Published online May 7, 2018. doi: 10.3748/wjg.v24.i17.1868
Peer-review started: March 25, 2018
First decision: April 18, 2018
Revised: April 23, 2018
Accepted: April 26, 2018
Article in press: April 26, 2018
Published online: May 7, 2018
Processing time: 44 Days and 6.3 Hours
In inflammatory bowel disease (IBD), tumor necrosis factor plays an important role in mediating inflammation, but several other pathways are also involved in eliciting an inflammatory response. One such pathway is the invasion of the intestinal mucosa by leukocytes. Leukocytes within the systemic circulation move to sites of inflammation, and blocking this pathway could be an important treatment strategy for IBD. Anti-integrin therapy blocks the action of integrin on the surface of circulating immune cells and endothelial cell adhesion molecules, thereby inhibiting the interactions between leukocytes and intestinal blood vessels. Natalizumab, which acts on α4-integrin, was the first such drug to be approved for Crohn’s disease, but its use is limited due to the risk of progressive multifocal leukoencephalopathy. Vedolizumab produces few systemic adverse effects because it acts on gut-trophic α4β7 integrin, and has been approved and is being used to treat IBD. Currently, several anti-integrin drugs, including etrolizumab, which acts on β7-integrin, and PF-00547569, which targets mucosal addressin cell adhesion molecule-1, are undergoing clinical trials and the results are being closely watched.
Core tip: Anti-integrin therapies have attracted attention as new therapeutic agents in inflammatory bowel disease. They inhibit the extravasation of leukocytes by blocking the interaction between integrins on immune cells and endothelial cell adhesion molecules. The use of the first developed anti-integrin agent, natalizumab is now limited due to the risk of progressive multifocal leukoencephalopathy. However, vedolizumab which acts selectively on the gut has shown few adverse events and is currently used in clinical practice. Newer anti-integrin drugs that act on different integrins-related targets, such as AJM300, abrilumab, etrolizumab, and PF-00547659 have also been developed and are in clinical trials.
- Citation: Park SC, Jeen YT. Anti-integrin therapy for inflammatory bowel disease. World J Gastroenterol 2018; 24(17): 1868-1880
- URL: https://www.wjgnet.com/1007-9327/full/v24/i17/1868.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i17.1868
Causes of inflammatory bowel disease (IBD) have not yet been clearly elucidated, but it is known that genetic susceptibility, altered gut microbiota, and environmental factors are all involved. It has also been reported that a combination of these factors causes an inappropriate immune response, resulting in impaired intestinal barrier function[1-3].
As continual research further reveals the immunopathogenesis of IBD, the treatment of IBD has shifted from conventional treatments, such as aminosalicylates, glucocorticoids, and immunomodulators (thiopurines and methotrexate), toward the biological drugs that target inflammation-related pathways[4]. Anti-tumor necrosis factor (TNF) agents were the first biologics used to treat IBD, and the objective of IBD treatment has shifted from controlling symptoms to changing the progression of disease and preserving the intestinal function. However, anti-TNF agents are not effective in all IBD patients, and a considerable number of patients experience relapse after stopping medication. The pathophysiology of IBD is very complex. This means that the most appropriate treatment method may vary for each patient, and therefore, constant efforts are being made to develop effective drugs[4]. In particular, new biologics that inhibit leukocyte trafficking to the site of inflammation have been developed and used. These drugs are called anti-integrin or anti-adhesion agents, or leukocyte-trafficking inhibitors because they block the actions of integrin, a cell surface protein expressed by circulating immune cells and endothelial cell adhesion molecules (CAMs), thereby selectively preventing the intestinal recruitment of lymphocytes to the site of inflammation[5]. Thus, unlike anti-TNF drugs, anti-integrin agents inhibit the interactions between leukocytes and the intestinal vasculature, and selectively prevent the influx of inflammatory cells, which mediate the inflammatory process in IBD, into intestinal lesions. In this report, we aim to discuss anti-integrin therapy, which is currently being highlighted as a new drug therapy for the treatment of IBD.
A variety of inflammatory and anti-inflammatory cytokines define and regulate various aspects of the inflammatory response and play an important role in the pathogenesis of IBD including Crohn’s disease (CD) and ulcerative colitis (UC), with the former mediated by type 1 T helper cells (TH1) and TH17, and the latter reportedly caused by an abnormal TH2 response. The immunopathogenesis of IBD is made more complex by imbalances in different T cell subsets, such as regulatory T cells, natural killer T cells, and TH9, as well as the interactions between these cell populations. Ultimately, the production of numerous cytokines is disturbed. These cytokines include the well-known TNF-α as well as IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17, IL-23, and transforming growth factor-α[3,6].
The use of TNF antagonists showed that just blocking a single cytokine could be sufficient to induce significant clinical remission. Until recently, in moderate-to-severe active IBD patients, especially if initial treatment with systemic corticosteroids or immunomodulators failed, anti-TNF agents were the only remaining treatment option.
Inspired by the treatment outcomes of the first generation anti-TNF agent infliximab, next-generation TNF antagonists, such as adalimumab, golimumab, and certolizumab pegol, were introduced for the treatment of IBD, drastically changing this treatment field; however, even these drugs did not show an effect in all IBD patients. Specifically, although reports differ slightly, anti-TNF agents produce primary non-response (PNR) in approximately 10%-30% of patients[7]. Several factors have been suggested as causes of PNR. One known cause of PNR is that TNF is not a major factor in the development of inflammation in some patients, and therefore, there is an increased need for drugs with new mechanisms[8].
Although anti-TNF agents show an initial effect, secondary non-response or loss of response (LOR) is seen in 23%-46%[7,9]. LOR is known to occur due to pharmacokinetic issues or the production of antibodies against the drug; however, it can also be caused by a shift in the inflammatory response pathway from TNF signaling to non-TNF signaling. Moreover, due to their comprehensive immunosuppressive effects, the use of anti-TNF agents can cause severe adverse reactions, including tuberculosis (TB), hepatitis B, pneumonia, herpes zoster, and other infections, as well as skin cancer, malignant lymphoma, psoriasis, lupus-like syndrome, demyelinating disease, congestive heart failure, and hepatotoxicity.
Although anti-TNF therapy has reduced the rate of surgery in IBD patients, a considerable number of patients experience a relapse of inflammation after as they stop anti-TNF[10]. After stopping TNF antagonist, the 12-mo relapse rate is 40% for CD and 28% for UC[9,11]. Therefore, there is an urgent need for drugs with novel mechanisms that are more effective and safer than anti-TNF agents, or in particular, that can be used when anti-TNF therapy is ineffective or causes an adverse reaction.
Since biological drugs have a high molecular weight, they are inevitably delivered by injection, and their immunogenicity leads to infusion reactions or LOR associated with the antidrug antibody. Therefore, one aspect of new drug development is to focus on small molecules of less than 1 kDa that could be taken orally, thereby increasing compliance, relatively inexpensive, and have almost no immunogenicity, allowing them to be taken safely on a long-term basis.
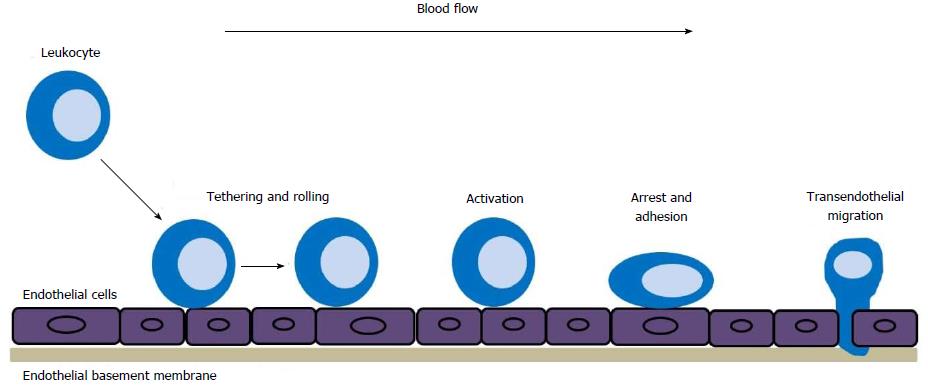
Innate and adaptive immune responses depend on the trafficking of immune cells to the organ targeted by the disease. During an inflammatory response, circulating leukocytes migrate to the target tissues through a homing process that takes place in several stages. Migrating leukocytes in the bloodstream begin tethering (capture) and rolling to a specific place, through the activation process, arrest and adhere to vascular endothelial cells, and finally undergo transendothelial migration (Figure 1). This process of leukocytes migration is mediated by interactions between leukocytes and adhesion molecules expressed by endothelial cells, which enables circulating leukocytes to migrate to the target tissues[12].
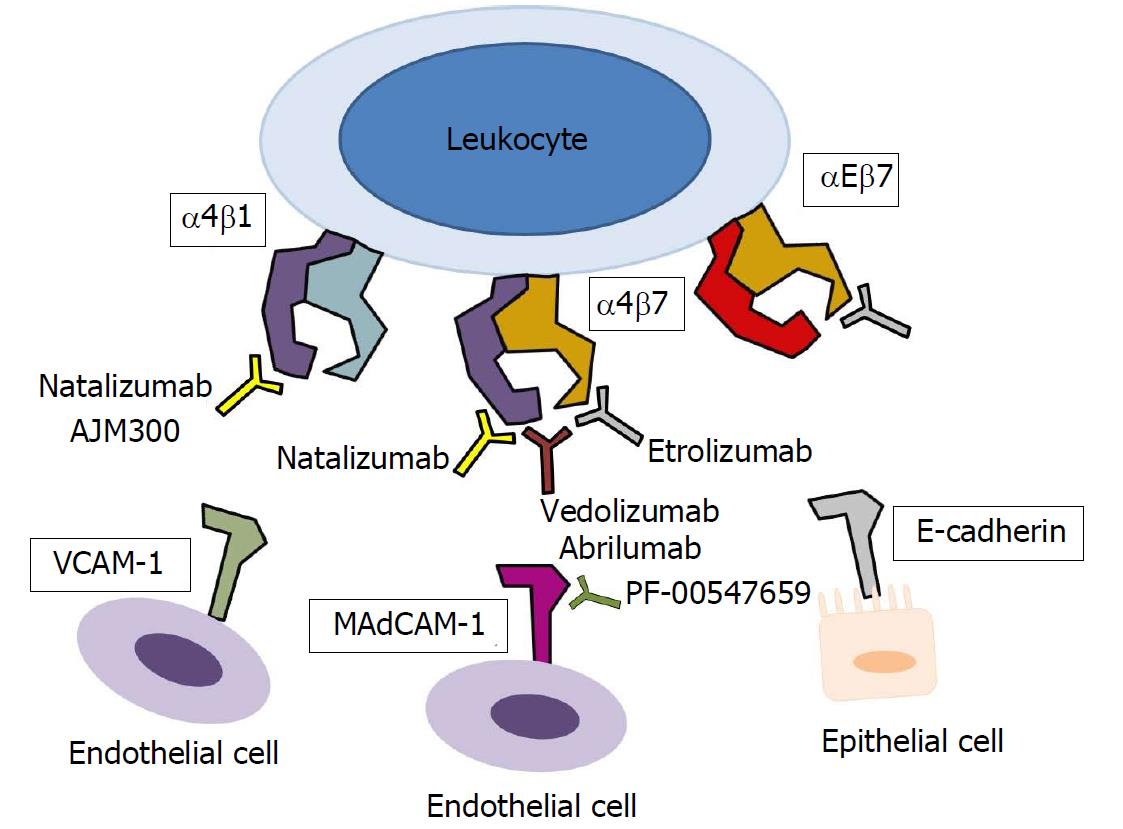
Leukocytes also express CAMs on the surface, called integrins which allow them to interact with the vascular endothelial cells or other cells. Integrin is a heterodimeric receptor formed from α and β subunits and is divided into several groups depending on the structure of the α and β subunit, and different populations of leukocytes express different integrins. These integrins include α4β1 (found on most leukocytes), α4β7 [found specifically on lymphocytes in the gastrointestinal (GI) tract], and αEβ7 (found on intraepithelial T cells, dendritic cells, mast cells or regulatory T cells)[13]. Integrins react with CAMs in the immunoglobulin (Ig) superfamily expressed by other cells to induce cell adhesion; α4β1, α4β7, and αEβ7 integrins bind to vascular cell adhesion molecule-1 (VCAM-1) on vascular endothelial cells, mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on intestinal endothelial cells, and E-cadherin on mucosal epithelial cells (Figure 2)[14].
The migration of leukocytes to the intestinal mucosa and the recruitment of immune cells to the site of inflammation due to increased expression of CAMs are essential to the development and maintenance of intestinal inflammation. Therefore, leukocyte trafficking to the gut is central to the immunopathogenesis of IBD, and its inhibition is recognized as an important goal in the development of anti-IBD drugs[5].
Anti-integrin therapies block the action of integrins, expressed by circulating immune cells, on endothelial CAMs, thereby decreasing the trafficking of immune cells to the endothelium and suppressing the recruitment of inflammatory cells such as lymphocytes to intestinal lesions. Table 1 shows the anti-integrin agents currently approved and in use or in clinical trials.
| Drug | Formula | Target | Route | Clinical studies | Summary |
| Natalizumab | Humanized IgG4 mAb | α4-integrin | i.v. | ENCORE | Induction and maintenance in CD |
| AJM300 | Small molecule | α4-integrin | Oral | Phase IIa | Induction in UC |
| Vedolizumab | Humanized IgG1 mAb | α4β7-integrin | i.v. | GEMINI 1 | Induction and maintenance in UC |
| GEMINI 2 | Induction and maintenance in CD | ||||
| GEMINI 3 | Induction in CD | ||||
| Abrilumab (AMG 181/MEDI 7183) | Fully human IgG2 mAb | α4β7-integrin | s.c. | Phase IIb | Induction in UC |
| Phase IIb | Induction in CD | ||||
| Etrolizumab | Humanized IgG1 mAb | β7-integrin | i.v./s.c. | EUCALYPTUS | Induction in UC |
| BERGAMOT | Induction in CD | ||||
| HICKORY | Induction in CD | ||||
| PF-00547659 (SHP647) | Fully human IgG2κ mAb | MAdCAM-1 | i.v./s.c. | TURANDOT | Induction in UC |
| OPERA | Induction in CD |
Natalizumab is a chimeric recombinant human IgG4 antibody that targets the α4 subunit in α4β7 and α4β1 integrins on leukocytes. α4β1 integrin interacts with VCAM-1. Natalizumab was first approved by the U.S. Food and Drug Administration (FDA) as a treatment for multiple sclerosis, which is an autoimmune disease of the central nervous system (CNS), and clinical trials were conducted to test its efficacy against CD.
In the phase III Efficacy of Natalizumab in Crohn’s disease Response and Remission (ENCORE) trial, 509 patients with moderate-to-severe activity and elevated C-reactive protein (CRP) (> 0.287 mg/dL) were allocated, in a 1:1 ratio, into groups receiving either 300 mg of natalizumab or placebo by intravenous injection at weeks 0, 4, and 8. The primary end point, which was the percentage of patients showing a clinical response [defined as a decrease of at least 70 points in CD activity index (CDAI) score] at week 8 and sustaining this response until week 12, was higher in the natalizumab group, at 48%, than in the placebo group, at 32% (P < 0.001)[15]. The percentage of patients showing sustained clinical remission (defined as a CDAI score under 150 points) at both week 8 and week 12 was also higher in the natalizumab group, at 26%, than in the placebo group, at 16% (P = 0.002). However, natalizumab prevents α4β1 integrin on leukocytes from binding VCAM-1 on vascular endothelial cells in the CNS as well as in the intestines; it has been reported that by reducing T cell trafficking to the brain, natalizumab can affect cerebral antiviral immunity, and in some cases, can cause a fatal brain infection called progressive multifocal leukoencephalopathy (PML) due to the reactivation of the John Cunningham (JC) virus[16,17]. Based on clinical trial data, the risk of PML after a mean of 17.9 mo of natalizumab treatment is approximately 1 case per 1000 patients[18]. The use of immunomodulators before natalizumab administration, a positive test for anti-JC virus antibody, and longer duration of natalizumab treatment are risk factors for PML[19]. Thus, natalizumab has been approved by the United States. FDA only in moderate-to-severe CD patients who did not respond to or were intolerant of conventional treatment or TNF inhibitor therapy; it has not been approved for use in Europe.
Despite safety issues for natalizumab, the oral α4 integrin antagonist AJM300 was developed and evaluated for use in UC. A phase IIa clinical trial was conducted in Japan on 102 patients with moderately active UC, who were intolerant or showed an inappropriate response to mesalamine or corticosteroids; when AJM300 960 mg or placebo was administered 3 times per day, the primary end point, which was the rate of clinical response (defined as a decrease of at least 3 points, and at least 30% compared to baseline, in the complete Mayo score, as well as a decrease of at least 1 point for the rectal bleeding or an absolute rectal bleeding subscore of 1 point or less) at week 8, was significantly higher in the AJM300 group, at 62.7%, than in the placebo group, at 25.5% (P = 0.0002)[20]. Meanwhile, the clinical remission (defined as a complete Mayo score of 0-2 points and no subscore higher than 1 point) rate was 23.5% in the AJM300 group and 3.9% in the placebo group (P = 0.0099), and the mucosal healing rate was 58.8% in the AJM300 group and 29.4% in the placebo group (P = 0.0014), both of which were significantly different. In this clinical study, serious adverse events did not occur, and adverse events were mild and self-limiting. However, considering that AJM300 shares the mechanism of natalizumab, and the number of subjects in this trial was small, and the study period was short, there are concerns about its practicality as a therapeutic drug. Nevertheless, the duration of effect for AJM300 is very short compared to that of natalizumab, and since it is an oral formulation, there is some expectation that it may cause fewer systemic adverse events.
Vedolizumab (VDZ; MLN0002) is a humanized monoclonal IgG1 antibody against α4β7-integrin that inhibits the adhesion of leukocytes to the endothelium by blocking the interaction between α4β7-integrin and MAdCAM-1 expressed on blood vessels and lymph nodes associated with the GI tract. The main difference between natalizumab and VDZ is that natalizumab inhibits leukocyte trafficking in multiple organs, including the brain, whereas VDZ acts specifically only on gut-trophic α4β7 heterodimers, and therefore, inhibits lymphocyte trafficking selectively in the intestine. Although MAdCAM-1 exists rarely at the blood-brain barrier, VDZ is known to have no effect on CNS immunity[21]. In a study in support of this idea, healthy volunteers were injected VDZ and when the cerebrospinal fluid (CSF) was tested 5 wk later, no change was observed in CSF lymphocyte counts or CD4:CD8 ratio following VDZ administration[22]. In another randomized controlled trial comparing VDZ with a placebo, the serum antibody response to a parenteral hepatitis B vaccine did not differ between the 2 groups, but the response to an oral cholera vaccine showed less antibody formation in the VDZ group compared to the placebo group, demonstrating that while VDZ has no effect on systemic immunity, it decreases immune surveillance in the GI tract[23].
The phase III GEMINI 1 trial, consisting of 2 cohorts, analyzed the efficacy of VDZ in 895 moderate-to-severe UC patients who had previously received steroid, immunomodulator, or anti-TNF therapy[24]. The 374 patients in cohort 1 were randomly allocated in a ratio of 3:2, with each group receiving 2 intravenous injections of VDZ 300 mg or placebo at week 0 and 2, and evaluated at week 6. The primary endpoint in the induction phase, which was the clinical response rate at week 6, was significantly higher in the VDZ group, at 47.1%, than in the placebo group, at 25.5% (P < 0.001). The clinical response rate at week 6 was also significantly higher in the VDZ group than in the placebo group among patients who had previously experienced treatment failure with anti-TNF agents (39.0% vs 20.6%, P = 0.01) or steroids (59.5% vs 20.0%, P < 0.001). Moreover, the clinical remission rate at week 6 was 16.9% in the VDZ group and 5.4% in the placebo group (P = 0.001), whereas the mucosal healing rate at week 6 was 40.9% in the VDZ group and 24.8% in the placebo group (P = 0.001), and these differences were statistically significant. To meet the required sample size for the maintenance phase, an additional 521 patients (cohort 2) were recruited for an open-label trial, and administered VDZ by the same method. In the maintenance phase, the 373 patients who achieved a clinical response with VDZ at week 6 were randomized in a 1:1:1 ratio, with each group receiving either a placebo, or VDZ 300 mg every four weeks, or every eight weeks. The trial lasted for a total of 52 wk. The primary endpoint in the maintenance phase, which was the clinical remission rate at week 52, was 15.9% in the placebo group, 41.8% in the VDZ every eight weeks group, and 44.8% in the VDZ every four weeks group, which showed that the effect was 2-fold higher in the VDZ groups than in the placebo group (P < 0.001). The durable clinical response (response at both week 6 and 52) was 23.8% in the placebo group, 56.6% in the VDZ every eight weeks group, and 52.0% in the VDZ every four weeks group, which was significantly different (P < 0.001). Similarly, mucosal healing at week 52 was 19.8% in the placebo group, 51.6% in the VDZ every eight weeks group, and 56.0% in the VDZ every four weeks group, which was also significantly different (P < 0.001). There was no significant difference in the efficacy of VDZ between the four-week and eight-week interval groups. Among patients who had experienced failure with anti-TNF therapy, the clinical remission rate was much lower in the placebo group, at 5.3%, than in the VDZ every eight weeks group, at 37.2%, and the VDZ every four weeks group, at 35.0% (P < 0.001). Therefore, VDZ demonstrated an effect against moderate-to-severe UC at week 6 and at week 52, irrespective of previous anti-TNF therapy. In the post-hoc analysis for the GEMINI I trial, patients were divided into those who were naïve to TNF antagonist (464 patients) and failed to TNF antagonist (367 patients)[25]. The treatment effect measured by the clinical response at week 6 was stronger in patients who were naïve to anti-TNF therapy [absolute difference (AD) between VDZ and placebo 26.4%] than in those who failed to anti-TNF therapy (AD 18.1%). In the maintenance phase, the ADs in week 52 clinical remission rates were 28.0% in patients who were naïve to anti-TNF therapy and 29.5% in patients who failed to anti-TNF therapy, respectively. Even among patients who had previously experienced failure with anti-TNF therapy, those who experienced LOR showed a lesser effect of VDZ than those who experienced PNR or intolerance.
The GEMINI 2 trial, consisting of 2 cohorts, analyzed the efficacy of VDZ in active CD patients[26]. The 368 patients in cohort 1 were randomly allocated in a 3:2 ratio, with each group receiving intravenous VDZ 300 mg or placebo at weeks 0 and 2, and evaluated at week 6. The primary endpoint, which was the clinical remission rate at week 6, was significantly higher in the VDZ group, at 14.5%, than in the placebo group, at 6.8% (P = 0.02). The other primary endpoint, the CDAI-100 response rate (defined as a decrease of at least 100 points in the CDAI score relative to baseline), was higher in the VDZ group, at 31.4%, than in the placebo group, at 25.7%; however, this difference was not statistically significant (P = 0.23). To meet the required sample size for the maintenance phase, an additional 747 patients (cohort 2) were recruited for an open-label trial, and administered VDZ by the same method. In the maintenance phase, 461 patients who had shown a clinical response to VDZ at week 6 which administered either placebo, or VDZ 300 mg every four weeks or every eight weeks. The primary endpoint in the maintenance phase, which was the clinical remission rate at week 52, was 21.6% in the placebo group, 39.0% in the VDZ every eight weeks group, and 36.4% in the VDZ every four weeks group, indicating that both the VDZ every eight weeks (P < 0.001) and VDZ every four weeks (P = 0.004) groups showed significantly higher clinical remission rates than the placebo group. Similarly, the CDAI-100 response rate at week 52 was 30.1% in the placebo group, 43.5% in the VDZ every eight weeks group, and 45.5% in the VDZ every four weeks group, indicating that the response rate was significantly higher in the VDZ every eight weeks group (P = 0.01) and the VDZ every four weeks group (P = 0.005) than in the placebo group. Among patients who had previously experienced failure with anti-TNF therapy, the remission rates at week 52 were 28.0%, 27.3%, and 12.8% for the VDZ every eight weeks, VDZ every four weeks, and placebo groups, respectively. This was significantly higher in the VDZ every eight weeks group (P = 0.01) and the VDZ every four weeks group (P = 0.02) than in the placebo group.
The GEMINI 3 trial was a phase III randomized controlled trial examining the efficacy and safety of VDZ in 416 moderate-to-severe CD patients[27]. Most of the participants (315 patients) had previously experienced failure with anti-TNF therapy (PNR, LOR, or intolerance). After the injection of VDZ 300 mg at weeks 0, 2, and 6, unlike the GEMINI I and II trials, the effects of VDZ were evaluated at week 10 as well as week 6. Among anti-TNF-naïve patients, the clinical remission rate at week 6 was 12.0% in the placebo group and 31.4% in the VDZ group, which was significantly different (P = 0.012). However, among patients with previous anti-TNF therapy failure, the clinical remission rate at week 6 was 12.1% in the placebo group and 15.2% in the VDZ group, which was not a statistically significant difference (P = 0.433), whereas the clinical remission rate at week 10 was significantly higher in the VDZ group, at 26.6%, than in the placebo group, at 12.1% (P = 0.001). Meanwhile, in patients with previous anti-TNF therapy failure, the CDAI-100 response rates at weeks 6 and 10 were 22.3% and 24.8%, respectively, in the placebo group, but were significantly higher in the VDZ group, at 39.2% and 46.8% (P = 0.001 and P < 0.001, respectively). These results show that patients who experience anti-TNF therapy failure take longer to show an effect from VDZ than anti-TNF-naïve patients. Notably, among the subjects in this trial, patients who had experienced anti-TNF therapy failure had a longer disease duration and more structural damage than anti-TNF-naïve patients, which could have affected the clinical effects of VDZ. In the post-hoc analyses for the GEMINI 2 and 3 trials, for patients in the VDZ group, the clinical remission rate at week 52 was 48.9% in patients who were naïve to anti-TNF therapy and 27.7% in patients who had experienced anti-TNF therapy failure, whereas the remission rates in the placebo group were 26.8% and 12.8%, respectively[28]. This shows that the clinical remission rates are higher in the VDZ group than in the placebo group, and that this effect is larger when patients have not previously been exposed to anti-TNF therapy.
Vedolizumab was approved by the FDA and the European Medicines Agency, for the treatment of moderate to severe ulcerative colitis and CD adult patients which are not responding to one or more conventional treatment such as steroids, immunosuppressive agents, or TNF antagonists. The results of the VDZ clinical trials showed different treatment effects in UC and CD. There are several theories to explain why the clinical effect of inhibiting leukocyte trafficking in CD appeared later than that in UC. CD can show systemic manifestations and affect the whole GI tract from the oral cavity to the anus, showing inflammation in all layers of the intestine; conversely, UC is limited to the colonic mucosa, which could explain the discrepancy in the treatment response. Recently, a study on IBD patients and a humanized mouse model found that VDZ treatment in CD reduced the expression of α4β1 in the peripheral blood and increased the expression of α4β1 in the intestine, suggesting that in CD, the VDZ-mediated inhibition of α4β7 could have been circumvented by homing to the ileum via α4β1 on effector T cells[29]. Thus, further in-depth research is required to better understand the pharmacokinetics and pharmacodynamics of VDZ in CD.
The GEMINI long-term safety (LTS) study examined the long-term safety and efficacy of VDZ[30,31]. Among patients in the phase II trial C13004, the GEMINI 1 trial, and VDZ-naïve UC patients who showed a response to VDZ at week 6 were switched to an open-label study and administered VDZ 300 mg continually at four-week intervals for 152 wk[30]. In an interim report on the efficacy of VDZ, the remission rates after 104 and 152 wk were 88% (120/136) and 96% (70/73), respectively, demonstrating a high maintenance of remission. Among patients who dropped out of the VDZ maintenance treatment at eight-week intervals before 52 wk in GEMINI I trial (n = 32), increased dosing frequency to every four weeks in GEMINI LTS improved clinical responses and remission rates from 19% and 6% to 41% and 28%, after 52 wk of GEMINI LTS, respectively. Similarly, among CD patients who had participated in the C13004, GEMINI 2, or GEMINI 3 trial, or were VDZ-naïve, those who showed a response to VDZ at week 6, when switched to an open-label study and monitored for 152 wk while receiving VDZ every four weeks, showed remission rates after 104 and 152 wk of 83% (100/120) and 89% (62/70), respectively[31]. Among patients who dropped out of the VDZ maintenance treatment at eight-week intervals before 52 wk in GEMINI 2 trial (n = 57), increased dosing frequency to every four weeks in GEMINI LTS improved clinical responses and remission rates from 39% and 4% to 47% and 32%, after 52 wk of GEMINI LTS, respectively. Therefore, for patients who show a response to VDZ every eight weeks in the induction phase, but show LOR in the maintenance phase, increasing the dosing frequency to every four weeks could produce a response again.
To examine mucosal and histological healing when VDZ was administered, prospective surveillance colonoscopy was performed in patients registered for the GEMINI LTS trial[32]. The follow-up period was over 1 year (1.1-6.1 years, median 3.2 years), the rate of mucosal healing with a Mayo score of 1 or less was 50% (17/34) for UC and 29% (7/24) for ulcer-free mucosal healing in CD patients. Histological healing with mucosal healing in UC and CD patients was 32% (11/34) and 21% (5/24), respectively.
The VERSIFY study was examined endoscopic mucosal healing at week 26 after VDZ treatment in 101 moderate-to-severe CD patients who had previously experienced failure with corticosteroids, immunomodulators, and/or anti-TNF agents. The endoscopic remission [simple endoscopic score for CD (SES-CD) ≤ 4] rate was 12% overall, 20% for patients who were naïve to anti-TNF therapy (n = 46), and 6% for patients who had previously experienced anti-TNF therapy failure (n = 55)[33]. The endoscopic response (SES-CD decrease of at least 50%) and complete endoscopic healing (no ulcerations) rates were, respectively, 25% and 15% overall, 28% and 24% for patients who were naïve to anti-TNF therapy, and 22% and 7% for patients who had failed at anti-TNF therapy. Thus, VDZ is effective at inducing endoscopic remission and healing in refractory CD patients, and the rates of endoscopic remission and healing are higher in anti-TNF-naïve patients than in those who have experienced anti-TNF therapy failure.
The US VICTORY Consortium provides data relating to VDZ from real-world experience; among 212 moderate-to-severe CD patients, 90% had exposed to anti-TNF therapy, and the median follow-up duration was 39 wk[34]. In responders, the median time to respond to VDZ was 19 wk. After 6, 12, and 18 mo of VDZ therapy, patients showed clinical remission rates of 18%, 35%, and 54%, respectively, and after 6 and 12 mo of treatment, showed cumulative mucosal healing rates of 20% and 63%, respectively, and cumulative deep remission (clinical remission and mucosal healing) rates of 14% and 26%, respectively. Higher disease activity, active perianal disease, smoking history, and prior TNF antagonist exposure were all factors that decreased the effectiveness of VDZ.
In a German cohort study including 115 active UC patients and 97 active CD patients, only 24.3% of UC patients and 5.2% of CD patients were naïve to TNF antagonist[35]. When these patients were treated with VDZ and monitored for 14 wk, at week 14, 23.5% of UC patients and 23.7% of CD patients achieved clinical remission, 57.4% of UC patients and 60.8% of CD patients showed a clinical response, and steroid-free remission was observed in 19.1% of UC patients and 19.6% of CD patients. Serum CRP and calprotectin levels were measured at weeks 0, 6, and 14; patients are showed decreased CRP levels, but this was not statistically significant, whereas calprotectin levels decreased significantly.
In the GETAID Cohort Data from France, the effects of VDZ treatment were analyzed in 121 UC patients and 173 CD patients who had failed with anti-TNF therapy. At week 6, the clinical remission rates were 32% and 31%, the steroid-free clinical remission rates were 21% and 19%, and the clinical response rates were 41% and 57% in the UC and CD patients, respectively[36]. At week 14, the clinical remission rates for UC and CD patients were respectively 39% and 36%, the steroid-free remission rates were 36% and 31%, and the clinical response rates were 57% and 64%, demonstrating that VDZ is effective for both UC and CD. The fact that a superior treatment response was observed at week 14 compared to week 6 re-confirms that it takes time for the effects of treatment to become apparent. When patients were monitored for 1 year, steroid-free remission at week 22 was 40% for UC patients and 34% for CD patients, indicating that remission rates gradually increased for both diseases, and that UC patients achieved steroid-free remission sooner than CD patients.
In summary, real-world data for VDZ treatment were similar to results of randomized controlled studies. In particular, it takes considerable time before the maximal effects of VDZ therapy can be observed, and corticosteroid treatment may be required during this period. The results of a network meta-analysis show that VDZ is more effective overall than anti-TNF therapy in the maintenance phase[37]. Thus, the effect of VDZ, once it becomes apparent, is maintained more strongly, and this sustained effect is considered its greatest advantage. In addition, for patients showing PNR, LOR, or intolerance to anti-TNF therapy, it is worth considering VDZ as a secondary treatment (Table 2).
| Anti-TNF therapy | Gut-specific anti-integrin therapy | |
| Mechanism of action | TNF-α inhibitor | α4β7-integrin inhibitor |
| Available agents | Infliximab (UC, CD) | Vedolizumab (UC, CD) |
| Adalimumab (UC, CD) | ||
| Certolizumab pegol (CD) | ||
| Golimumab (UC) | ||
| Therapeutic efficacy | Frequent loss of response during maintenance therapy | Modest effect on induction therapy for CD |
| Side effects | Infections, reactivation of latent tuberculosis, potential risk of lymphoma | Nasopharyngitis, arthralgia, headache, nausea |
| Immunogenicity | Measure the ADA if available | No significant immunogenicity |
| Add immunomodulator (infliximab) |
Because VDZ acts selectively on the intestine, it causes relatively little systemic immunosuppression, and this is expected to result in fewer adverse events. In the GEMINI 1 and 2 trials, the most commonly reported adverse reactions to VDZ (incidence ≥ 5%) were nausea, nasopharyngitis, upper respiratory tract infection, arthralgia, fever, fatigue, headache, and cough[24,26]. In safety data from the 6 VDZ clinical trials (placebo-controlled trials C13002, GEMINI 1, 2, and 3, and open-label trials C13004 and GEMINI LTS), VDZ showed no significant difference from the placebo in overall adverse reactions[38]. In particular, the exposure-adjusted incidence rates of infections and serious infections, which is a problem in anti-TNF therapy, were 63.5/100 person-years (PYs) and 4.3/100 PYs in patients receiving VDZ, respectively, and 82.9/100 PYs and 3.8/100 PYs in the placebo group, respectively. However, the rates of gastroenteritis and Clostridium difficile infection were low but higher in VDZ-treated patients (4.0/100 PYs and 0.4/100 PYs, respectively) than those in the placebo group (1.4/100 PYs and 0.0/100 PYs, respectively), and further studies will be required to determine whether these results are due to gut-selective immune suppression by VDZ. In safety data from the 6 VDZ clinical trials, 18 patients developed malignancy, including GI cancer (6 patients), skin cancer (5 patients), lung cancer (2 patients), genitourinary cancer (2 patients), breast cancer (2 patients), and B cell lymphoma (1 patient). Colon cancer (0.1/100 PYs) was the most common type of GI cancer, but its incidence was lower than that observed in IBD patients in the HealthCore Integrated Research Database (2.1/1000 PYs; 95%CI: 1.3-3.2)[38,39]. Infusion-related reactions were reported with a low incidence of less than 5% in patients who received VDZ[38]. VDZ does not affect α4β1-related nervous system leukocyte trafficking, and no cases of PML were observed in the clinical trials. Therefore, VDZ can be considered as a primary biological drug in elderly patients with a high risk of opportunistic infections or cancer and in young male patients at risk of hepatosplenic T cell lymphoma. Especially in countries with a high prevalence of TB, such as Korea, China, and India, the risk of TB needs to be considered when selecting a therapeutic drug. VDZ is expected to be a very low-risk drug in this regard, with only 4 TB cases out of approximately 3000 patients who received VDZ (0.1%). Another advantage of VDZ is that it can be used even in the presence of comorbidities that contraindicate anti-TNF therapy, such as demyelinating disease, congestive heart failure, and lymphoma.
Nevertheless, due to the gut selectivity of VDZ, it may not be expected to be effective in patients with extraintestinal symptoms. Recently, a case of CD involving the pleura and lungs after 3 doses of VDZ has been reported[40]. After isolating peripheral blood mononuclear cells from the patient, flow cytometry revealed an upregulation of β1 integrin, which is required for homing of lymphocytes to the lungs, and the condition of the patient improved after prednisolone treatment. This shows that the shift in integrin expression triggered by VDZ can cause immune cells to migrate to organs other than the gut, thereby increasing the risk of extraintestinal autoimmune manifestations in CD.
Anti-VDZ antibodies (AVAs) were detected in 56 out of 1434 patients (4%) who were treated with VDZ up to week 52 in the GEMINI 1 and 2 trials, but of these, only 9 patients (0.6%) continued to show AVA positivity, and 33 patients (2.6%) developed neutralizing antibodies[38]. In the GEMINI LTS trial, the immunogenicity rate did not increase over time. When VDZ was administered in combination with immunosuppressants at baseline, the AVA positivity rate was 3%, which was 1% lower than the AVA positivity rate of 4%. However, these measurements were taken when the patients had a high serum drug concentration, which could have interfered with the assay. Therefore, VDZ seems to have low immunogenicity and could be used without immunosuppressants; however, further research is required.
VDZ may be expected to have a positive effect on fistula closure rate in CD. The phase IV ENTERPRISE trial (NCT02630966), which is currently underway, focuses on fistula healing at week 30 after 22 wk of VDZ medication in patients with fistulizing CD.
Research on combination therapy has so far been limited to case reports. One report found that VDZ + etanercept, the soluble TNF receptor, combination therapy is effective at controlling severe pouchitis and spondylarthritis that developed in a patient with UC; one UC patient who showed no response to treatment with methotrexate, adalimumab, infliximab, azathioprine, cyclosporine A, or golimumab showed clinical remission and mucosal healing when treated with a combination of VDZ + certolizumab pegol and monitored for 21 mo[41,42]. These reports indicate that combination therapy using VDZ and an anti-TNF agent can provide additional clinical benefits, and an open-label study is currently underway to examine the effects of three-drug combination therapy using VDZ, adalimumab, and methotrexate in high-risk CD patients (NCT02764762).
Recently, a study was published on biomarkers that can predict response to VDZ[43]. Using VDZ labeled with fluorescein isothiocyanate, α4β7-expressing cells were detected by confocal laser endomicroscopy; clinical response and endoscopic remission to VDZ were observed in patients who showed pericryptal α4β7+ cells in the mucosa, whereas patients without α4β7+ cells did not respond to VDZ.
Abrilumab (AMG 181/MEDI 7183) is a fully human monoclonal IgG2 antibody against α4β7 integrin that has recently been used in several clinical trials.
In a phase IIb study to evaluate the efficacy and safety of abrilumab in 354 moderate-to-severe UC patients who showed an inappropriate response or LOR to anti-TNFs, immunomodulators, or corticosteroid therapy, patients were divided into a placebo group, groups receiving subcutaneous abrilumab 7, 21, or 70 mg at weeks 0, 2, and 4, followed by its administration once every four weeks, and a group receiving a single subcutaneous 210 mg dose of abrilumab[44]. The primary endpoint, which was remission rate at week 8, was 1.6%, 2.9%, 13.5%, and 13.4% in the abrilumab 7 mg, 21 mg, 70 mg, and 210 mg groups, respectively, and was 4.4% in the placebo group; the abrilumab 70 mg group (P = 0.021) and 210 mg group (P = 0.030) both showed a significantly higher remission rate than the placebo group. Abrilumab increased α4β7-high central memory CD4+ T cell counts in the peripheral blood, and high trough abrilumab concentrations were associated with increased remission rate. No PML or severe adverse events were observed in the abrilumab groups through week 24 and no patients developed neutralizing antibodies to abrilumab. Thus, abrilumab showed advantageous pharmacokinetics, pharmacodynamics, very low immunogenicity, and an acceptable safety profile; further results are expected in the future.
A phase IIb trial was conducted to evaluate the efficacy and safety of abrilumab in 249 patients with moderate-to-severe CD who showed evidence of active inflammation and an inappropriate response, LOR, or intolerance to immunosuppressants, anti-TNFs, or corticosteroid therapy[45]. Patients were divided into a placebo group and groups receiving abrilumab 21 mg or 70 mg at weeks 0, 2, and 4, followed by once every four weeks, and a group receiving a single 210 mg dose of abrilumab. The primary endpoint, which was CDAI remission (CDAI score of < 150 points) rate at week 8, was 23.1%, 14.4%, and 21.9% in the abrilumab 21 mg, 70 mg, and 210 mg groups, respectively, and 12.8% in the placebo group; there were no statistically significant differences between the abrilumab groups and the placebo group. However, among patients who had previously experienced anti-TNF treatment failure, CDAI remission rates at week 12 were 22.9%, 17.4%, and 24.8% in the abrilumab 21 mg, 70 mg, and 210 mg groups, respectively, which were all significantly higher than the remission rate of 8.2% in the placebo group (P < 0.01). Also, in patients with prior anti-TNF failure, the CDAI response (decrease of at least 100 points in CDAI score compared to baseline) rates at week 12 in the abrilumab 21 mg, 70 mg, and 210 mg groups were 30.0%, 39.4%, and 37.4%, respectively, and these values in the abrilumab 70 mg and 210 mg groups were significantly higher than the response rate of 14.2% in the placebo group (P < 0.01). Adverse events up to week 24 were the same in the abrilumab groups and the placebo group, and there were no cases of PML or death in any of the abrilumab groups. Thus, in CD, although abrilumab did not show a significant improvement in the primary endpoint, it could show useful effects.
Etrolizumab (rhuMAb β7) is a humanized monoclonal IgG1 antibody against the β7 subunit of α4β7 and αEβ7 that blocks not only the interaction between α4β7 and MAdCAM-1, but also the interaction between αEβ7 and E-cadherin expressed mostly by epithelial cells. Thus, etrolizumab suppresses the trafficking of lymphocytes into the gut and the retention of lymphocytes in the intraepithelial compartment.
The phase II EUCALYPTUS induction study was conducted on 124 moderate-to-severe UC patients who showed no response to conventional therapy[46]. Patients were randomly allocated in a 1:1:1 ratio into a placebo group, a group administered subcutaneous etrolizumab 100 mg at weeks 0, 4, and 8 (and placebo at week 2), and a group administered a loading dose (LD) of subcutaneous etrolizumab 420 mg, followed by subcutaneous doses of 300 mg at weeks 2, 4, and 8. The primary endpoint, which was clinical remission rate at week 10, was 0% in the placebo group, 20.5% in the etrolizumab 100 mg group (P = 0.004), and 10.3% in the etrolizumab 300 mg plus LD group (P = 0.048); the clinical remission rate was higher in the etrolizumab groups than in the placebo group. In a subgroup analysis, among anti-TNF-naïve patients, the clinical remission rates in the etrolizumab 100 mg group and the etrolizumab 300 mg plus LD group were 44% and 25%, respectively; however, among patients who had not responded to anti-TNF therapy, the clinical remission rates were 5% and 4%. Although there were no cases of severe infection in the etrolizumab-treated groups, and there was no significant difference in the rate of adverse reactions sufficient to stop medication in the three groups, influenza-like illness (7% vs 0% and 2%) arthralgia (15% vs 5% and 9%), and rash (7% vs 3% and 2%) were observed more frequently in the etrolizumab 100 mg group than the etrolizumab 300 mg plus LD group or the placebo group. However, these adverse events were all mild or moderate, demonstrating that etrolizumab is safe and tolerable.
One notable aspect of this study is that when quantitative PCR and immunohistochemistry were used to measure the number of αE gene (ITGAE)-expressing and αE-positive cells in the colonic mucosa, higher αE expression was associated with a higher rate of clinical remission at week 10 in patients treated with etrolizumab, suggesting that αE expression could be used as a biomarker in etrolizumab treatment[46]. The subsequent study was conducted on colon tissues taken by biopsies from the UC patients in this phase II trial, as well as the patients with UC and a control group without IBD in an observational study[47]. Here, the mRNA for granzyme A (GZMA), a serine protease that promotes cell migration and is associated with the secretion of inflammatory cytokines such as IL-1β and TNF-α, showed high expression in colonic CD4+ integrin αE+ cells; higher levels of GZMA mRNA or ITGAE mRNA were associated with a higher likelihood of responding to etrolizumab, and their expression after etrolizumab treatment decreased significantly by 40%-80%.
Currently, there are 5 ongoing phase III randomized controlled trials (HIBISCUS I, HIBISCUS II, GARDENIA, LAUREL, and HICKORY) and 1 rollover open-label extension trial (COTTONWOOD) on UC.
The phase III BERGAMOT trial aimed to evaluate the safety and efficacy of etrolizumab in 300 moderate-to-severe CD patients who were previously refractory or intolerant to anti-TNFs, immunomodulators, and/or corticosteroid therapy[48]. The patients were randomly allocated in a ratio of 1:2:2 into a placebo group, a group receiving subcutaneous etrolizumab 105 mg every four weeks, and a group receiving subcutaneous etrolizumab 210 mg at weeks 0, 2, 4, 8, and 12. The symptomatic remission (abdominal pain ≤ 1 and unweighted stool frequency ≤ 3) rates at week 6 were 15.0% and 25.6% in the etrolizumab 105 mg and etrolizumab 210 mg groups, respectively, which were higher than the rate of 8.5% in the placebo group. Similarly, the symptomatic remission rates at week 10 were higher in the etrolizumab 105 mg and etrolizumab 210 mg groups, at 15.8% and 27.3%, than the placebo group, at 8.5%, and the symptomatic remission rates at week 14 were still higher in the etrolizumab 105 mg and 210 mg groups, at 20.8% and 24.8%, than in the placebo group, at 11.9%. The endoscopic improvement (decrease of at least 50% in SES-CD compared to baseline) rates at week 14 were also higher in the etrolizumab 105 mg and 210 mg groups, at 21.0% and 17.4%, than in the placebo group, at 3.4%. There were no significant differences between the placebo group and the etrolizumab groups in adverse events. Thus, etrolizumab showed a rapid effect at week 6 in the treatment of moderate-to-severe CD, and research is underway investigating the maintenance phase.
The phase III HICKORY open-label induction trial aimed to investigate the efficacy of etrolizumab in 130 moderate-to-severe UC patients who showed intolerance or no response to anti-TNFs[49]. After patients were administered etrolizumab 105 mg by subcutaneous injections for 14 wk, at four-week intervals, the clinical response and remission rates at week 14 were 50.8% and 12.3%, respectively, and 43.9% of patients receiving etrolizumab showed an endoscopic improvement, represented by a decrease of at least 1 point in endoscopy score compared to baseline. HICKORY including double blind induction phase and maintenance phase is currently ongoing (NCT02100696).
αEβ7 and α4β7 are differentially expressed in T lymphocyte effector subsets in the peripheral blood and intestines of IBD patients; T cell receptor stimulation and transforming growth factor-β treatment increased the expression of αEβ7, especially in CD8+ lymphocytes[50]. When used in a humanized mouse model of colitis, etrolizumab surrogate antibody decreased the accumulation of CD8+ and CD4+ Th9 cells in the intestine more strongly than VDZ; this seems to be because etrolizumab had an additional inhibitory effect on the αEβ7-mediated retention of lymphocytes[50].
If β7 integrin is blocked, it could reduce gut specificity; this is because αEβ7 is expressed by T cells in other tissues as well as in the intestines; therefore, problems can arise with the control of local infection[51]. Therefore, in the ongoing phase III trials, it is important to determine whether latent infection is a significant adverse effect of etrolizumab.
PF-00547659 (SHP647) is a fully human monoclonal IgG2κ antibody targeting MAdCAM-1, an intestinal endothelial CAM that binds α4β7 integrin on lymphocytes. This is another strategy for inhibiting leukocyte adhesion by blocking the endothelial CAM from binding to the integrin ligand.
The phase II TURANDOT trial analyzed 357 moderate-to-severe UC patients who had either shown failure or intolerance for at least one conventional therapy[52]. PF-00547569 was administered every four weeks by subcutaneous injection at either one of the 4 different doses (7.5, 22.5, 75, or 225 mg) and the outcomes were compared with a placebo. The primary endpoint, which was clinical remission rate at week 12, was 2.7% in the placebo group, 11.3% in the PF-00547569 7.5 mg group (P = 0.0425), 16.7% in the 22.5 mg group (P = 0.0099), 15.5% in the 75 mg group (P = 0.0119), and 5.7% in the 225 mg group (P = 0.1803), indicating that the remission rate was significantly higher in the PF-00547569 7.5 mg, 22.5 mg, and 75 mg groups than in the placebo group, and the efficacy was the highest in the 22.5 mg and 75 groups. The mucosal healing rate at week 12 was 8.2% in the placebo group, 15.5% in the 7.5 mg group (P = 0.0099), 27.8% in the 22.5 mg group (P = 0.0038), 25.4% in the 75 mg group (P = 0.0080), and 14.3% in the 225 mg group (P = 0.0099), showing the highest value in the PF-00547569 22.5 mg and 75 mg groups. In a subgroup analysis, among patients experiencing anti-TNF therapy failure, the remission rate at week 12 was 0% in the placebo group, 7.3% in the 7.5 mg group (P = 0.0425), 9.8% in the 22.5 mg group (P = 0.0099), 9.8% in the 75 mg group (P = 0.0119), and 2.5% in the 225 mg group (P = 0.1803), showing significantly higher values than the placebo group in the PF-00547569 7.5 mg, 22.5 mg, and 75 mg groups. The reason that the clinical effect of PF-00547659 at the highest dose decreased may be because of study design or the depletion of the anti-inflammatory regulatory T cells to the intestine, and further research is needed[53]. There were no significant differences between the placebo group and the PF-00547569 groups in the frequency of adverse events, and there were no cases of severe infection or PML. When GI side effects were investigated considering the gut selectivity of PF-00547659, Clostridium difficile infection, anal abscess and anal fistula were observed in patients treated with PF-00547659 and one patient was diagnosed with adenocarcinoma of colon during the study period. Therefore, special attention should be paid to GI complications in the treatment of PF-00547659, and additional data is necessary to establish its safety. A large-scale phase III clinical trial is currently underway in patients with UC (NCT03259334).
The phase II OPERA trial aimed to evaluate the efficacy and safety of PF-00547569 in 265 moderate-to-severe CD patients who had previously shown no response or intolerance for anti-TNFs and/or immunosuppressants[54]. Patients were randomly allocated, in a 1:1:1:1 ratio, to a placebo group and groups receiving PF-00547569 at either one of the 3 doses (22.5, 75, or 225 mg). The CDAI-70 response rates at week 8 showed no significant differences, at 47.7% in the placebo group and 52.7%, 60.1%, and 62.7% in the PF-00547569 22.5, 75, and 225 mg groups, respectively. Similarly, the CDAI-70 response rates at week 12 also showed no significant differences, at 58.6% in the placebo group and 62.8%, 64.7%, and 57.5% in the PF-00547569 22.5, 75, and 225 mg groups, respectively. However, among patients with high baseline CRP levels (> 5 mg/dL or > 18.8 mg/dL), the CDAI remission rates at week 8 or 12 were higher in the PF-00547569 groups than in the placebo group. Moreover, in the PF-00547569 groups, soluble MAdCAM level decreased significantly at week 2 in a dose-dependent manner and circulating β7+ CD4+ central memory T lymphocytes increased at weeks 8 and 12. Therefore, although the high clinical response rate in the placebo group indicated that there was no significant difference between the PF-00547569 groups and the placebo group, PF-00547569 seems to be effective in patients with active inflammation.
Given the clinical success of drugs that block α4β7 integrin, antibodies against MAdCAM-1 should produce a similar clinical effect. However, this is not reflected in the study results because α4β7 not only binds MAdCAM-1, but also has epitopes for binding VCAM-1 and fibronectin, though it is known that VDZ does not affect the adhesion of α4β7 to VCAM-1[55].
The introduction of anti-TNF drugs in IBD treatment demonstrated superior therapeutic effects compared to conventional treatment. However, the development of new drugs is required for several reasons, including inadequate response, LOR or intolerance. The aim of developing new treatments for UC and CD is to produce targeted drugs that can enhance the clinical effect while reducing systemic adverse events. In this regard, anti-integrin agents are one of the most promising drug classes for IBD after anti-TNF agents. Anti-integrin agents inhibit the extravasation of lymphocytes by blocking the interactions between integrin and CAMs. The use of the initially developed natalizumab is limited use due to the risk of PML; however, VDZ developed later acts selectively on the intestine and shows few systemic adverse effects. VDZ has been approved and is currently used in clinical practice. Newer anti-integrin drugs that act on different targets associated with integrin, such as AJM300, abrilumab, etrolizumab, and PF-00547659 are also being developed and currently undergoing clinical trials.
In the future, clinical trials of anti-integrin drugs are expected to demonstrate their clinical efficacy, their place in the treatment of IBD, and their associated adverse effects. This will widen the range of drugs available to physicians and patients for treating IBD, and is an important step toward truly personalized treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Katsanos KH, Maric I, Papamichail K, Zouiten-Mekki L S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Atreya R, Neurath MF. IBD pathogenesis in 2014: Molecular pathways controlling barrier function in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol Ther. 2015;149:191-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1120] [Article Influence: 124.4] [Reference Citation Analysis (1)] |
| 4. | Coskun M, Steenholdt C, de Boer NK, Nielsen OH. Pharmacology and Optimization of Thiopurines and Methotrexate in Inflammatory Bowel Disease. Clin Pharmacokinet. 2016;55:257-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Danese S, Panés J. Development of drugs to target interactions between leukocytes and endothelial cells and treatment algorithms for inflammatory bowel diseases. Gastroenterology. 2014;147:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1978] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 7. | Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol. 2016;7:e135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 522] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 8. | Billiet T, Rutgeerts P, Ferrante M, Van Assche G, Vermeire S. Targeting TNF-α for the treatment of inflammatory bowel disease. Expert Opin Biol Ther. 2014;14:75-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 11. | Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic Review of Effects of Withdrawal of Immunomodulators or Biologic Agents From Patients With Inflammatory Bowel Disease. Gastroenterology. 2015;149:1716-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3136] [Article Influence: 174.2] [Reference Citation Analysis (1)] |
| 13. | Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Zundler S, Becker E, Weidinger C, Siegmund B. Anti-Adhesion Therapies in Inflammatory Bowel Disease-Molecular and Clinical Aspects. Front Immunol. 2017;8:891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M, Wolf DC, Hernandez C, Bornstein J, Sandborn WJ; International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 752] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 18. | Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 19. | Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 909] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 20. | Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, Iwakiri R, Watanabe K, Hibi T; AJM300 Study Group. Safety and Efficacy of AJM300, an Oral Antagonist of α4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology. 2015;149:1775-1783.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Döring A, Pfeiffer F, Meier M, Dehouck B, Tauber S, Deutsch U, Engelhardt B. TET inducible expression of the α4β7-integrin ligand MAdCAM-1 on the blood-brain barrier does not influence the immunopathogenesis of experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Milch C, Wyant T, Xu J, Parikh A, Kent W, Fox I , Berger J. Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol. 2013;264:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Wyant T, Leach T, Sankoh S, Wang Y, Paolino J, Pasetti MF, Feagan BG, Parikh A. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 25. | Feagan BG, Rubin DT, Danese S, Vermeire S, Abhyankar B, Sankoh S, James A, Smyth M. Efficacy of Vedolizumab Induction and Maintenance Therapy in Patients With Ulcerative Colitis, Regardless of Prior Exposure to Tumor Necrosis Factor Antagonists. Clin Gastroenterol Hepatol. 2017;15:229-239.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 26. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1568] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 27. | Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D'Haens G, Ben-Horin S, Xu J, Rosario M. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627 e613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Sands BE, Sandborn WJ, Van Assche G, Lukas M, Xu J, James A, Abhyankar B, Lasch K. Vedolizumab as Induction and Maintenance Therapy for Crohn's Disease in Patients Naive to or Who Have Failed Tumor Necrosis Factor Antagonist Therapy. Inflammatory bowel diseases. 2017;23:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 29. | Zundler S, Fischer A, Schillinger D, Binder MT, Atreya R, Rath T, Lopez-Pósadas R, Voskens CJ, Watson A, Atreya I . The α4β1 Homing Pathway Is Essential for Ileal Homing of Crohn’s Disease Effector T Cells In Vivo. Inflamm Bowel Dis. 2017;23:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Loftus EV Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D’Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Vermeire S, Loftus EV Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BE, Danese S, D’Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Crohn’s Disease. J Crohns Colitis. 2017;11:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Noman M, Ferrante M, Bisschops R, De Hertogh G, Van den Broeck K, Rans K, Rutgeerts P, Vermeire S, Van Assche G. Vedolizumab Induces Long-term Mucosal Healing in Patients With Crohn’s Disease and Ulcerative Colitis. J Crohns Colitis. 2017;11:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Danese S, Feagan B, Sandborn WJ, Colombel J-F, Vermeire S, Jones S, Brennan K, Bornstein J. OP023 A phase 3b open-label multicentre study (VERSIFY) of the efficacy of vedolizumab on endoscopic healing in moderately to severely active Crohn’s disease (CD). J Crohns Colitis. 2018;12 Suppl 1:S16-S17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 35. | Baumgart DC, Bokemeyer B, Drabik A, Stallmach A, Schreiber S; Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice--a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 36. | Amiot A, Grimaud JC, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R. Effectiveness and Safety of Vedolizumab Induction Therapy for Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2016;14:1593-1601.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 37. | Vickers AD, Ainsworth C, Mody R, Bergman A, Ling CS, Medjedovic J, Smyth M. Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis. PLoS One. 2016;11:e0165435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 39. | McAuliffe ME, Lanes S, Leach T, Parikh A, Faich G, Porter J, Holick C, Esposito D, Zhao Y, Fox I . Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin. 2015;31:1655-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Lissner D, Glauben R, Allers K, Sonnenberg E, Loddenkemper C, Schneider T, Siegmund B. Pulmonary Manifestation of Crohn’s Disease Developed Under Treatment With Vedolizumab. Am J Gastroenterol. 2018;113:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Bethge J, Meffert S, Ellrichmann M, Conrad C, Nikolaus S, Schreiber S. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4:e000127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Fischer S, Rath T, Geppert CI, Manger B, Schett G, Neurath MF, Atreya R. Long-term Combination Therapy with Anti-TNF plus Vedolizumab Induces and Maintains Remission in Therapy-refractory Ulcerative Colitis. Am J Gastroenterol. 2017;112:1621-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Rath T, Bojarski C, Neurath MF, Atreya R. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest Endosc. 2017;86:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Sandborn WJ, Cyrille M, Hansen MB, Feagan BG, Loftus Jr. EV, G. R, Vermeire S, Cruz ML, Yang J, Sullivan BA. OP034 Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: results of a phase 2b, randomised, double-blind, multiple-dose, placebo-controlled study. J Crohns Colitis. 2017;11 Suppl 1:S21-S22. [DOI] [Full Text] |
| 45. | Sandborn WJ, Cyrille M, Berner Hansen M, Feagan BG, Loftus Jr. EV, Vermeire S, Cruz ML, Sullivan BA, Reinisch W. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J Crohns Colitis. 2017;11 Suppl 1:S22-S23. [DOI] [Full Text] |
| 46. | Vermeire S, O’Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 354] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 47. | Tew GW, Hackney JA, Gibbons D, Lamb CA, Luca D, Egen JG, Diehl L, Eastham Anderson J, Vermeire S, Mansfield JC. Association Between Response to Etrolizumab and Expression of Integrin αE and Granzyme A in Colon Biopsies of Patients With Ulcerative Colitis. Gastroenterology. 2016;150:477-487.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 48. | Sandborn WJ, Panes J, Hassanali A, Jacob R, Sharafali Z, Oh YS, Tole S. LB03 Etrolizumab as Induction Therapy in Moderate to Severe Crohn’s Disease: Results From BERGAMOT Cohort 1. United Eur Gastroent. 2017;5 Suppl 1:1139. |
| 49. | Peyrin-Biroulet L, Rubin DT, Feagan BG, Oh YS, Arulmani U, Tyrrell H, Maciuca R, Williams S, Tole S, Thommes J. LB02 Etrolizumab induction therapy improved endoscopic score, patient-reported outcomes, and inflammatory biomarkers in patients with moderate to severe UC who had failed the antagonist therapy: results from the HICKORY open-label induction (OLI) trial. United Eur Gastroent. 2017;5 Suppl 1:1138-1139. |
| 50. | Zundler S, Schillinger D, Fischer A, Atreya R, López-Posadas R, Watson A, Neufert C, Atreya I , Neurath MF. Blockade of αEβ7 integrin suppresses accumulation of CD8+ and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut. 2017;66:1936-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Vermeire S, Sandborn WJ, Danese S, Hébuterne X, Salzberg BA, Klopocka M, Tarabar D, Vanasek T, Greguš M, Hellstern PA. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 53. | Laharie D. Towards therapeutic choices in ulcerative colitis. Lancet. 2017;390:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Sandborn WJ, Lee SD, Tarabar D, Louis E, Klopocka M, Klaus J, Reinisch W, Hébuterne X, Park DI, Schreiber S. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut. 2017;pii:gutjnl-2016-313457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 55. | Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |