-CCCTACTGTTGTTTGTGTTGGA-3′; GAPDH (NM_017008, 92 bp), 5′-GACATGCCGCCTGGAGAAAC-3′ and 5′-AGCCCAGGATGCCCTTTAGT-3′. Real-time PCR was performed at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 60 s. To ensure specific amplification, a melting curve was generated at the end of the PCR for each sample. Hepatocyte growth factor (HGF) mRNA quantities were determined by comparative CT method by the Light Cycler software.
The biochemical indices of liver function, including alanine aminotransferase aspartate (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) in serum, and prothrombin time (PT) and activated partial thromboplastin time (APTT) in plasma, were detected by using an automated analyzer (LX20; Beckman Coulter, Brea, CA, United States).
All data were presented as means ± SD and analyzed using SPSS 17.0 statistical software. The differences between the mean values of each group were compared by the one-way analysis of variance (ANOVA) or Kruskal-Wallis test as appropriate, and considered to be statistically significant when the adjusted P values were < 0.05 (two-tailed).
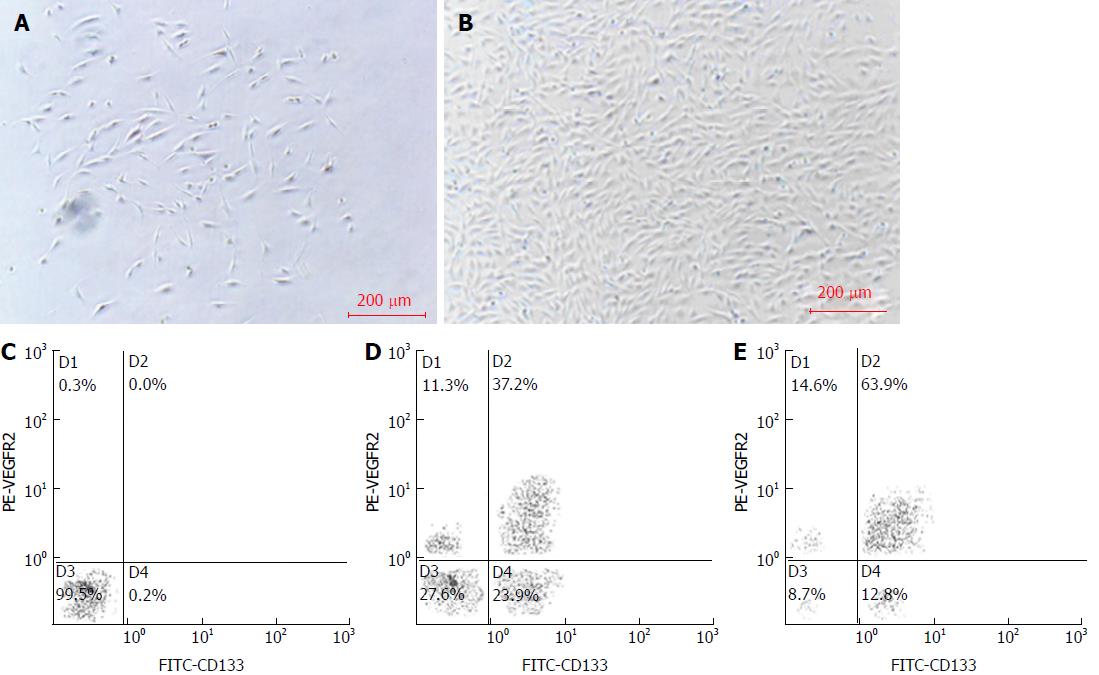
After 4 d of culture inductions, BM-EPCs were adherent (Figure 1A). After 10 d, BM-EPCs formed colonies (Figure 1B), and expression of CD133 and VEGFR2 were detected (Figure 1E). The co-expression level was 63.9% ± 2.15%, which was significantly higher than that of cells cultured without induction (37.2% ± 1.35%, P < 0.05) (Figure 1D).
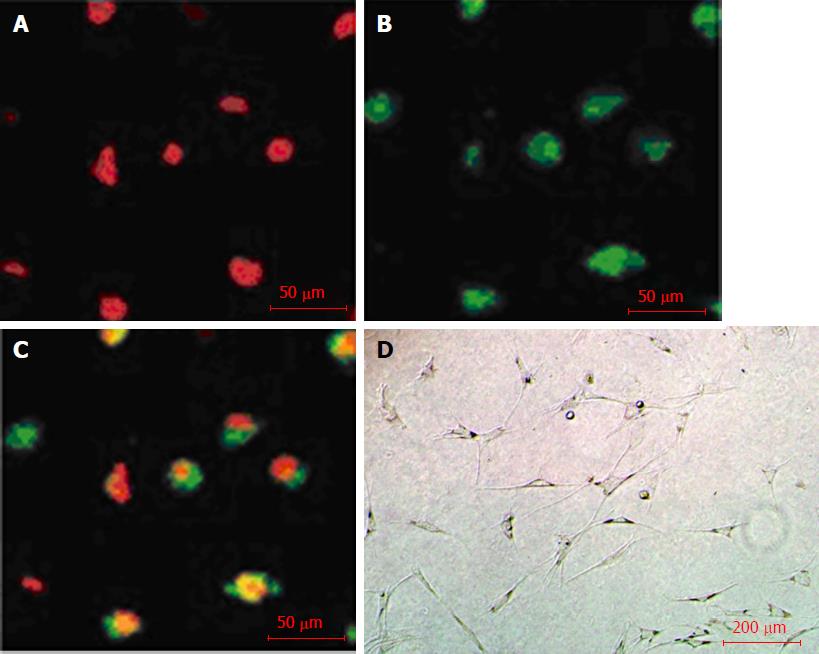
After 10 d of culture induction, uptake of Dil-ac-LDL (red in Figure 2A) and binding with FTIC-UEA-1 (green in Figure 2B) to BM-EPCs were observed. The cells with yellow double-fluorescence were differentiating EPCs (Figure 2C). Furthermore, BM-EPCs formed a stable vascular network-like structure on Matrigel (Figure 2D).
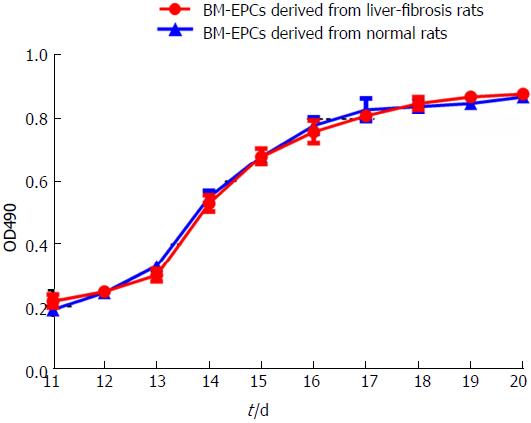
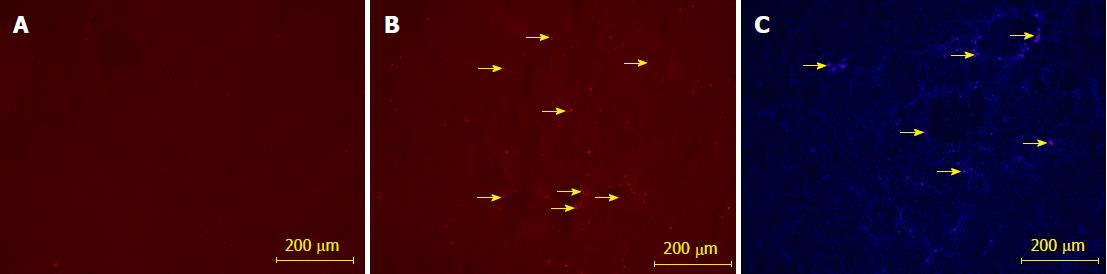
The growth curve of BM-EPCs from rats with liver fibrosis was similar to that of BM-EPCs of normal rats from day 11 to day 20 post induction. The logarithmic growth phase of cells was 3-7 d. The cells grew slowly and plateaued at day 18 (Figure 3). We implanted BM-EPCs in rats with fibrotic liver. We did not detect significant red fluorescence in the fibrotic liver tissue without implantation. BM-EPCs labeled with PKH26 were implanted in the fibrotic liver, and BM-EPCs were located primarily in/near hepatic sinusoids as indicated by nuclear staining with DAPI (data not shown).
BM-EPCs labeled with PKH26 implanted in the fibrotic liver (red in Figure 4B). The DAPI nuclear staining result showed that BM-EPCs were located primarily in/near hepatic sinusoids (Figure 4C).
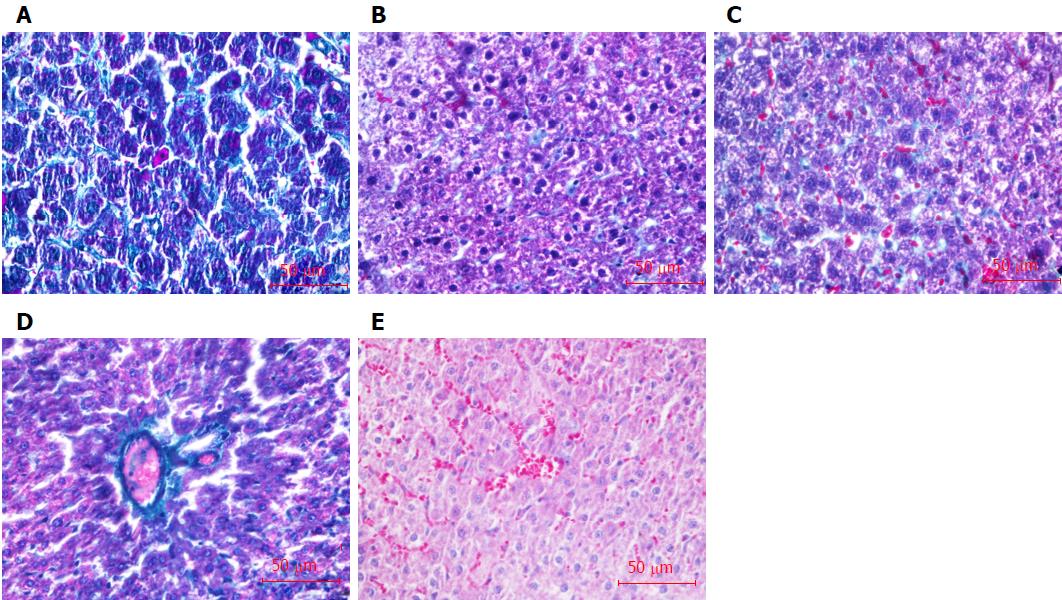
Masson staining results showed there were significant numbers of collagen fibers (green) formed in the liver tissue of liver fibrosis rats in comparison to that in normal liver tissue (Figure 5A and B), and the staging score of liver fibrosis was 3.00 ± 0.19, which was dramatically higher than that in normal rats (0, P < 0.05). Transplantations of BDHSCs or BM-EPCs alone and combination of both suppressed the formation of collagen fibers (Figure 5C-E). There was no significant difference between the staging scores of liver fibrosis in the BDHSCs (2.88 ± 0.23) or BM-EPCs (2.75 ± 0.16) groups and that of the model group with only CCl4 treatment (P > 0.05). However, transplantation of BM-EPCs/BDHSCs combination, significantly reduced the degree of liver fibrosis (staging score of 1.75 ± 0.25, P < 0.05) (Table 1).
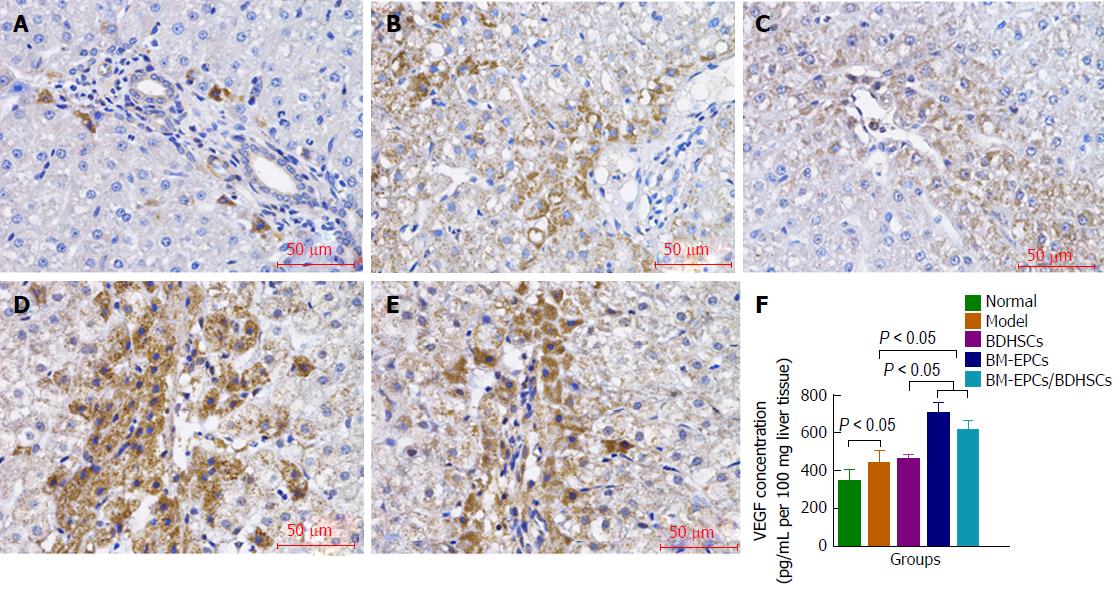
Immunohistochemistry (Figure 6A-E) and ELISA (Figure 6F) results showed that VEGF levels in the liver tissues in the model group with CCl4 treatment alone were higher than that in the normal group (440.95 pg/mL ± 65.11 pg/mL vs 349.70 pg/mL ± 56.50 pg/mL per 100 mg liver tissue, P < 0.05). There were no significant differences between those in the model group and the BDHSCs group (461.28 ± 23.78, P > 0.05). The VEGF levels were increased in the BM-EPCs (707.10 ± 54.32) and BM-EPCs/BDHSCs group (615.42 ± 42.96), compared with those in the model group and BDHSCs group (P < 0.05). BM-EPCs transplantation slightly increased VEGF level but not significantly, as compared with that of combined transplantation of BM-EPCs and BDHSCs (P > 0.05).
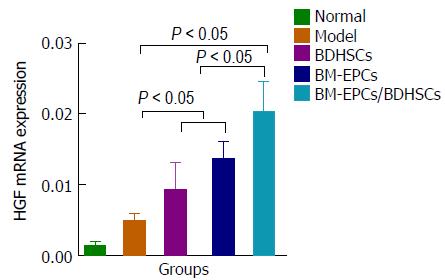
We evaluated liver regeneration after transplantation by detecting the expressions of PCNA protein and HGF gene in the liver. We found that compared with those in the normal group, the expressions of PCNA protein (Figure 7A-F) and HGF mRNA (Figure 8) in the model group was slightly but not significantly increased (P > 0.05). Significant increases in the levels of PCNA protein and HGF mRNA were observed in the BDHSCs, BM-EPCs and BM-EPCs/BDHSCs compared with those in the model group (P < 0.05), and combination of BM-EPCs/BDHSCs transplantation induced maximal up-regulation of PCNA protein and HGF mRNA levels.
As shown in Table 2, the levels of ALT, AST, TBIT, PT and APTT were increased in the model group, compared with those in the normal group (P < 0.05). Compared with the model group, transplantation of BDHSCs reduced the levels of ALT, TBIL and APTT (P < 0.05), but failed to improve the levels of AST and PT (P > 0.05). Furthermore, levels of ALT, AST and APTT in the group with BM-EPCs transplantation were lower than that in the model group (P < 0.05), but there was no significant difference in levels of TBIL and PT between the two groups. The levels of ALT, AST, TBIT, PT and APTT in the BM-EPCs/BDHSCs group were significantly improved, to be equivalent to normal levels (P > 0.05) compared with those in the BDHSC (AST, TBIL and PT, P < 0.05) and BM-EPCs (TBIL and PT, P < 0.05) groups (Table 2).
| Group | n | ALT, IU/L | AST, IU/L | TBIL, μmol/L | PT, s | APTT, s |
| Normal | 8 | 62.5 ± 12.7 | 153.5 ± 13.6 | 6.6 ± 0.3 | 16.6 ± 1.0 | 21.9 ± 1.5 |
| Model | 8 | 144.8 ± 61.5a | 269.6 ± 83.3a | 17.3 ± 2.6a | 20.5 ± 1.1a | 26.2 ± 2.8a |
| BDHSCs | 8 | 70.4 ± 19.5c | 260.4 ± 84.2a | 13.5 ± 1.8ac | 19.5 ± 1.3a | 20.1 ± 2.7c |
| BM-EPCs | 8 | 65.8 ± 26.5c | 159.3 ± 41.3c | 14.3 ± 2.3ag | 19.4 ± 1.4a | 19.9 ± 2.2c |
| BM-EPCs/BDHSCs | 8 | 61.6 ± 13.3c | 135.0 ± 33.3ce | 6.5 ± 1.1ceg | 16.6 ± 1.0ceg | 19.8 ± 2.3c |
Hepatic neovascularization and endothelial/sinusoidal remodeling are critical for the treatment of liver fibrosis, in addition to liver regeneration[24,25], and EPCs have been shown to play this role in liver fibrosis[10,13]. The majority of EPCs exist in bone marrow and peripheral blood. EPCs are rare in blood, since their proportion is only 0.005%-0.01% of that of white blood cells in blood[26]. Bone marrow contains numerous hematopoietic stem cells and mesenchymal stem cells, which can differentiate into EPCs[27]. Therefore, bone marrow may be the major source of EPCs in potential clinical treatment of liver fibrosis. BM-EPCs used in recent therapeutic studies were derived from healthy individuals[11-15]. Thus, the clinical application of EPCs transplantation is limited, due to rare EPCs in peripheral blood and shortage of normal allogeneic bone marrow.
Whether sufficient normal EPCs can be obtained from bone marrow cells in liver fibrosis pathoenvironment is a critical issue for the clinical application of autogenous EPCs therapy. A recent study showed that the functional homeostasis of bone marrow-derived c-Kit+, Sca-1+ and Lin− (BM-KSL) cells, as a stem cell fraction of BM-EPCs, was disrupted in liver fibrosis, indicating a weakened regenerative capability and decreased differentiation potential in liver fibrosis mice[28]. It is predicted that disturbed EPC differentiation might give rise to inadequate production for angiogenesis and tissue regeneration[28].
Whether normal EPCs can be obtained by in vitro culture from bone marrow in liver fibrosis rats remains uncertain. In the present study, abundant EPCs that possess specific phenotypes (VEGFR2 and CD133) and functions (phagocytosis and vasculogenesis) as well as normal proliferation and differentiation abilities were successfully obtained from bone marrow in liver fibrosis rats by collection and culture induction of plastic-nonadherent cells in vitro. EPCs do not easily attach on the bottom of surface of a culture plate, and thus different-rate adherent culture is a way to obtain EPC-differentiated cells[27,28]. BM-KSL cells are only a small portion of plastic-nonadherent cells[29]. Therefore, only some of the BMSCs in the liver fibrosis pathoenvironment might be hypophrenic. These hypophrenic cells in BMSCs could be eliminated automatically in the process of different-speed adherent and culture induction in vitro.
In the present study, transplanted BM-EPCs were located primarily in/near hepatic sinusoids, suggesting in the in vivo pathoenvironment of liver fibrosis, exogenous BM-EPCs derived from liver fibrosis rats had the ability to reside at sites of vessels in the liver.
In order to achieve the dual effects of hepatic neovascularization and liver regeneration, we next compared the effects of transplantations of BM-EPCs, BDHSCs and the combination of both on liver fibrosis. The increased levels of VEGF were observed in both BM-EPCs and BM-EPCs/BDHSCs groups, but not in the BDHSCs group. It has been reported that EPCs release vasoactive substances that promote angiogenesis by autocrine and paracrine signaling, such as VEGF, EGF and platelet-derived growth factor[11,14]. Among them, VEGF plays an essential role in both the neovascularization and the enhancement of sinusoidal density in the liver[27]. Studies have shown that the release of VEGF is essential for permeabilization of liver sinusoidal endothelial cells and integration of transplanted cells in the liver parenchyma[30,31]. Conversely, this study has shown that the effect of BM-EPCs transplantation on promoting VEGF secretion was slightly stronger than that of combined transplantation of BM-EPCs and BDHSCs. This result suggested that the release of VEGF in the liver might be controlled mainly by EPCs, but not BDHSCs.
Furthermore, an enhancement of liver regeneration manifested by increased HGF levels was observed in the rats with combined transplantation of BM-EPCs and BDHSCs[32], and this effect was greater than that of transplantation of BDHSCs or BM-EPCs alone. We deduced that BDHSCs might promote self-proliferation and mobilize endogenous liver regeneration through secretion of growth factors such as HGF[33,34], and EPCs can restore the permeabilization of the endothelial barrier interposed between liver sinusoids and parenchyma by releasing vasoactive substances and vasodilator-related molecules[30,31].
The integral effect of BM-EPCs transplantation on suppression of hepatic fibrogenesis and improvement of liver function was similar to that of BDHSCs transplantation. However, the combined effect of transplantation of BM-EPCs and BDHSCs exceeded individual transplantations significantly.
In summary, our study demonstrated that it was feasible to obtain normal EPCs from bone marrow in liver fibrosis rats and that combined transplantation of BM-EPCs and BDHSCs are effective for treating liver fibrosis. These findings highlight the clinical value of EPCs autotransplantation and the potential application of a novel strategy of therapy for liver fibrosis that involves combined transplantation of BM-EPCs and BDHSCs. Further studies are need to be carried out to explore the reciprocal mechanism of BM-EPCs and BDHSCs in liver fibrosis. In addition, whether BM-EPCs might promote tumor angiogenesis and cause potential tumor growth[2] in the process of treating liver fibrosis should also be evaluated in further study.
Hepatic neovascularization and endothelial/sinusoidal remodeling are critical for the treatment of liver fibrosis, in addition to liver regeneration. Transplantation of bone marrow-derived endothelial progenitor cells (BM-EPCs) has potential roles in hepatic neovascularization, endothelial/sinusoidal remodeling and increased hepatic blood flow for the treatment of liver fibrosis. However, BM-EPCs were all derived from healthy rats in these studies. We sought to evaluate the feasibility of clinical application of autogenous EPCs therapy by using normal BM-EPCs obtained from liver fibrosis rats, and evaluated the effectiveness of combined transplantation of BM-EPCs and BDHSCs in vivo for the treatment of liver fibrosis.
The author observed the feasibility of obtaining normal BM-EPCs from the liver fibrosis environment in rats. We evaluated the effectiveness for treating liver fibrosis by combined transplantation of BM-EPCs and bone marrow-derived hepatocyte stem cells (BDHSCs) in order to achieve the dual effects of hepatic neovascularization and liver regeneration. These findings highlight the clinical value of EPCs autotransplantation, and potential application of a novel strategy of therapy for liver fibrosis that involves combined transplantation of BM-EPCs and BDHSCs.
Our research objectives were to explore the feasibility of obtaining normal BM-EPCs from the liver fibrosis environment and the effectiveness for treating liver fibrosis by combined transplantation of BM-EPCs and BDHSCs. The significance of realizing these objectives were to highlight the clinical value of EPCs autotransplantation and potential application of a novel strategy of therapy for liver fibrosis that involves combined transplantation of BM-EPCs and BDHSCs.
For the treatment of liver fibrosis, BDHSCs, which can promote hepatic cell regeneration, combined with BM-EPCs, which can promote hepatic revascularization, were transplanted into rats with liver fibrosis. The normal functional BM-EPCs and BDHSCs were both successfully obtained from liver fibrosis rats. Tracing analysis was conducted by labeling EPCs with PKH26 in vitro to show EPCs’ location in the liver. After transplantation of BM-EPCs, BDHSCs or both into liver fibrosis rats, the indicators of hepatic neovascularization, liver regeneration, collagen formation, liver fibrosis degree and liver function were observed and detected.
Normal functional BM-EPCs from liver fibrosis rats were successfully obtained. The co-expression level of CD133 and VEGFR2 was 63.9% ± 2.15%. Transplanted BM-EPCs were located primarily in/near hepatic sinusoids. The combined transplantation of BM-EPCs and BDHSCs promoted hepatic neovascularization, liver regeneration and liver function, and decreased collagen formation and liver fibrosis degree.
This study demonstrated that it was feasible to obtain normal BM-EPCs “filtered” by in vivo pathological environment from bone marrow of a liver fibrosis rat model. The combined transplantation of BM-EPCs and BDHSCs was performed in order to achieve the dual effects of hepatic neovascularization and liver regeneration for liver fibrosis. The combined transplantation exhibited maximal therapeutic effect compared to that of transplantation of BM-EPCs or BDHSCs alone. These findings highlight the clinical value of EPCs autotransplantation and the potential application of a novel strategy of therapy for liver fibrosis that involves combined transplantation of BM-EPCs and BDHSCs.
Our research is still at the stage of animal experiments. Further studies are needed to explore the reciprocal mechanism of BM-EPCs and BDHSCs in liver fibrosis. Clinical experiments should be carry out gradually. In addition, whether BM-EPCs might promote tumor angiogenesis and cause potential tumor growth in the process of treating liver fibrosis should also be evaluated in further study.
The authors are grateful to Dr. Zheng-Guo Liu (Department of Pathology, the People’s Hospital, Zhengzhou University, Zhengzhou, China) and Dr. Xiu-Hua Ren (Basic Medical College, Zhengzhou University, Zhengzhou, China) for their contributions in staining and examination of the liver tissue sections. The authors also appreciate Dr. Adam Clemens (Division of Biology and Biomedical Sciences, Washington University, St. Louis, MO, United States) for his critical reading of the manuscript.
Manuscript source: Unsolicited manuscript
P- Reviewer: Skrypnyk IN, Sugimura H S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Rautou PE. Endothelial progenitor cells in cirrhosis: the more, the merrier? J Hepatol. 2012;57:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Shackel N, Rockey D. In pursuit of the “Holy Grail”--stem cells, hepatic injury, fibrogenesis and repair. Hepatology. 2005;41:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45:144-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol. 2015;62:S157-S169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Ishikawa T, Terai S, Urata Y, Marumoto Y, Aoyama K, Sakaida I, Murata T, Nishina H, Shinoda K, Uchimura S. Fibroblast growth factor 2 facilitates the differentiation of transplanted bone marrow cells into hepatocytes. Cell Tissue Res. 2006;323:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Khoo CP, Pozzilli P, Alison MR. Endothelial progenitor cells and their potential therapeutic applications. Regen Med. 2008;3:863-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Sakamoto M, Nakamura T, Torimura T, Iwamoto H, Masuda H, Koga H, Abe M, Hashimoto O, Ueno T, Sata M. Transplantation of endothelial progenitor cells ameliorates vascular dysfunction and portal hypertension in carbon tetrachloride-induced rat liver cirrhotic model. J Gastroenterol Hepatol. 2013;28:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Nakamura T, Torimura T, Sakamoto M, Hashimoto O, Taniguchi E, Inoue K, Sakata R, Kumashiro R, Murohara T, Ueno T. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:91-107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Lian J, Lu Y, Xu P, Ai A, Zhou G, Liu W, Cao Y, Zhang WJ. Prevention of liver fibrosis by intrasplenic injection of high-density cultured bone marrow cells in a rat chronic liver injury model. PLoS One. 2014;9:e103603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Nakamura T, Torimura T, Iwamoto H, Masuda H, Naitou M, Koga H, Abe M, Hashimoto O, Tsutsumi V, Ueno T. Prevention of liver fibrosis and liver reconstitution of DMN-treated rat liver by transplanted EPCs. Eur J Clin Invest. 2012;42:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Taniguchi E, Kin M, Torimura T, Nakamura T, Kumemura H, Hanada S, Hisamoto T, Yoshida T, Kawaguchi T, Baba S. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology. 2006;130:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Liu F, Liu ZD, Wu N, Cong X, Fei R, Chen HS, Wei L. Transplanted endothelial progenitor cells ameliorate carbon tetrachloride-induced liver cirrhosis in rats. Liver Transpl. 2009;15:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2163] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 17. | García-Bravo M, Morán-Jiménez MJ, Quintana-Bustamante O, Méndez M, Gutiérrez-Vera I, Bueren J, Salido E, Segovia JC, Fontanellas A, de Salamanca RE. Bone marrow-derived cells promote liver regeneration in mice with erythropoietic protoporphyria. Transplantation. 2009;88:1332-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Yu L, Chen S, Luo N, He S. The C-terminus domain of the hepatitis B virus x protein stimulates the proliferation of mouse foetal hepatic progenitor cells, although it is not required for the formation of spheroids. Int J Mol Med. 2017;40:400-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lan L, Chen Y, Sun C, Sun Q, Hu J, Li D. Transplantation of bone marrow-derived hepatocyte stem cells transduced with adenovirus-mediated IL-10 gene reverses liver fibrosis in rats. Transpl Int. 2008;21:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Inderbitzin D, Avital I, Gloor B, Keogh A, Candinas D. Functional comparison of bone marrow-derived liver stem cells: selection strategy for cell-based therapy. J Gastrointest Surg. 2005;9:1340-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Wu XL, Zeng WZ, Wang PL, Lei CT, Jiang MD, Chen XB, Zhang Y, Xu H, Wang Z. Effect of compound rhodiola sachalinensis A Bor on CCl4-induced liver fibrosis in rats and its probable molecular mechanisms. World J Gastroenterol. 2003;9:1559-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Ueno T, Nakamura T, Torimura T, Sata M. Angiogenic cell therapy for hepatic fibrosis. Med Mol Morphol. 2006;39:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 25. | Casamassimi A, Balestrieri ML, Fiorito C, Schiano C, Maione C, Rossiello R, Grimaldi V, Del Giudice V, Balestrieri C, Farzati B. Comparison between total endothelial progenitor cell isolation versus enriched Cd133+ culture. J Biochem. 2007;141:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Shirakura K, Masuda H, Kwon SM, Obi S, Ito R, Shizuno T, Kurihara Y, Mine T, Asahara T. Impaired function of bone marrow-derived endothelial progenitor cells in murine liver fibrosis. Biosci Trends. 2011;5:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Smadja DM, Cornet A, Emmerich J, Aiach M, Gaussem P. Endothelial progenitor cells: characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007;23:223-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Bahde R, Kapoor S, Viswanathan P, Spiegel HU, Gupta S. Endothelin-1 receptor A blocker darusentan decreases hepatic changes and improves liver repopulation after cell transplantation in rats. Hepatology. 2014;59:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S. Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver. Hepatology. 2013;57:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Al-Rasheed NM, Attia HA, Mohamad RA, Al-Rasheed NM, Al Fayez M, Al-Amin MA. Date fruits inhibit hepatocyte apoptosis and modulate the expression of hepatocyte growth factor, cytochrome P450 2E1 and heme oxygenase-1 in carbon tetrachloride-induced liver fibrosis. Arch Physiol Biochem. 2017;123:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |