Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.2009
Peer-review started: March 10, 2018
First decision: April 11, 2018
Revised: April 20, 2018
Accepted: April 23, 2018
Article in press: April 23, 2018
Published online: May 14, 2018
Processing time: 63 Days and 1.9 Hours
To investigate viability assessment of segmental small bowel ischemia/reperfusion in a porcine model.
In 15 pigs, five or six 30-cm segments of jejunum were simultaneously made ischemic by clamping the mesenteric arteries and veins for 1 to 16 h. Reperfusion was initiated after different intervals of ischemia (1-8 h) and subsequently monitored for 5-15 h. The intestinal segments were regularly photographed and assessed visually and by palpation. Intraluminal lactate and glycerol concentrations were measured by microdialysis, and samples were collected for light microscopy and transmission electron microscopy. The histological changes were described and graded.
Using light microscopy, the jejunum was considered as viable until 6 h of ischemia, while with transmission electron microscopy the ischemic muscularis propria was considered viable until 5 h of ischemia. However, following ≥ 1 h of reperfusion, only segments that had been ischemic for ≤ 3 h appeared viable, suggesting a possible upper limit for viability in the porcine mesenteric occlusion model. Although intraluminal microdialysis allowed us to closely monitor the onset and duration of ischemia and the onset of reperfusion, we were unable to find sufficient level of association between tissue viability and metabolic markers to conclude that microdialysis is clinically relevant for viability assessment. Evaluation of color and motility appears to be poor indicators of intestinal viability.
Three hours of total ischemia of the small bowel followed by reperfusion appears to be the upper limit for viability in this porcine mesenteric ischemia model.
Core tip: Research on experimental methods to improve the surgeon’s assessment of viability of ischemic bowel with higher accuracy than currently possible, requires an accurate reference model. We investigated viability assessment in a porcine model of warm ischemia on jejunum with mesenteric occlusion, followed by reperfusion. Our aim was to determine the time point of irreversible damage, to provide a reference model. We created parallel segmental models on the jejunum in 15 pigs and compared the results from visual inspection with histology and microdialysis. Three hours of ischemia followed by reperfusion appeared to be the upper limit for viability in this model.
- Citation: Strand-Amundsen RJ, Reims HM, Reinholt FP, Ruud TE, Yang R, Høgetveit JO, Tønnessen TI. Ischemia/reperfusion injury in porcine intestine - Viability assessment. World J Gastroenterol 2018; 24(18): 2009-2023
- URL: https://www.wjgnet.com/1007-9327/full/v24/i18/2009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i18.2009
Evaluation of intestinal viability is essential in surgical decision-making in patients with acute intestinal ischemia[1-3], but can be challenging as the appearance of the ischemic or reperfused intestine can be deceptive[4]. The standard clinical method for intraoperative assessment of intestinal viability is evaluation of color, motility and bleeding of cut ends[3]. This method is not very specific and requires a high level of clinical experience[4,5].
There is a risk of short bowel syndrome if resection is performed too extensively, and on the other hand, a risk of peritonitis, sepsis and death if non-viable intestine is not removed[6]. The gold standard for determination of bowel viability is a second-look laparotomy (within 48 h) to reinspect areas of questionable viability[7]. Up to 57% of patients need further bowel resection at a later time, and this number includes patients undergoing second look surgery (40% of the patients)[8].
The intestinal wall consists of several tissue layers that have varying ability to tolerate ischemic insults. While the mucosa has a lower tolerance for ischemic damage than the muscularis propria, the mucosa has a very potent ability for rapid regeneration and repair[9]. When the muscularis propria and the muscularis mucosae are damaged, peristalsis and the movement of the villi will be lost. Regenerated scar tissue might not uphold sufficient peristalsis, and may lead to later stricture[2].
While intestinal ischemia may have a number of underlying causes, an early and essential element of the clinical treatment in nearly all cases is the restoration of perfusion[10]. However, it may cause both local and systemic responses, potentially creating damage far beyond the direct ischemic injury[11-13]. The extent of ischemia/reperfusion injury is variable and dependent on the underlying mechanisms, the duration of ischemia, the length of the affected segment and hypoxic tolerance of the tissue[10,14].
Experimental studies on intestinal viability have reported that the time before irreversible damage occurs varies between species, between anatomical locations (e.g. jejunum, ileum, or colon), and between the ischemia models used[15-17]. Rat intestine is reported to be irreversibly damaged after 45 min of ischemia[18], whereas in juvenile pig jejunum irreversible damage to mucosal regeneration has been reported after 6.5 h of ischemia[19]. To judge the accuracy of clinical and experimental methods in the assessment of intestinal viability, histological analysis and/or patient outcome approaches have been used as the standard for comparison[4].
There is presently no standard classification method for the histological assessment of ischemia/reperfusion damage in the gut[20] and several approaches have been proposed, focusing on different aspects of the damage process[21]. Many previous studies of intestinal viability have concentrated on mucosal injury[13,22-25]. A commonly used histological classification system for ischemic mucosal lesions is based on the grading system proposed by Chiu et al[22], including modifications proposed by Park et al[26] to include evaluation of damage in the deeper layers of the intestine. Swerdlow et al[21] proposed a classification system, suggesting that mixing etiologic and morphologic terms should be avoided. This classification system has later been modified[27,28].
Microdialysis has been suggested as a way to monitor bowel ischemia[29], and can be used to measure changes in local metabolic substrate concentrations related to ischemia/reperfusion injury[30-32]. The principle is to place a tubular microdialysis membrane in the tissue of interest, to pump a slow and steady flow of isotonic fluid through the inside of the membrane and on to a sampling vial. The tubular semi-permeable membrane will allow low molecular weight substances in the area surrounding the probe to diffuse through the porous membrane due to differences in concentration gradient[33]. When using intraluminal microdialysis in the small intestine, the substrates of interest are primarily lactate and glycerol. The anaerobic metabolism in the ischemic cells leads to an increase in lactate, and glycerol is released as cell membranes deteriorate. Ischemia/reperfusion experiments have shown, however, that intraluminal microdialysis measurements of glucose and pyruvate can be unreliable[34,35].
In this study, we compared the results from visual inspection, intraluminal microdialysis and histology (light and transmission electron microscopy) with the aim of assessing the viability of porcine jejunum following segmental mesenteric occlusion with warm ischemia and further reperfusion. We evaluated the injury occurring in all layers of the intestinal wall. The overall aim was to determine when irreversible damage occurs, and to establish a reference for use with experimental approaches of viability assessment on the porcine jejunum.
The animal protocol was designed to minimize pain or discomfort to the animals and reduce the overall number of animals used. The experiment was approved by the Norwegian Food Safety Authority (FOTS ID 8304 and 12695) and conducted in accordance with Norwegian animal welfare guidelines (FOR-2015-06-18-761) and EU directive (2010/63/EU). We conducted the study on 15 Norwegian Landrace pigs, with a weight range 44.3-58.6 kg, 11 were females. Food was withheld 12 h prior to surgery. We used a segmental mesenteric occlusion (SMO) model utilizing several small bowel segments in the same pig[12,19,36,37], selecting 30 cm segments of the jejunum, starting 30 cm distal from the duodenum. More than 30 cm free intervals were maintained between the segments. Local ischemia was induced by atraumatic clamping of the arteries and veins of the jejunal mesentery on the selected segments[17,19], resulting in a 20-cm central zone of warm ischemia and two surrounding approximately 5 cm edge zones of marginal tissue hypoxia[38]. Reperfusion was initiated by releasing the clamps and verified by observing the return of color in the previously ischemic segments. We conducted a series of ischemia/reperfusion intervals (ischemia 1-16 h, reperfusion for 5-15 h post 1-8 h of ischemia, control 1-16 h) in order determine the occurrence of irreversible injury. At the end of the experiment, the animals were sacrificed by a lethal dose of potassium chloride (100 mmol).
Anesthesia was induced with intramuscular ketamine (Warner Lambert, Morris Plains, NJ, United States) 15 mg/kg, azaperone (Janssen-Cilag Pharma, Austria) 1 mg/kg, and atropine (Nycomed Pharma, Asker, Norway) 0.02 mg/kg. Tracheotomy was performed, and anesthesia was maintained with isoflurane (Abbott Scandinavia AB, Kista, Sweden) (1%-1.5%) and a mixture of air and O2 to obtain an FIO2 of 30%. Morphine (Alpharma, Oslo, Norway) 0.4-0.7 mg/kg/h was administered as a continuous intravenous infusion. Ventilation was adjusted to a pCO2 of 5-6 kPa (37.5-45.0 mmHg). A continuous infusion of Ringer acetate 10-30 mL/kg/h was administered as fluid replacement.
Surgery was performed under sterile conditions. Tracheostomy was performed initially for mechanical ventilation. The left internal jugular vein was cannulated with a triple lumen catheter for blood sampling, measuring of central venous pressure and infusion of fluids. Arterial pressure was measured through a catheter placed in a carotid artery, the urinary bladder temperature was measured with a thermistor probe. Arterial and venous blood gases were regularly measured throughout the experimental period. Pulse oximetry, heart rate, respiratory rate and expiratory pCO2 were continuously monitored. The jejunum was made accessible through midline laparotomy. The mesentery of the selected jejunal segments were marked and clamped using Satinsky clamps[39].
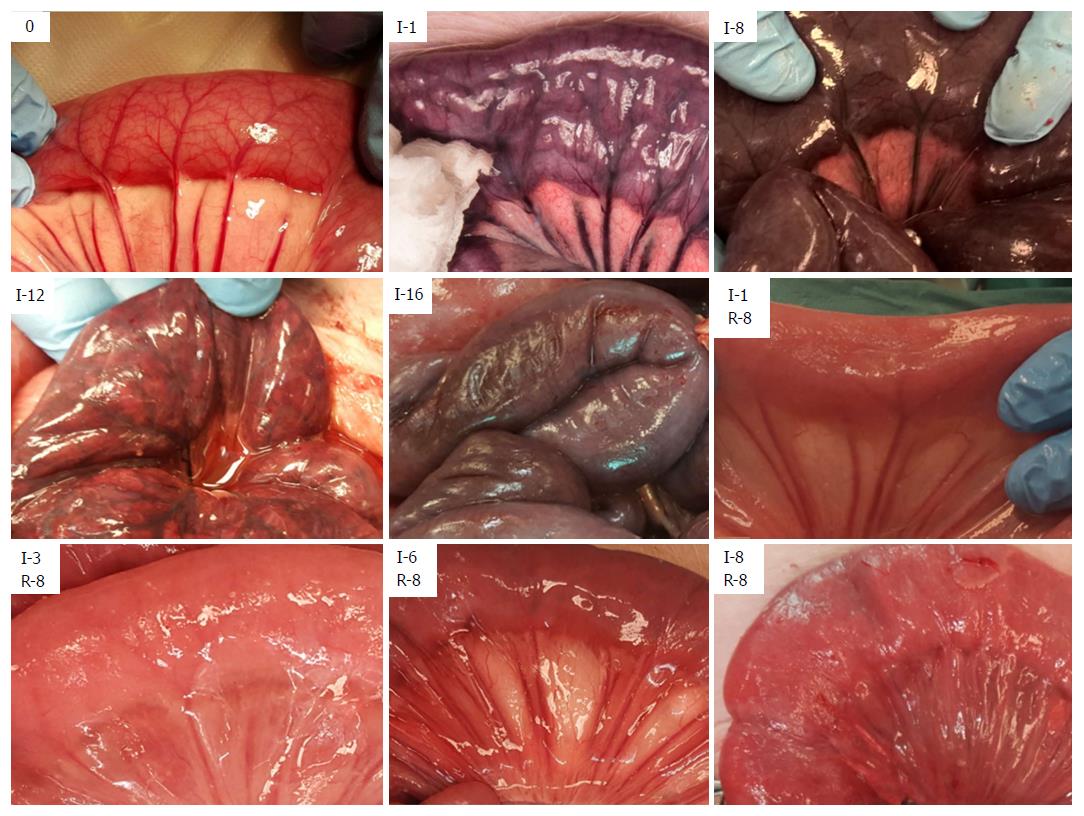
The presence of peristalsis in the bowel segments was monitored by visual observation and palpation, and registered hourly for the duration of the experiments. We photographed the intestinal segments hourly to monitor alterations in color.
CMA65 Custom made Microdialysis Catheter (65CMC) with 30 mm membrane length, 100 kDa cut-off (M Dialysis AB, Stockholm, Sweden) was perfused with 60 mg/mL Voluven (Fresenius Kabi Norge AS, Halden, Norway) for 30 min, before being inserted into the lumen of the selected jejunal segments, with a split-needle technique. The flow rate was adjusted to 1 μL/min using CMA 107 microdialysis pumps (CMA Microdialysis, Stockholm, Sweden). A baseline measurement was obtained (30 min) before the initiation of ischemia, and then for every hour during the experiment duration. An ISCUSflex Microdialysis Analyzer (M Dialysis AB, Stockholm, Sweden) was used to analyze the samples continuously after sampling, using Reagent set A (M Dialysis AB, Stockholm, Sweden). The reagent set was used to analyze glucose, lactate, pyruvate and glycerol. The ISCUSflex was set to normal linear range, 0.1-12 mmol/L (lactate) and 10-1500 μmol/L (glycerol). After a period of ischemia our results reached values above the linear range. Seven of the microdialysis catheters failed to operate normally and were excluded from the study.
We collected a total of 128 intestinal tissue samples from 5 pigs for light microscopy (LM) at selected time intervals from control jejunum, ischemic jejunum and reperfused jejunum. The biopsies were fixed overnight in buffered 10% formalin. The samples were then processed according to a routine protocol and embedded in paraffin wax, and 2-3 histological sections from each sample were stained with hematoxylin and eosin. The sections were reviewed with LM by two pathologists (HMR & FPR) and pathological changes in each layer of the intestine were assessed. The intestinal tissue damage was also classified using a system devised by Antonioli and Swerdlow, as modified by Hegde et al[27,28] and a modification of the grading system devised by Chiu[22], proposed by Park et al[26] (Table 1).
| Grade | Modified Swerdlow | Park/Chiu |
| 0 | No pathological change | Normal mucosa |
| 1 | Focal loss of surface epithelium | Subepithelial space at villus tips |
| 2 | Mucosal infarction (extensive loss of surface epithelium, loss of variable amounts of lamina propria, sparing of basal glands, intact muscularis mucosae) | Extension of subepithelial space with moderate lifting |
| 3 | Submucosal infarction (variable necrosis of submucosa, complete mucosal necrosis, intact muscularis mucosae) | Massive lifting down the sides of the villi, some denuded tips |
| 4 | Mural infarction (loss of muscularis mucosae, complete necrosis of mucosa and submucosa) | Denuded villi, dilated capillaries |
| 5 | Mural infarction (involvement of inner layer of muscularis propria, complete necrosis of mucosa and submucosa) | Disintegration of lamina propria |
| 6 | Transmural infarction (complete necrosis of the bowel wall) | Crypt layer injury |
| 7 | Transmucosal infarction | |
| 8 | Transmural infarction |
In addition, 58 samples at selected time intervals from 3 pigs were collected and fixed in a phosphate-buffered mixture of 2% glutaraldehyde and 0.5% paraformaldehyde overnight. From each sample, four specimens were subsequently embedded in an epoxy resin according to a standard protocol. Toluidine blue-stained semi-thin sections were used to select areas of interest and ultra-thin sectioning of one block. The ultra-thin sections were examined by transmission electron (TEM) microscopy by one pathologist (FPR). The focus was on cellular and subcellular changes as a basis of estimating tissue viability in the muscularis propria.
The microdialysis data was analyzed for distribution, skewness, kurtosis and homogeneity of variance to assess distribution. Continuous data were described with mean and SD and categorical data with counts and proportions. Comparisons of the intraluminal lactate and glycerol levels between the control and ischemia/reperfusion segments of jejunum were made using two-way repeated measures analysis of variance (RM ANOVA). For the ANOVA’s the responses of interest were lactate and glycerol level, and the factors used were “case” (control, ischemic, reperfusion) and time duration [h]. P-values were adjusted for multiple comparisons using Holm-Sidak’s correction. The ANOVA’s were run using GraphPad Prism version 7.00 (GraphPad Software, United States).
All the animals (n = 15) were hemodynamically stable during the experiments. 10-20 min after reperfusion was initiated in a segment of the jejunum after a period of ischemia, there was an increase in heart rate (+20 to 60 beats per minute) that lasted for 5 to 30 min (increasing with the late reperfusion intervals), and there was also an initial decrease in mean arterial blood pressure (5-25 Torr) lasting for 5-15 min, before returning to normal after increased fluid administration. SpO2 (measured at the pig tail) was above 98% in all animals during the entire experiment. Mean body temperature increased from 38.5 °C at the start of the experiments to 40.5 °C by the end of the experiment.
After initiating ischemia of a bowel segment, we observed a period of hyperperistalsis that lasted for approximately 30-40 min. Ischemia leads to a change in color of the involved tissue (Figure 1 and Table 2), and edema is the hallmark of reperfusion. Upon reperfusion, peristalsis was visible in all jejunal segments that had been ischemic for ≤ 5 h and most of the segments that had been ischemic for 6 h. We observed an initial hyperemia, and a return of color even in the jejunum that had been ischemic for 8 h. In the samples that had been ischemic for ≥ 2 h there was a gradual formation of a fibrinous exudate on the serosa after reperfusion. Following reperfusion, we observed the formation of small fluid droplets on the surface of the samples that had been ischemic for ≥ 3 h, which was associated with a gradual increase in peritoneal fluid. We observed a darker “internal hue” in the samples that were reperfused after ≥ 4 h of ischemia.
| Ischemia (h) | Observations on the ischemic jejunum | Minutes after reperfusion before color has returned (mean ± SD) | Observable peristalsis in No. of pigs | Reperfusion (h) | Observations on the reperfused jejunum | No. of pigs |
| 0 | Normal color | 15 | ||||
| 1 | Purple | 0.9 ± 0.1 | 15 of 15 | 8 | Edema | 15 |
| 2 | Darker purple | 2 ± 0.1 | 2 of 2 | 8 | Edema, slight fibrinous coating | 2 |
| 3 | Darker purple | 4 ± 0.3 | 13 of 13 | 8 | Edema, fluid droplets, slight fibrinous coating | 13 |
| 4 | Darker purple | 6 ± 0.7 | 4 of 4 | 8 | Edema, fluid droplets, fibrinous coating, darker internal hue | 4 |
| 5 | Darker purple | 15 ± 1.6 | 11 of 11 | 8 | Edema, fluid droplets, fibrinous coating, darker internal hue | 11 |
| 6 | Darker purple | 26 ± 3.3 | 3 of 4 | 8 | Edema, fluid droplets, fibrinous coating, deeper red color, darker internal hue | 4 |
| 8 | Black | 49 ± 91 | 0 of 4 | 8 | Edema, fluid droplets, fibrinous coating, deeper red color, darker internal hue | 4 |
| 12 | Patches of paler color | 4 | ||||
| 16 | Necrotic | 4 |
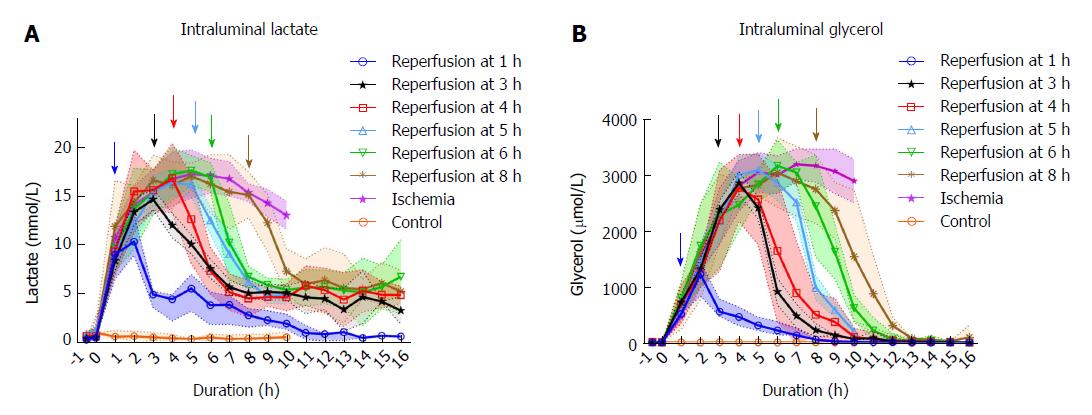
Levels of the intraluminal lactate increased significantly during the first hour of ischemia (P < 0.001) from mean (SD) 0.65 (0.28) to 8.54 (3.43) mmol/L, peaking around 4-5 h of ischemia compared to the control (Figure 2). Following reperfusion after 1 h of ischemia, the intraluminal lactate level showed little change during the first hour of reperfusion with 10.42 (1.97) mmol/L compared to 13.69 (2.33) mmol/L in the ischemic tissue. In the second hour of reperfusion the lactate levels decreased significantly to 4.64 (1.36) mmol/L compared to 15.43 (2.47) mmol/L in the ischemic tissue (P < 0.001). In the series with ischemia duration > 1 h, the lactate levels decreased over the first hour following reperfusion. In the tissue that was ischemic for the whole duration of the experiment there was a gradual decrease in lactate level from mean (SD) 17.22 (3.48) mmol/L at 6 h of ischemic duration to 12.96 (2.01) mmol/L by the end of the experiment. Only in the jejunum that was reperfused after 1 hour of ischemia, did the lactate values approach pre-ischemic levels during the experiment. There was no significant change in arterial lactate throughout the experiment (data not shown).
The intraluminal glycerol level increased significantly from mean (SD) 5.7 (2.0) to 554.1 (215) μmmol/L during the first hour after the initiation of ischemia (P < 0.001), peaking around 6-8 h of ischemia compared to the control (Figure 2). In the segments that were reperfused after 1-3 h of ischemia, the glycerol levels continued to increase during the first hour of reperfusion, while decreasing during the first hour of reperfusion following longer ischemia intervals. The glycerol levels in the lumen of the reperfused intestinal segments approached the control level after 6 to 7 h of reperfusion, regardless of previous ischemic exposure. In the tissue that was ischemic for the duration of the experiment, there was a gradual decrease in glycerol level from mean (SD) 3180.4 (382.8) μmmol/L at 7 h of ischemia to 2780.2 (471.0) μmmol/L by the end of the experiment.
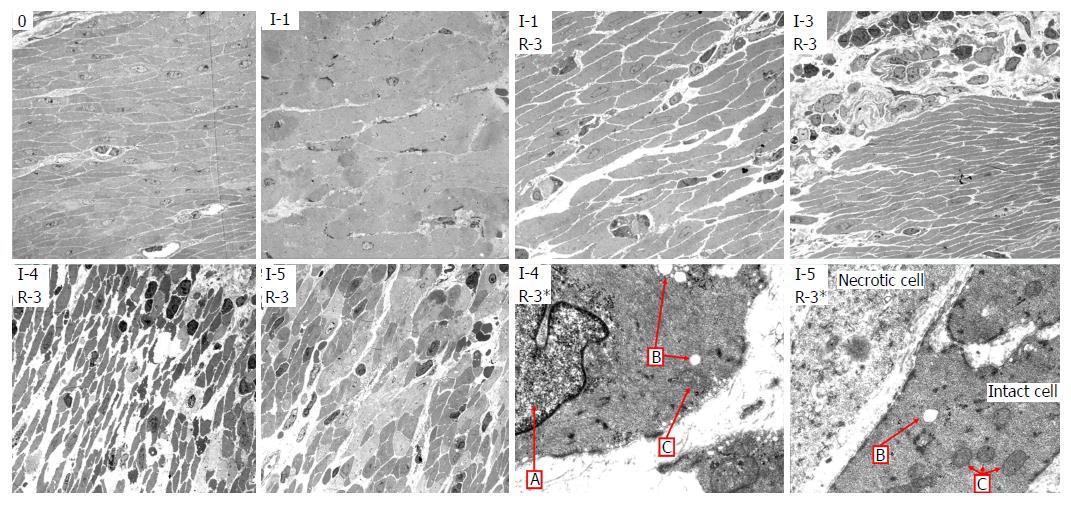
Light microscopy: LM of cross-sections of jejunum showed gradually increasing signs of injury in the ischemic tissue with time, and more pronounced injury following reperfusion. There was some variation in the pattern and extent of pathological changes between different samples from the same time point, and between different areas within the same samples, but the lesions were reproducible and the predominant findings at each time point are shown in Table 3. Based on the observations of total loss of crypt epithelium and pronounced smooth muscle cell shrinkage in the muscle layers, the samples from tissue exposed only to ischemia were considered irreversibly damaged by ischemia at 6 h exposure.
| Ischemia | 1 h isc, 8 h rep | 2 h isc, 8 h rep | 3 h isc, 8 h rep | 4 h isc, 8 h rep | 6 h isc, 8 h rep | 8 h isc, 8 h rep | Control |
| I-1: Early loss of SE1 | I-1: Early loss of SE1 | I-2: Total loss of SE2 | I-3: Early loss of CE, congestion and bleeding LP2 | I-4: Total loss of SE, focal damage to outer layer of MP2 | I-6: Total loss of CE, damage to LP, MM. Bacteria in LP2 | I-8: Damage to all components3 | N-0: Normal1 |
| I-2: Total loss of SE2 | I-1/R-1: Total loss of SE, apoptosis in CE, light N2 | I-2/R-1: Apoptosis in CE, light N, congestion and focal bleeding in LP2 | I-3/R-1: Apoptosis in CE, N, wavy myocytes in MP2 | I-4/R-1: Focal damage to both layers of MP (most to outer layer)2 | I-6/R-1: Damage to all components3 | I-8/R-1: Damage to all components3 | N-6: Few instances of apoptosis in CE, light N and light edema in MP1 |
| I-3: Early loss of CE2 | I-1/R-3: Focal damage to outer layer of MP2 | I-2/R-3: Early regeneration of SE, congestion, bleeding and necrosis in LP, apoptosis in CE, interstitial inflammation in MP2 | I-3/R-3: Edema, inflammation, and focal necrosis in outer layer of MP2 | I-4/R-3: Total loss of CE, NGR, cell disintegration in MM and MP3 | I-6/R-3: Damage to all components3 | I-8/R-3: Damage to all components3 | N-12: Few instances of apoptosis in CE, light N and light edema in MP1 |
| I-4: Focal damage to outer layer of MP2 | I-1/R-6: SE regenerated. Focal damage to outer layer of MP1 | I-2/R-6: Regeneration of SE, wavy myocytes and focal necrosis in MP2 | I-3/R-6: Most of CE is lost, wavy myocytes and focal necrosis in MP2 | I-4/R-6: Total loss of CE, NGR, loss of myocytes, disintegration3 | I-6/R-6: Damage to all components3 | I-8/R-6: Damage to all components3 | |
| I-5: Damage to inner layer of MP2 | I-1/R-8: SE regenerated. Focal damage to outer layer of MP1 | I-2/R-8: Regeneration of SE with focal loss and erosion, focal damage to the MP with wavy myocytes and necrosis2 | I-3/R-8: Most of CE is lost, wavy myocytes and focal necrosis in both layers of MP2 | I-4/R-8: Damaged SE, CE, MM, submucosa, MP, PM3 | I-6/R-8: Damage to all components3 | I-8/R-8: Damage to all components3 | |
| I-6: Total loss of CE, damage to LP, MM and bacteria in LP3 | |||||||
| I-7: Hemorrhage in subserosa, peritonitis, and damage to all components3 | |||||||
| I ≥ 8: Damage to all components3 |
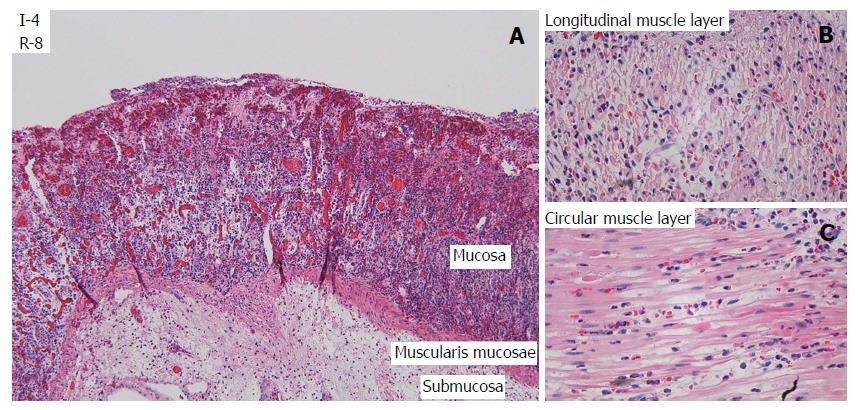
After one hour of ischemia and 8 h of reperfusion, there was increased apoptosis in the crypt epithelium, mild inflammation with neutrophils mainly in capillaries in all layers of the intestine, edema in the subserosa and submucosa and signs of focal injury to the outer layer of the muscularis propria. After 3 h of ischemia and 8 h of reperfusion there was focal damage to all layers. After 4 h of ischemia and 8 h of reperfusion there was a total loss of crypt epithelium, extensive shrinkage and loss of myocytes in the outer layer of the muscularis propria, suggesting likely irreversible damage (Figure 3). In intestine subjected to 6-8 h of ischemia followed by 1 h of reperfusion, there were signs of damage to all components of the intestinal wall.
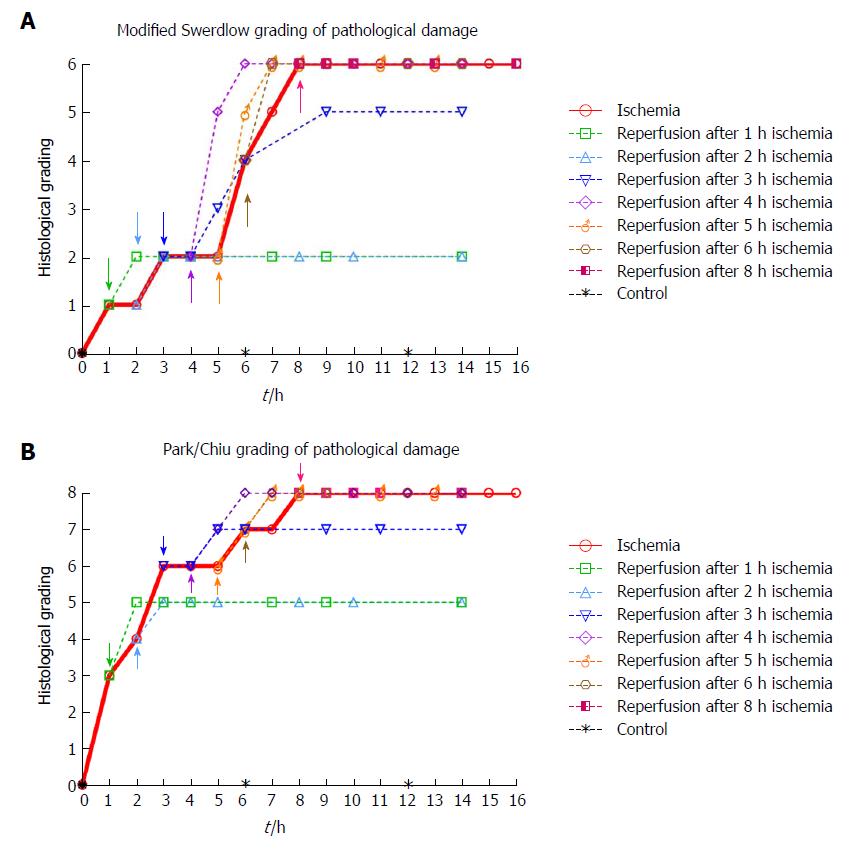
Histological damage according to grading systems: The predominant findings at each time point are shown in Figure 4. The highest score in both grading systems was reached after 8 h of ischemia. The reperfused tissue received a full score for intervals ≥ 4 h of ischemia followed by 2 h of reperfusion. The observed sequence of ischemia/reperfusion injury did not necessarily follow the outwards direction from the mucosa to the outer muscular layer, as the grading systems suggest (compare Tables 3 and 4 with Table 1 and Figure 4).
| Ischemia (h) | Observations | Ischemia/reperfusion (h/h) | Observations |
| 0 | Intact musculature. Some variation in the electron density in the muscle cells, focal swollen mitochondria’s with vacuolized matrixes1 | ||
| 1 | Intact musculature. Discrete intercellular edema. Lymphocytes in the interstitial space. Increased variation in the electron density in the muscle cells. Some cells have increased electron density (darker). Some of the mitochondria are more prominent. Some minimal fat vacuoles are visible2 | 1-3 | Inflammation, cell death, sparse fine-vacuolization of the sarcoplasm, slightly swollen mitochondria2 |
| 2 | More prominent variation in electron density between muscle cells. Increased number of vacuoles, some of them are fat vacuoles. Focal edema, thickening of the mitochondrial cristae. Some lysosomes with membrane fragments2 | 2-3 | Inflammation, cell death, more comprehensive fine-vacuolization of the sarcoplasm, slightly swollen mitochondria2 |
| 3 | Same results as at 2 h, but a few more interstitial immune response cells are visible. Monocytes, macrophages, and a few granulocytes. Vacuoles in the sarcoplasm. Slightly swollen mitochondria2 | 3-3 | Inflammation, cell death, more comprehensive fine-vacuolization of the sarcoplasm, slightly swollen mitochondria, focal single cell necrosis, swollen cell nuclei2 |
| 4 | Same changes as at 3 h, but the changes are more prominent as the cells with higher electron density are more condensed, and there are more vacuoles around the mitochondria2 | 4-3 | Pronounced cell shrinking/cell death, swollen cell nuclei, loss of cohesion, interstitial edema3 |
| 5 | Focal edema, variations in electron density, thickening of the mitochondrial cristae, vacuoles in the sarcoplasm, swollen mitochondria, interstitial lymphocytes/monocytes/granulocytes, loss of plasma-membrane and coherence, focal single cell necrosis3 | 5-3 | Increased cell shrinking/cell death, swollen cell nuclei, loss of cohesion, interstitial edema3 |
| 6 | Necrosis, focal large vacuoles in some mitochondria3 | 6-3 | Increased cell shrinking/cell death, swollen cell nuclei, loss of cohesion, interstitial edema3 |
| 7 | Necrosis with macrophages. Non-necrotic cells appear like the cells at time intervals 3-6 h3 | ||
| 8 | Like the results at 7 h3 |
Transmission electron microscopy: Using TEM on the muscularis propria and serosa we observed a gradual increase in damage to the cell structures during ischemia (Table 4, left columns), with probably irreversible damage in the muscularis propria after 5 h of ischemia. Interestingly, even at 7 to 8 h of ischemia, focal areas of muscle cells still appeared viable, illustrating heterogeneity in the development of ischemic damage to the muscularis propria.
There was reperfusion induced inflammation and cell death of varying degrees in all the tissue that had been subjected to ischemia. After 3 h of ischemia and 3 h of reperfusion (Table 4, left), there was inflammation, cell death, slightly swollen mitochondria, and swollen cell nuclei, and the muscle tissue appeared to be approaching irreversible damage. After 4 h of ischemia and 3 h of reperfusion (Table 4, right), there was pronounced cell shrinking/death, swollen cell nuclei, loss of cohesion, substantial interstitial edema and the muscle tissue no longer appeared viable. Figure 5 shows TEM images with typical observations described in Table 4.
The viability of ischemic small bowel is determined in a clinical setting by observation of color, peristalsis and bleeding from cut ends. As this method is not very specific and requires a high level of clinical experience[5], there is a need for increased accuracy of the viability assessment[4]. Intraoperatively, decision on the resection margin is the most important factor contributing to postoperative mortality and morbidity[40,41]. We approached the question of viability assessment in ischemic and reperfused porcine jejunum by using microdialysis and by histological assessment of pathological changes. Microdialysis allowed monitoring of metabolic changes related to ischemia and reperfusion. Presumed irreversible tissue damage was detected after shorter duration of ischemia using TEM than with LM. Subsequent reperfusion aggravated ischemic damage to the jejunum. Likely irreversible damage (when including the effects of reperfusion) occurs between 3 and 4 h of full mesenteric warm ischemia in the porcine jejunum, indicating a time limit for viability in the model.
While return of color and peristalsis does not correlate uniformly with intestinal viability[2,42], these are the most common criteria in the clinical assessment of intestinal viability[3]. A small variation in the nuance of darkness was the only change in color from 2-9 h of full occlusion ischemia, showing that intestinal color alone is a poor indicator of viability. The later change in appearance from dark (8 h), to patchy colored (11-12 h), to necrotic (15-16 h), indicates the time window between the initiation of full occlusion warm ischemia and the presence of pronounced necrotic bowel in the SMO model.
We observed return of color and peristalsis (Table 2) in intestine that histologically contained areas of probably irreversible damage (Table 3). Following reperfusion, the increase in time before return of color associated with an increase in ischemic exposure, indicating that the time before return of color is affected by the level of tissue injury. However, confounding effects such as internal bleeding and edema in the intestinal wall may have reduced the accuracy of the return of color assessment after the long reperfusion intervals.
Macroscopically, fibrin exudate was seen on the serosal surface (Table 2) on the segments that had been ischemic for more than 1 hour. In addition to being triggered by ischemia/reperfusion[43], the formation of fibrinous exudate on the serosa was probably exacerbated by handling and exposure of the intestine to foreign material during the course of the experiment[44].
Using microdialysis to measure intraluminal lactate and glycerol, we were able to closely monitor the onset and duration of ischemia, and the onset of reperfusion (Figure 2). In the segments that were reperfused after ≥ 6 h of ischemia, we observed increasing leakage of fluid from the intestines into the abdominal cavity and increasing amounts of fluid accumulating inside the lumen. Granger et al[45] reported a doubling of vascular permeability during ischemia and a fourfold increase in vascular permeability after reperfusion. This probably dilutes the luminal lactate and glycerol concentrations, limiting the accuracy of intraluminal microdialysis during prolonged ischemia/reperfusion experiments[46]. The phenomenon is expressed by a gradual decrease in lactate and glycerol levels in the ischemic intestine past the 6-h duration.
Intraluminal lactate and glycerol levels have been reported to mirror the permeability (polyethylene glycol 4000) of the intestinal mucosa after ischemia, and lactate more precisely so than glycerol[47]. The lactate and glycerol levels started to decrease before reperfusion and dropped after reperfusion even in severely ischemic intestine (8 h), where we observed histological damage to all layers (Table 3). This suggests that the relationship between permeability and lactate/glycerol levels may be valid only after shorter periods of ischemia, and that our late results may be confounded by the dilution effect of leakage into the lumen.
In comparison to previous experiments using intraluminal microdialysis in ischemia/reperfusion of the small intestine in pigs[30,34,35,48,49], we have monitored the intestine over a longer period of ischemic time and over more ischemia/reperfusion intervals than previously reported. Interestingly, Solligard et al[47] monitored a single clamp for 9 h of reperfusion after 1 h of ischemia with similar results as ours.
After start of reperfusion, there appears to be no clear difference in the time course of metabolic marker concentration between reversibly and irreversibly damaged tissue, indicating that prediction of viability based on intraluminal microdialysis alone is unreliable. Ideally, placement of microdialysis catheters into the intestinal wall would be preferable, as this would circumvent the late ischemia/reperfusion effects related to intraluminal leakage and dilution. Still, intraluminal microdialysis has been recommended over microdialysis catheters inserted into the intestinal wall, because of the reported poor reliability of the latter method[30,35,47,49-53]. The present results confirm that intraluminal microdialysis has high specificity and sensitivity for detecting and monitoring ischemia in the small intestine.
LM (Figure 3, Table 3) and TEM (Figure 5, Table 4) showed a gradual increase in injury in the ischemic tissue with probable irreversible damage appearing around 6 h and 5 h, respectively, indicating that the pathological changes related to viability are visible somewhat earlier on the ultrastructural level than with LM. Tissue that still appeared viable after 4 h of ischemia was considered irreversibly injured after subsequent 3 h of reperfusion, indicating the limit of viability in the model.
When investigating what others have reported with respect to a viability limit in the porcine jejunum, we did not find much information. In most papers discussing viability in the small intestine, observations are reported as histological grading scores or as morphological observations[20], but few contain explicit statements about viability. The most common time duration reported for porcine intestine related to viability is that it takes approximately 8 h of full ischemia to induce transmural necrosis[22,54]. We observed the same result in the present study (Table 3, Figure 4).
Chan et al[18], reporting that irreversible damage in porcine jejunum, defined as lack of mucosal regeneration in samples taken 24 h after reperfusion, occurred after 6.5 h of ischemia followed by reperfusion[19]. We acknowledge that mucosal necrosis will heal completely in most cases, except in cases with necrosis of long mucosal segments with substantial damage to the crypt layer, where there is a risk of complications due to hemorrhage and fluid loss[15,21]. The mucosa can regenerate on injured segments of intestine that do not develop into transmural infarction. However, such segments may develop persistent injury with large degree of fibrosis and stricture formation[21]. The exacerbation of injury following reperfusion indicates that reperfusion is a major contributor to injury in the porcine SMO model.
As the Park/Chiu grading system was created to be sensitive to early mucosal changes, the initial grading after one hour of ischemia is 3, indicating that a finer resolution than 1 h of ischemia should be used to utilize its potential. The grading system may have been designed for assessment of inflammatory diseases and the status of cold preserved tissue for transplantation, rather than with respect to overall viability. The Swerdlow grading system has a more evenly distributed resolution with respect to injury in the whole intestinal wall, including two levels of injury with respect to mural infarction. Nevertheless, both systems arrive at similar results, as the structures are similar. We agree with Quaedackers et al[20] that a better description of the last grades of the Park/Chiu system would further strengthen its suitability.
We found that more than 3 h of ischemia gave a full score in both grading systems within two hours following reperfusion (Figure 4). This indicates that to assess jejunal viability using histology after an ischemic event of unknown duration, at least two hours of reperfusion is needed before the histological sampling will accurately illustrate the outcome. We generally observed slightly higher levels of injury than Blikslager et al[55] in a similar model used on the ileum in pigs, and Chan et al[19] in a similar model on the jejunum in two juvenile pigs. As the ileum is more resistant to ischemic damage than the jejunum we expected a slightly higher injury grade in the jejunum.
In the samples from the reperfused tissue that had been exposed to only one hour of ischemia, there was visible regeneration of the epithelial cells after 3 h of reperfusion, with a large degree of regeneration after 6 h. This is similar to what has been reported previously both in humans[56] and pigs[9,57].
An important observation from the present study is that the sequence of ischemia/reperfusion injury using the SMO model does not necessarily follow the outwards direction from the mucosa to the outer muscular layer, as most grading and classification systems suggest[16,20]. Rather, the ischemic damage may be patchy and somewhat unpredictable, as we observed tissue damage in the outer layer of the muscularis propria while the inner muscular layer still appeared viable. This is illustrated when comparing Figure 4 (histological grading) with Table 3 (morphologic observations).
Evaluation of tissue viability based on histological assessment is difficult[58], as the samples are small and lesions are heterogeneous in composition and distribution[59] with areas of viable and necrotic tissue in the same tissue sample. Predicting the healing potential of the various intestinal layers after ischemia/reperfusion is also challenging. Although we observed injury to the jejunal wall that we considered irreversible, the ability to regenerate is likely to vary with the total volume of damaged tissue, making exact assessments from tissue samples difficult. With respect to the observation of heterogeneous injury, Guan et al[60] speculated that this may be related to difference in the flow in the mesenteric versus antimesenteric side of the small intestine.
We selected the pig model for viability assessment of the small intestine, as it has important anatomical and physiological similarities to humans[61], the pathophysiology of ischemia/reperfusion in the porcine model is similar to humans[12], and because the pig model has been suggested as a reference standard in intestinal transplantation research. The SMO model[17] was selected as it provides a well-defined area of ischemic injury affecting the whole intestinal wall in the occluded segment[12], as opposed to the commonly used intestinal ischemia model of occlusion of the superior mesenteric artery[17,62]. The SMO model simulates ischemic injury as caused by strangulation-ileus.
A 50 kg pig has approximately 15 meters of small intestine[63], allowing for the creation of several parallel SMO models[19,36,37], reducing the total number of animals needed for the experiment. However, there are some disadvantages with parallel ischemia/reperfusion models in the same pig. Previous studies have shown that the cytokine levels are reduced when reperfusion of segments is continued in the same pig, due to increasing tolerance levels[64,65]. In addition, we observed periods of increasing heart rate, decreasing blood pressure, fever, and increasing permeability of the intestines, following the late reperfusion intervals. Increased heart rate, decreased blood pressure and fever may be systemic responses related to the release of increasing quantities of harmful substances following the late reperfusion intervals[66,67]. The increasing permeability[45] was visible as fluid droplets on the surface of the reperfused segments and increasing amounts of peritoneal fluid.
Inspection of the control tissue after 12 h gave an indication of the systematic effects on the surrounding perfused jejunum. LM showed mild reactive and inflammatory changes (Table 3), while TEM of the muscularis propria and serosa showed cells with some swollen mitochondria with vacuolated matrices. The microdialysis results and the histological grading systems did not indicate any changes in the control specimens. So, although some minor changes could be observed in the control intestine, we find it unlikely that this had any confounding effects on the outcome of the experiments. Thus, the observed ischemic changes in each occluded segment in the same pig are likely independent of systemic effects until the onset of reperfusion.
In conclusion, in the present porcine model with segmental occlusion of the jejunal mesentery, the intestinal tissue was judged to be probably irreversibly damaged when exposed to ≥ 4 h of ischemia and then reperfused. Using microdialysis to monitor intraluminal lactate and glycerol allowed us to closely monitor the onset and duration of ischemia, and the onset of reperfusion, but we were unable to find sufficient level of association between tissue viability and metabolic markers to be clinically relevant. The sequence of ischemia/reperfusion injury using the SMO model does not follow the outwards direction from the mucosa to the outer muscular layer, as most current histological grading and classification system suggest. Evaluation of intestinal viability based on return of color and the presence of peristalsis did not match well with histologic assessment of tissue viability.
The clinical gold standard used on humans for assessment of intestinal viability is still based on palpation, visual inspection, bleeding from cut ends and the use of second look operations. The high mortality rates related to acute mesenteric ischemia have not been reduced drastically since the 1980’s.
We are investigating methods to improve the accuracy of intraoperative surgical decision making with respect to assessment of the viability of ischemic/reperfused intestine. To assess the accuracy of these methods we need a reference for the limits of intestinal tissue viability. As the pathophysiology of ischemia/reperfusion in the porcine model is similar to humans, and because the pig model has been suggested as a reference standard in intestinal transplantation research, we decided to investigate the jejunal viability limit in a pig model. Our hypothesis is that the results with a pig model can have translational relevance for humans.
We investigated viability assessment in a porcine model of warm ischemia on jejunum with mesenteric occlusion, followed by reperfusion. Our aim was to determine the time point of irreversible damage, to provide a reference for experimental approaches to intestinal viability assessment.
We created parallel segmental models on the jejunum in 15 pigs, by clamping the mesenteric arteries and veins for 1 to 16 h. Reperfusion was initiated after different intervals of ischemia (1-8 h) and subsequently monitored for 5-15 h. We compared the results from visual inspection with histology (light microscopy and transmission electron microscopy) and intraluminal microdialysis. The intestinal injury was graded using Park/Chiu and modified Swerdlow grading.
Only jejunal segments that had been ischemic for ≤ 3 h appeared viable (following ≥ 1 h of reperfusion). The jejunal segments that had been ischemic for 4 h showed (following ≥ 1 h of reperfusion) a total loss of crypt epithelium, extensive shrinkage and loss of myocytes in the outer layer of the muscularis propria. Intraluminal microdialysis allowed us to closely monitor the onset and duration of ischemia and the onset of reperfusion. We observed return of color and peristalsis in intestine that histologically contained areas of probably irreversible damage. The sequence of ischemia/reperfusion injury using the SMO model does not follow the outwards direction from the mucosa to the outer muscular layer, as most current histological grading and classification system suggest.
In the present porcine model with segmental occlusion of the jejunal mesentery, the intestinal tissue was judged to be probably irreversibly damaged when exposed to ≥ 4 h of ischemia and then reperfused. Three hours of ischemia followed by reperfusion appeared to be the upper limit for viability in this model. We were unable to find sufficient level of association between tissue viability and metabolic markers to conclude that microdialysis is clinically relevant for viability assessment. Evaluation of color and motility appears to be poor indicators of intestinal viability.
Segmental mesenteric occlusion provides reproducible injury in porcine jejunum and appears to be a relevant model for studies on viability assessment. Future studies should consider viability assessment in settings where the various etiologic factors related to acute mesenteric ischemia (emboli, arterial and venous thrombus and nonocclusive ischemia) can be evaluated, as good reference models are needed for each etiology.
We want to thank Rune Veddegjerde at Sensocure, and the medical staff at the Department of Emergencies and Critical Care at Oslo University Hospital, for invaluable assistance during the animal experiments. We want to thank Sheraz Yacub at the Department of HPB Surgery at Oslo University Hospital for suggestions and guidance. We want to thank Ellen Hellesylt and her staff at the Department of Pathology at Oslo University Hospital for preparation of the histological samples for light microscopy. We also want to thank Sverre-Henning Brorson at The Core Facility for Electron Microscopy, Oslo University Hospital, for his work with preparation of samples for transmission electron microscopy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Salvadori M, Zhu X S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 2. | Horgan PG, Gorey TF. Operative assessment of intestinal viability. Surg Clin North Am. 1992;72:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Tilsed JV, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, Ponchietti L, Shamiyeh A, Al-Ayoubi F, Barco LA. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42:253-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Urbanavičius L, Pattyn P, de Putte DV, Venskutonis D. How to assess intestinal viability during surgery: A review of techniques. World J Gastrointest Surg. 2011;3:59-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 5. | Bulkley GB, Zuidema GD, Hamilton SR, O’Mara CS, Klacsmann PG, Horn SD. Intraoperative determination of small intestinal viability following ischemic injury: a prospective, controlled trial of two adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment. Ann Surg. 1981;193:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115-1134, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Yanar H, Taviloglu K, Ertekin C, Ozcinar B, Yanar F, Guloglu R, Kurtoglu M. Planned second-look laparoscopy in the management of acute mesenteric ischemia. World J Gastroenterol. 2007;13:3350-3353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 9. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1524] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 11. | Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Yandza T, Tauc M, Saint-Paul MC, Ouaissi M, Gugenheim J, Hébuterne X. The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J Surg Res. 2012;178:807-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749-G753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Liao YF, Zhu W, Li DP, Zhu X. Heme oxygenase-1 and gut ischemia/reperfusion injury: A short review. World J Gastroenterol. 2013;19:3555-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 15. | Brolin RE, Bibbo C, Petschenik A, Reddell MT, Semmlow JL. Comparison of ischemic and reperfusion injury in canine bowel viability assessment. J Gastrointest Surg. 1997;1:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand. 1987;153:321-324. [PubMed] |
| 17. | Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308:G63-G75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Illyés G, Hamar J. Sequence of morphological alterations in a small intestinal ischaemia/reperfusion model of the anesthetized rat. A light microscopy study. Int J Exp Pathol. 1992;73:161-172. [PubMed] |
| 19. | Chan KL, Chan KW, Tam PKH. Segmental small bowel allograft—Ischemic injury and regeneration. J Pediatr Surg. 1998;33:1703-1706. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Quaedackers JS, Beuk RJ, Bennet L, Charlton A, oude Egbrink MG, Gunn AJ, Heineman E. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proc. 2000;32:1307-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Swerdlow SH, Antonioli DA, Goldman H. Intestinal infarction: a new classification. Arch Pathol Lab Med. 1981;105:218. [PubMed] |
| 22. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1426] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Ahrén C, Haglund U. Mucosal lesions in the small intestine of the cat during low flow. Acta Physiol Scand. 1973;88:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 47] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Clark ET, Gewertz BL. Intermittent ischemia potentiates intestinal reperfusion injury. J Vasc Surg. 1991;13:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Weixiong H, Aneman A, Nilsson U, Lundgren O. Quantification of tissue damage in the feline small intestine during ischaemia-reperfusion: the importance of free radicals. Acta Physiol Scand. 1994;150:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574-580. [PubMed] |
| 27. | Plonka AJ, Schentag JJ, Messinger S, Adelman MH, Francis KL, Williams JS. Effects of enteral and intravenous antimicrobial treatment on survival following intestinal ischemia in rats. J Surg Res. 1989;46:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Hegde SS, Seidel SA, Ladipo JK, Bradshaw LA, Halter S, Richards WO. Effects of mesenteric ischemia and reperfusion on small bowel electrical activity. J Surg Res. 1998;74:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Deeba S, Corcoles EP, Hanna GB, Pareskevas P, Aziz O, Boutelle MG, Darzi A. Use of rapid sampling microdialysis for intraoperative monitoring of bowel ischemia. Dis Colon Rectum. 2008;51:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Tenhunen JJ, Kosunen H, Alhava E, Tuomisto L, Takala JA. Intestinal luminal microdialysis: a new approach to assess gut mucosal ischemia. Anesthesiology. 1999;91:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Waelgaard L, Dahl BM, Kvarstein G, Tønnessen TI. Tissue gas tensions and tissue metabolites for detection of organ hypoperfusion and ischemia. Acta Anaesthesiol Scand. 2012;56:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Pischke SE, Tronstad C, Holhjem L, Line PD, Haugaa H, Tønnessen TI. Hepatic and abdominal carbon dioxide measurements detect and distinguish hepatic artery occlusion and portal vein occlusion in pigs. Liver Transpl. 2012;18:1485-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Sommer T. Microdialysis of the bowel: the possibility of monitoring intestinal ischemia. Expert Rev Med Devices. 2005;2:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Högberg N, Carlsson PO, Hillered L, Meurling S, Stenbäck A. Intestinal ischemia measured by intraluminal microdialysis. Scand J Clin Lab Invest. 2012;72:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Sommer T, Larsen JF. Intraperitoneal and intraluminal microdialysis in the detection of experimental regional intestinal ischaemia. Br J Surg. 2004;91:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Cook VL, Jones Shults J, McDowell M, Campbell NB, Davis JL, Blikslager AT. Attenuation of ischaemic injury in the equine jejunum by administration of systemic lidocaine. Equine Vet J. 2008;40:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol. 2007;292:G647-G656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Noer RJ, Derr JW. Revascularization following experimental mesenteric vascular occlusion. Arch Surg. 1949;58:576-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Strand-Amundsen RJ, Tronstad C, Kalvøy H, Gundersen Y, Krohn CD, Aasen AO, Holhjem L, Reims HM, Martinsen ØG, Høgetveit JO, Ruud TE, Tønnessen TI. In vivo characterization of ischemic small intestine using bioimpedance measurements. Physiol Meas. 2016;37:257-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Karakaş BR, Sırcan-Küçüksayan A, Elpek OE, Canpolat M. Investigating viability of intestine using spectroscopy: a pilot study. J Surg Res. 2014;191:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 42. | Glotzer DJ, Villegas AH, Anekamaya S, Shaw RS. Healing of the intestine in experimental bowel infarction. Ann Surg. 1962;155:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Schoots IG, Levi M, Roossink EH, Bijlsma PB, van Gulik TM. Local intravascular coagulation and fibrin deposition on intestinal ischemia-reperfusion in rats. Surgery. 2003;133:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Torre M, Favre A, Pini Prato A, Brizzolara A, Martucciello G. Histologic study of peritoneal adhesions in children and in a rat model. Pediatr Surg Int. 2002;18:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269-H1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 163] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Haglund U. Gut ischaemia. Gut. 1994;35:S73-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Solligård E, Juel IS, Spigset O, Romundstad P, Grønbech JE, Aadahl P. Gut luminal lactate measured by microdialysis mirrors permeability of the intestinal mucosa after ischemia. Shock. 2008;29:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Tenhunen JJ, Jakob SM, Takala JA. Gut luminal lactate release during gradual intestinal ischemia. Intensive Care Med. 2001;27:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Solligård E, Juel IS, Bakkelund K, Johnsen H, Saether OD, Grønbech JE, Aadahl P. Gut barrier dysfunction as detected by intestinal luminal microdialysis. Intensive Care Med. 2004;30:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Pynnönen L, Minkkinen M, Perner A, Räty S, Nordback I, Sand J, Tenhunen J. Validation of intraluminal and intraperitoneal microdialysis in ischemic small intestine. BMC Gastroenterol. 2013;13:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Emmertsen KJ, Wara P, Soerensen FB, Stolle LB. Intestinal microdialysis--applicability, reproducibility and local tissue response in a pig model. Scand J Surg. 2005;94:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Davies MI, Lunte CE. Microdialysis sampling for hepatic metabolism studies. Impact of microdialysis probe design and implantation technique on liver tissue. Drug Metab Dispos. 1995;23:1072-1079. [PubMed] |
| 53. | Anderson C, Andersson T, Wårdell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Amano H, Bulkley GB, Gorey T, Hamilton SR, Horn SD, Zuidema GD. Role of Micro-Vascular Patency in the Recovery of Small-Intestine from Ischemic-Injury. Surg Forum. 1980;31:157-159. |
| 55. | Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery. 1997;121:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, Heineman E, van Dam RM, Dejong CH, Buurman WA. Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation. PLoS One. 2008;3:e3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Gayle J, Jones SL, Argenzio RA, Blikslager AT. Neutrophils increase paracellular permeability of restituted ischemic-injured porcine ileum. Surgery. 2002;132:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Hillman H. Limitations of clinical and biological histology. Med Hypotheses. 2000;54:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Dabareiner RM, Sullins KE, White NA, Snyder JR. Serosal injury in the equine jejunum and ascending colon after ischemia-reperfusion or intraluminal distention and decompression. Vet Surg. 2001;30:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Guan Y, Worrell RT, Pritts TA, Montrose MH. Intestinal ischemia-reperfusion injury: reversible and irreversible damage imaged in vivo. Am J Physiol Gastrointest Liver Physiol. 2009;297:G187-G196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Douglas WR. Of pigs and men and research: a review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972;3:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | McCance RA. The effect of age on the weights and lengths of pigs’ intestines. J Anat. 1974;117:475-479. [PubMed] |
| 64. | Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 65. | Ruud TE, Gundersen Y, Wang JE, Foster SJ, Thiemermann C, Aasen AO. Activation of cytokine synthesis by systemic infusions of lipopolysaccharide and peptidoglycan in a porcine model in vivo and in vitro. Surg Infect (Larchmt). 2007;8:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Willerson JT. Pharmacologic approaches to reperfusion injury. Adv Pharmacol. 1997;39:291-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |