Published online Apr 21, 2018. doi: 10.3748/wjg.v24.i15.1666
Peer-review started: February 6, 2018
First decision: February 24, 2018
Revised: March 8, 2018
Accepted: March 18, 2018
Article in press: March 18, 2018
Published online: April 21, 2018
Processing time: 72 Days and 19.5 Hours
To evaluate the impact of enhanced recovery after surgery (ERAS) programs on postoperative complications of pancreatic surgery.
Computer searches were performed in databases (including PubMed, Cochrane Library and Embase) for randomized controlled trials or case-control studies describing ERAS programs in patients undergoing pancreatic surgery published between January 1995 and August 2017. Two researchers independently evaluated the quality of the studies’ extracted data that met the inclusion criteria and performed a meta-analysis using RevMan5.3.5 software. Forest plots, demonstrating the outcomes of the ERAS group vs the control group after pancreatic surgery, and funnel plots were used to evaluate potential publication bias.
Twenty case-control studies including 3694 patients, published between January 1995 and August 2017, were selected for the meta-analysis. This study included the ERAS group (n = 1886) and the control group (n = 1808), which adopted the traditional perioperative management. Compared to the control group, the ERAS group had lower delayed gastric emptying rates [odds ratio (OR) = 0.58, 95% confidence interval (CI): 0.48-0.72, P < 0.00001], lower postoperative complication rates (OR = 0.57, 95%CI: 0.45-0.72, P < 0.00001), particularly for the mild postoperative complications (Clavien-Dindo I-II) (OR = 0.71, 95%CI: 0.58-0.88, P = 0.002), lower abdominal infection rates (OR = 0.70, 95%CI: 0.54-0.90, P = 0.006), and shorter postoperative length of hospital stay (PLOS) (WMD = -4.45, 95%CI: -5.99 to -2.91, P < 0.00001). However, there were no significant differences in complications, such as, postoperative pancreatic fistulas, moderate to severe complications (Clavien-Dindo III- V), mortality, readmission and unintended reoperation, in both groups.
The perioperative implementation of ERAS programs in pancreatic surgery is safe and effective, can decrease postoperative complication rates, and can promote recovery for patients.
Core tip: Enhanced recovery after surgery (ERAS) programs have been launched in a variety of surgical fields, including colorectal, orthopedics, urology, esophageal and gynecology, demonstrating favorable outcomes. Pancreatic surgery is considered a high-risk abdominal surgery, due to increased surgical trauma and high incidence of postoperative complications. In this meta-analysis we aimed to evaluate the impact of ERAS on complications of pancreatic surgery. The present study demonstrates that ERAS could reduce complication rates, especially of mild complications, delayed gastric emptying, abdominal infection and postoperative length of hospital stay, while not affecting the rates of postoperative pancreatic fistulas, reoperation, readmission and mortality during the perioperative period.
- Citation: Ji HB, Zhu WT, Wei Q, Wang XX, Wang HB, Chen QP. Impact of enhanced recovery after surgery programs on pancreatic surgery: A meta-analysis. World J Gastroenterol 2018; 24(15): 1666-1678
- URL: https://www.wjgnet.com/1007-9327/full/v24/i15/1666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i15.1666
Enhanced recovery after surgery (ERAS; also called ‘fast-track surgery’) was first introduced by Kehlet H, a Danish surgeon, in 1997[1]. ERAS is a multidisciplinary and evidence-based framework developed to decrease perioperative surgical stress, accelerate postoperative recovery and significantly reduce the postoperative length of hospital stay (PLOS). ERAS programs were initially implemented in colorectal surgery and have been shown to be effective for reducing PLOS and complications[2]. Subsequently, ERAS programs have been published in numerous areas of surgery, such as orthopedics, urology, esophageal, gynecology, breast and hepatobiliary[3-8].
An array of studies has shown that the perioperative implementation of ERAS programs can reduce PLOS without increasing complications or mortality. However, pancreatic surgery is still considered a high-risk abdominal surgery, due to the anatomical location of the pancreas and high rate of complications (30%-60%). Postoperative complications, such as postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE), abdominal infection, and so on, are the main reasons for delayed recovery and the frequent need for additional interventions, without which the complications are potentially life threatening. For these reasons, the implementation of ERAS programs has lagged for pancreatic surgeries.
There had been an increasing number of ERAS programs implemented in pancreatic surgery when the ERAS group published evidence-based consensus recommendations for pancreatic surgery in 2012[9]. The benefit of implementing ERAS programs on postoperative complications in pancreatic surgery has not reached consensus. For this reason, we performed a meta-analysis of the available studies on ERAS programs compared with traditional perioperative management in patients undergoing pancreatic surgery.
A search was performed by two researchers (Ji HB and Wang XX) in August 2017 of the PubMed, Cochrane Library and Embase database, spanning the period from January 1995 to August 2017. The search language was restricted to English, using the search terms “enhanced recovery after surgery”, “fast track surgery”, “ERAS”, “clinical pathways”, “pancreatectomy”, “pancreatoduodenectomy” and “duodenopancreatectomy”, and using the Boolean operators “AND” and “OR”. Synonyms of all these terms were used in this search. The PubMed search strategy for the meta-analysis is shown in Table 1.
| Search number | Description | Number of publications |
| 1 | Enhanced recovery after surgery [Title/Abstract] OR ERAS [Title/Abstract] OR fast track surgery [Title/Abstract] | 3333 |
| 2 | Clinical pathways [MeSH Terms] | 5848 |
| 3 | 1 OR 2 | 9130 |
| 4 | Pancreatectomy [MeSH Terms] OR Pancreatectomy* [Title/Abstract] OR Pancreatoduodenectomy [MeSH Terms] OR Pancreatoduodenectom* [Title/Abstract] OR duodenopancreatectomy [MeSH Terms] OR duodenopancreatectom* [Title/Abstract] | 21497 |
| 5 | 3 AND 4 NOT (animals[mh] NOT humans[mh]) | 69 |
| 6 | 5 limited to English | 68 |
Studies meeting all of the following selection criteria were eligible for inclusion: (1) studies concerning patients undergoing pancreatic surgery; (2) the ERAS group implemented ERAS programs management, and the control group adopted traditional perioperative management; (3) measures in perioperative management were described in both groups; and (4) studies reported at least the following outcome measures, POPF, DGE, abdominal infection, mortality and PLOS, and explained their diagnostic criteria for postoperative complications.
Exclusion criteria were (1) sample size of less than 10; (2) comments, guidelines, reviews, case reports, abstracts, letters and non-comparative studies; (3) repeated publication of the same study population; and (4) incomplete clinical data.
The outcomes of interest were POPF, DGE, PLOS, abdominal infection, mortality, readmission, unintended reoperation and occurrence of any complication within a postoperative period of 30 d. POPF was defined using the International Study Group of Pancreatic Fistula (ISGPF) guidelines describing a drain output of any measurable volume of fluid on or after postoperative day (POD) 3, with an amylase content greater than three times the serum amylase activity or as defined by the study’s authors[10]. DGE was defined according to the International Study Group of Pancreatic Surgery’s (ISGPS) recommendation that patients needing maintenance of a nasogastric tube (NGT) for > 3 d, needing to reinsert the NGT for persistent vomiting after POD 3, or unable to tolerate a solid diet by POD 7, should be considered DGE. In addition, there are another two widely used definitions for DGE after pancreatic resection (1) Yeo defined DGE as an NGT left in place for ≥ 10 d plus one of the following, or for < 10 d plus two of the following (a) repeated emesis after removal of the NGT, (b) need for prokinetic agents after POD 10, (c) need for reinsertion of the NGT, or (d) failure to progress with the diet. (2) Van Berge Henegouwen et al[11] defined DGE as gastric stasis requiring NGT for ≥ 10 d or the inability to tolerate a regular diet after POD 14. PLOS was defined as the span from the day of surgery to the day of actual discharge from the hospital. Abdominal infection was defined by the study’s authors. Mortality was defined as the range from the day of hospitalization to the first 30 d after actual discharge. Readmission was defined as the patient needing medical attention again within 30 d after discharge. Overall postoperative complications included any complication from the time of surgery to discharge, or within 30 d, with severity grading and classification relying on the Clavien-Dindo system[12]. Unintended reoperation was defined as patients with complications or other reasons that required reoperation within 30 d after discharge.
Data were extracted from each study by two authors (Ji HB and Wei Q) independently. The main parameters included common information (time of study publication, country, study type, and authors), characteristics of the study population (sex and age), elements of ERAS programs, and postoperative outcomes (overall complications, POPF, DGE, abdominal infection, PLOS, mortality, readmission, and unintended reoperation). All continuous outcome variables were described using the means and standard deviations for this meta-analysis. We needed to estimate means and standard deviations via the methodologies reported by Hozo et al[13] if the original data were expressed as medians or ranges.
The quality assessment of each study was done by two authors (Zhu WT and Ji HB) independently via the Methodological Index for Non-Randomized Studies (MINORS) checklist. It was then summarized by a French surgeon, and if there was a disagreement, the third researcher was involved in the negotiation or adjudication, until a consensus was achieved. The MINORS checklist includes eight methodological items for non-comparative studies and an additional four items for comparative studies. The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The overall ideal scores were 24 for comparative studies.
The meta-analysis was performed using RevMan5.3.5 software (Ji HB and Wang HB). Continuous and categorical variables were calculated as weighted mean differences (WMDs) or odds ratios (ORs) with their corresponding 95% confidence interval (CI), respectively. Heterogeneity was assessed using a chi-square test, where P > 0.05 was considered non-significant. I2 values were used for the evaluation of statistical heterogeneity, and the I2 value of 50% or more indicated the presence of heterogeneity. The fixed-effects model was used for studies of homogeneity (I2 < 50%), and the random-effects model was applied when studies indicated heterogeneity (I2 ≥ 50%). In addition, funnel plots were used to evaluate potential publication bias based on the incidence of POPF and mortality.
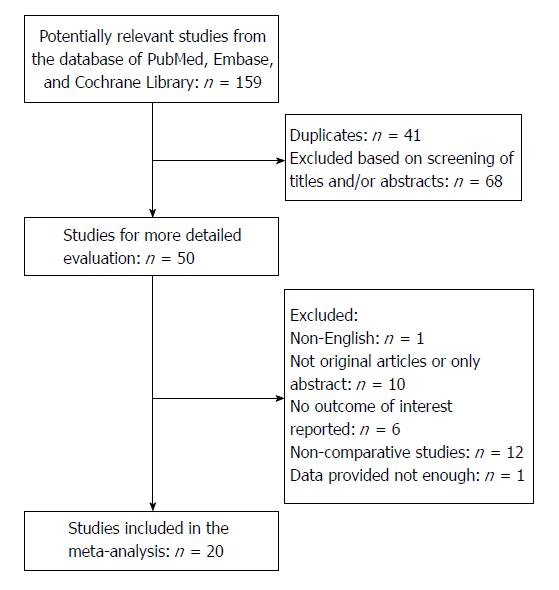
The search strategy initially identified 159 relevant studies. No randomized control trials were identified. Figure 1 shows the process of selecting the studies for meta-analysis. After removing duplicates, the titles and abstracts of 118 studies were reviewed. Of these, 68 studies were not related to ERAS in pancreatic surgery, 12 studies did not have a control group, 6 studies did not have the outcomes of interest reported, 10 studies only had an abstract or we were unable to get the full text, 1 study did not have enough data, and 1 study was published in a language other than English. A total of 20 studies met the inclusion criteria for the meta-analysis.
The characteristics and quality assessments of the included studies are shown in Table 2[14-33]. All studies clearly described an ERAS program. The major components are summarized in Table 2. All of the studies used a retrospective case-control model, and of those, there were 16 studies that had sample sizes greater than 100. A total of 3694 patients were included, of which there were 1886 patients and 1808 patients included in the ERAS group and control group, respectively. In addition, there were 17 studies with MINORS scores > 12.
| Study | Year | Country | Study design | Sample size | ERAS programs1 | MINORS Score | |
| ERAS group | Control group | ||||||
| Kennedy et al[14] | 2007 | United States | Case-control | 91 | 44 | e, f, g, h | 16/24 |
| Vanounou et al[15] | 2007 | United States | Case-control | 145 | 64 | c, d, g, h | 15/24 |
| Balzano et al[16] | 2008 | Italy | Case-control | 252 | 252 | d, e, f, g, h | 13/24 |
| Kennedy et al[17] | 2009 | United States | Case-control | 71 | 40 | d, e, f, g, h | 11/24 |
| Abu Hilal et al[18] | 2013 | Britain | Case-control | 20 | 24 | b, e, f, g, h | 15/24 |
| Braga et al[19] | 2014 | Italy | Case-control | 115 | 115 | a, b, c, d, e, f, g, h | 17/24 |
| Pillai et al[20] | 2014 | India | Case-control | 20 | 20 | c, d, e, f, g, h | 17/24 |
| Coolsen et al[21] | 2014 | Holland | Case-control | 86 | 97 | b, c, d, e, f, g, h | 12/24 |
| Nussbaum et al[22] | 2014 | United States | Case-control | 100 | 142 | c, e, f, g, h | 11/24 |
| Yui et al[23] | 2014 | Japan | Case-control | 57 | 52 | e, g, h | 13/24 |
| Nussbaum et al[24] | 2014 | United States | Case-control | 50 | 100 | c, e, f, g, h | 16/24 |
| Kobayashi et al[25] | 2014 | Japan | Case-control | 100 | 90 | a, e, g, h | 13/24 |
| Shao et al[26] | 2015 | China | Case-control | 325 | 310 | d, e, f, g, h | 15/24 |
| Joliat et al[27] | 2015 | Switzerland | Case-control | 74 | 87 | a, b, c, d, e, f, g, h | 15/24 |
| Partelli et al[28] | 2015 | Italy | Case-control | 22 | 66 | a, c, d, e, f, g, h | 13/24 |
| Williamsson et al[29] | 2015 | Sweden | Case-control | 50 | 50 | c, d, e, f, g, h | 17/24 |
| Morales Soriano et al[30] | 2015 | Spain | Case-control | 41 | 44 | a, b, c, d, e, f, g, h | 17/24 |
| Bai et al[31] | 2016 | China | Case-control | 124 | 63 | a, d, e, f, g, h | 15/24 |
| Zouros et al[32] | 2016 | Greece | Case-control | 75 | 50 | a, b, c, d, e, f, g, h | 16/24 |
| Dai et al[33] | 2017 | China | Case-control | 68 | 98 | a, b, c, e, f, g, h | 15/24 |
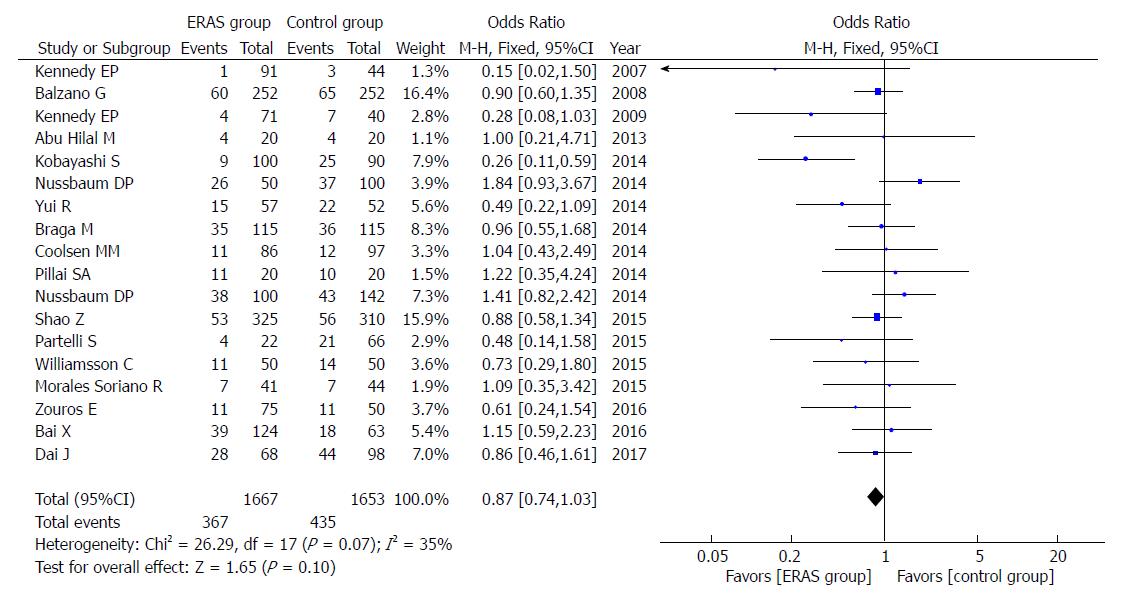
Eighteen studies reported the rates of POPF. The overall results (OR = 0.87, 95%CI: 0.74-1.03, P = 0.10; Figure 2), or only those using the ISGPF definition (OR = 0.90, 95%CI: 0.76-1.07, P = 0.24), showed that there were no significant differences present in either group. Furthermore, there was no significant difference in A (OR = 1.05, 95%CI: 0.81-1.36, P = 0.71), B (OR = 1.13, 95%CI: 0.85-1.51, P = 0.40), and C (OR = 0.90, 95%CI: 0.60-1.33, P = 0.59) grade of POPF between the ERAS group and control group.
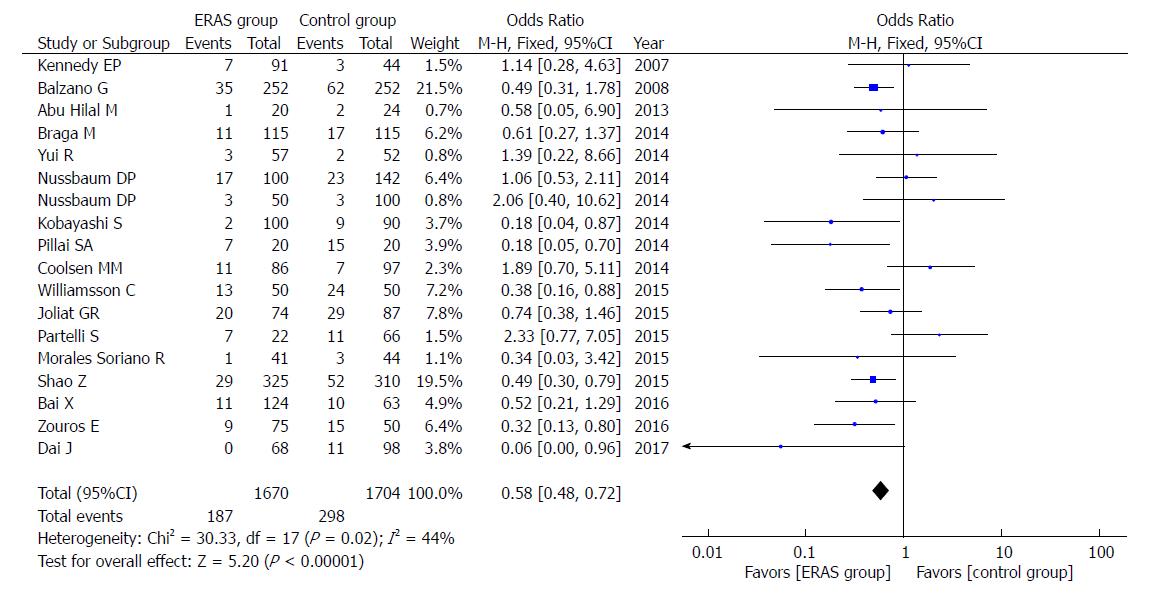
Eighteen studies reported the rates of DGE. Compared to the control group, the ERAS group had a lower incidence of DGE (OR = 0.58, 95%CI: 0.48-0.72, P < 0.00001; Figure 3). The difference persisted when including only studies that adopted the ISGPS definition (OR = 0.50, 95%CI: 0.39-0.65, P < 0.00001).
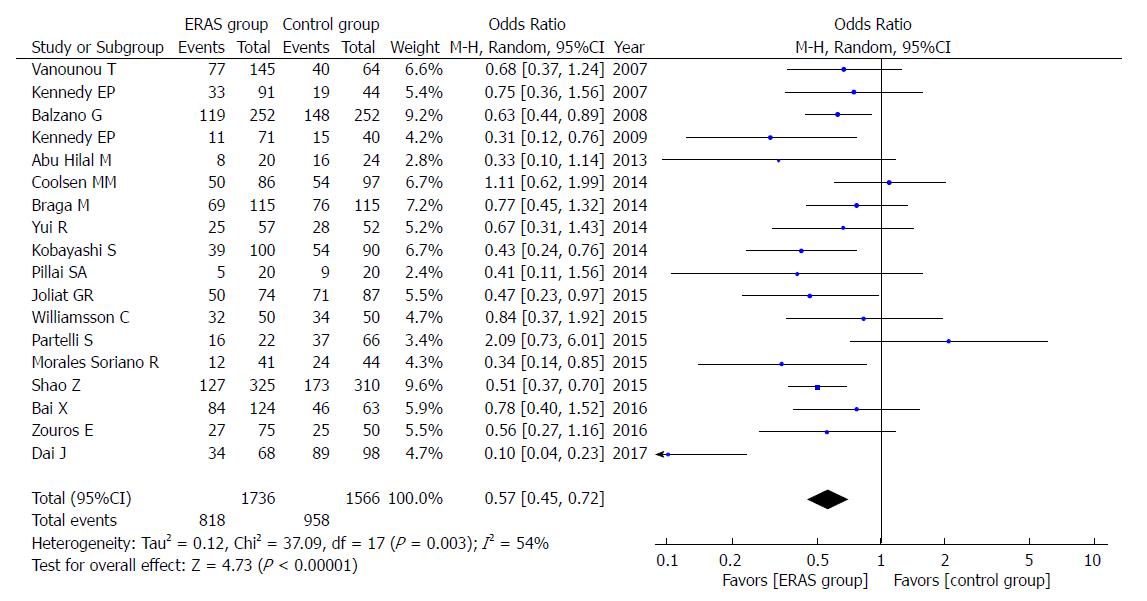
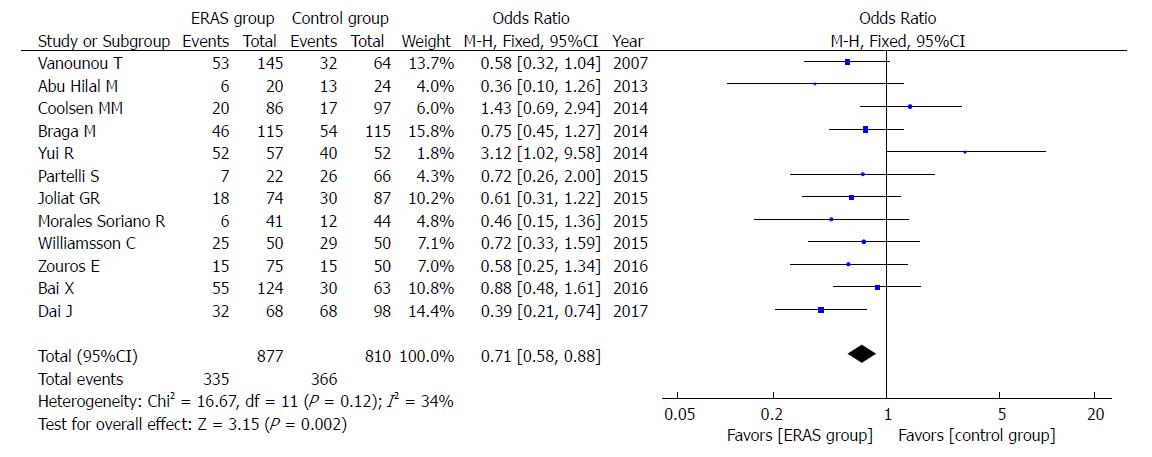
The rate of overall postoperative complications was lower in the ERAS group (OR = 0.57, 95%CI: 0.45-0.72, P < 0.00001; Figure 4). Additionally, the incidence of mild postoperative complications (Clavien-Dindo I-II), which relies on the Clavien-Dindo definition of severity and classification, was lower in the ERAS group (OR = 0.71, 95%CI: 0.58-0.88, P = 0.002; Figure 5). There were no statistical differences in the moderate to severe complication rates (Clavien-Dindo III-V) between the ERAS group and control group (OR = 0.90, 95%CI: 0.73-1.11, P = 0.32).
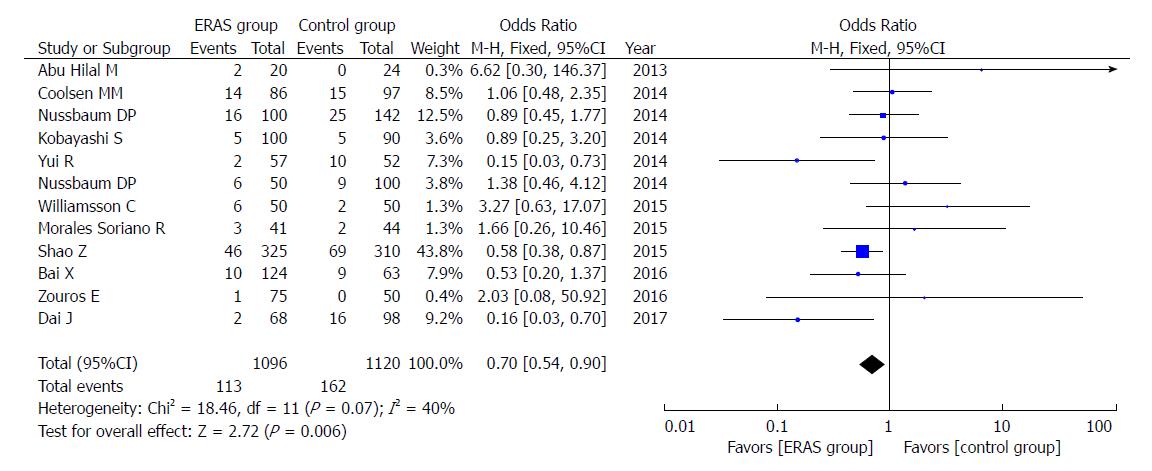
A total of 12 studies reported the rates of abdominal infection. The incidence of abdominal infection was lower (OR = 0.70, 95%CI: 0.54-0.90, P = 0.006; Figure 6) in the ERAS group.
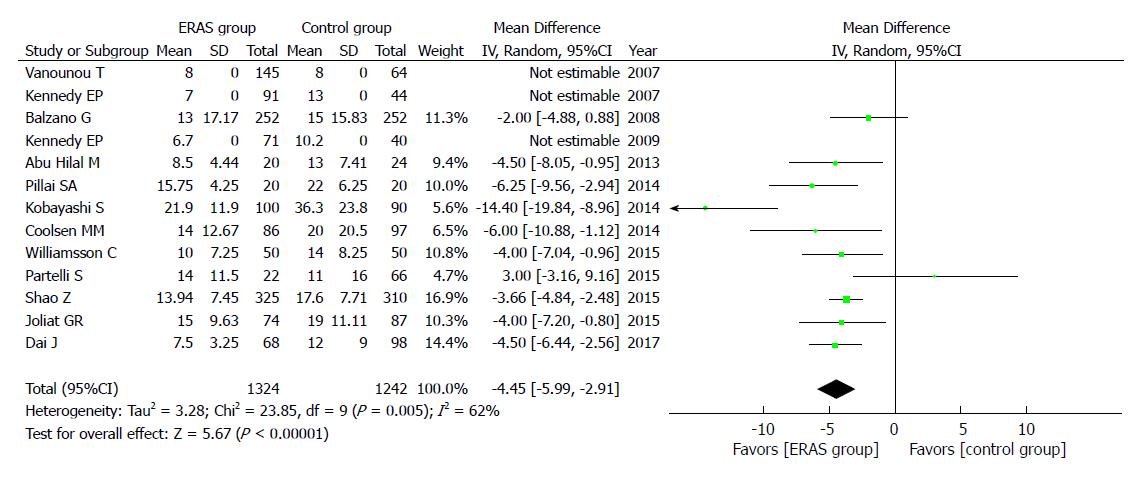
A total of 13 studies reported the PLOS, and they showed that the ERAS group had shorter PLOS (WMD = -4.45, 95%CI: -5.99 to -2.91, P < 0.00001; Figure 7) than the control group.
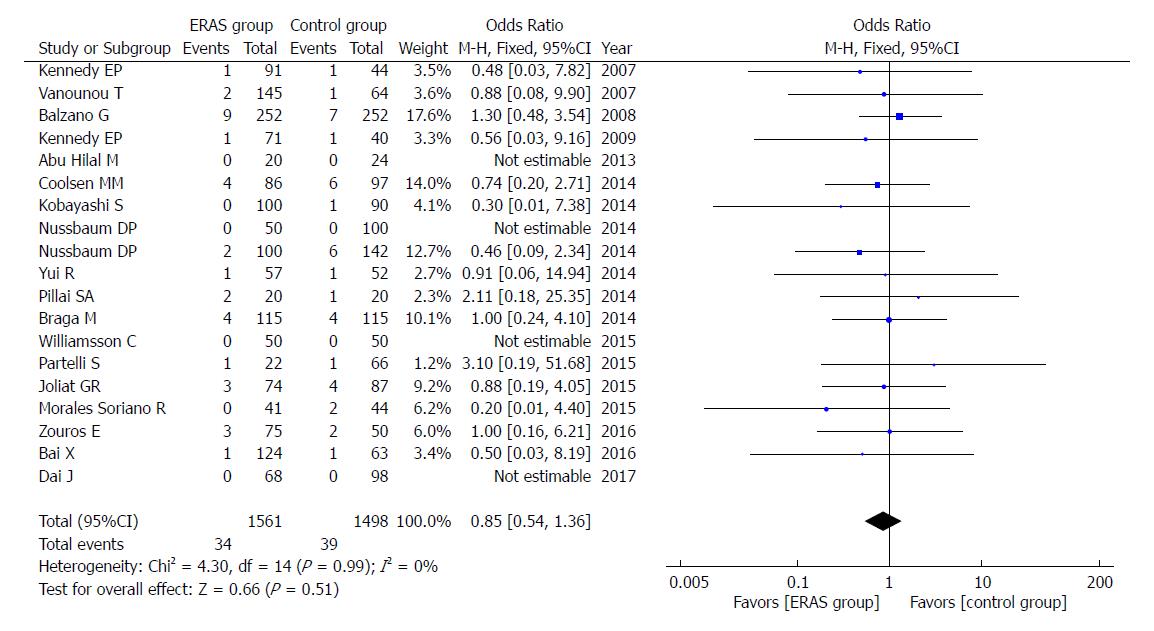
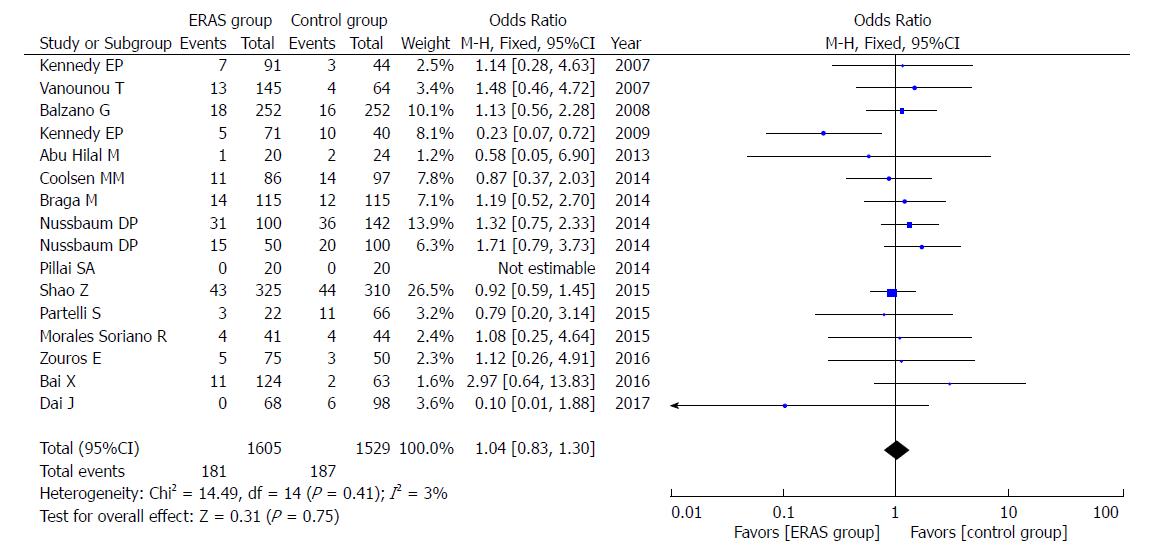
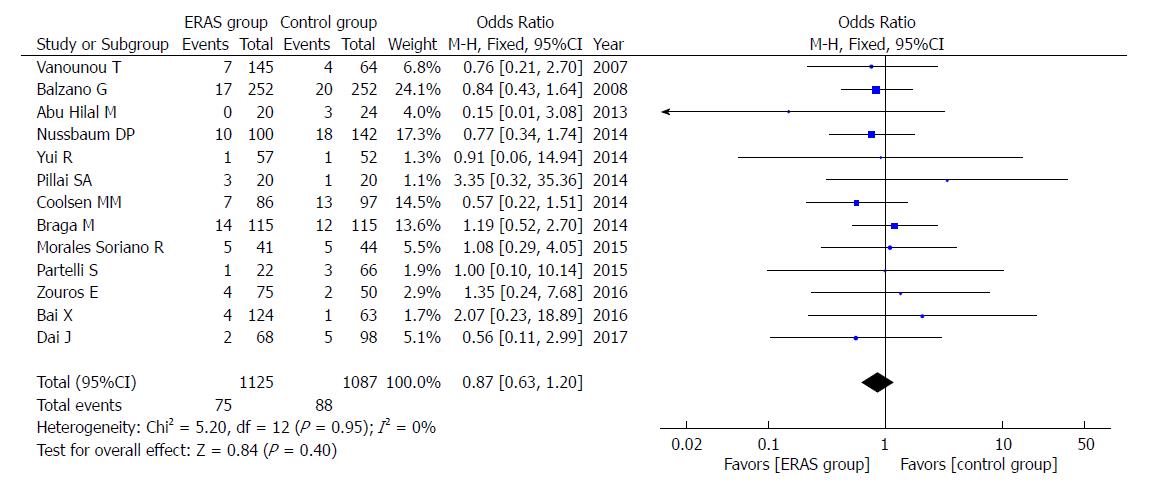
In addition, there were no significant differences in rates of mortality (OR = 0.85, 95%CI: 0.54-1.36, P = 0.51; Figure 8), readmission (OR = 1.04, 95%CI: 0.83-1.30, P = 0.75; Figure 9), and unintended reoperation (OR = 0.87, 95%CI: 0.63-1.20, P = 0.40; Figure 10).
The subgroup analysis, which included only larger size studies (n ≥ 100) generated similar results in postoperative outcomes (Table 3). Furthermore, the analysis of only high-quality studies (MINORS score > 12) also yielded parallel results in postoperative outcomes (Table 3). However, the heterogeneity for overall complications and PLOS still exists in larger studies and high-quality studies.
| Outcomes of interest | Studies | Patients | OR/WMD | 95%CI | P-value | Heterogeneity P-value | I2, % |
| Studies with cases ≥ 100 | |||||||
| POPF | 14 | 3067 | 0.87 | 0.73-1.03 | 0.11 | 0.02 | 48 |
| DGE | 14 | 3117 | 0.58 | 0.47-0.71 | < 0.00001 | 0.07 | 39 |
| Overall complications | 14 | 3045 | 0.57 | 0.45-0.72 | < 0.00001 | 0.006 | 55 |
| Mild complications | 9 | 1470 | 0.74 | 0.59-0.93 | 0.009 | 0.06 | 46 |
| Abdominal infection | 10 | 2087 | 0.67 | 0.51-0.87 | 0.003 | 0.07 | 42 |
| PLOS | 10 | 2394 | -4.64 | -6.37 to -2.91 | < 0.00001 | 0.009 | 65 |
| Mortality | 15 | 2802 | 0.83 | 0.51-1.37 | 0.47 | 1 | 0 |
| Readmission | 12 | 2877 | 1.05 | 0.83-1.33 | 0.68 | 0.23 | 22 |
| Unintended reoperation | 9 | 1955 | 0.85 | 0.60-1.21 | 0.38 | 0.96 | 0 |
| MINORS score > 12 | |||||||
| POPF | 15 | 2784 | 0.84 | 0.70-1.00 | 0.05 | 0.13 | 30 |
| DGE | 16 | 2949 | 0.52 | 0.42-0.64 | < 0.00001 | 0.12 | 31 |
| Overall complications | 16 | 3008 | 0.56 | 0.44-0.71 | < 0.00001 | 0.01 | 51 |
| Mild complications | 11 | 1504 | 0.67 | 0.54-0.83 | 0.0003 | 0.24 | 21 |
| Abdominal infection | 10 | 1791 | 0.63 | 0.46-0.85 | 0.002 | 0.05 | 46 |
| PLOS | 11 | 2272 | -4.35 | -5.97 to -2.72 | < 0.00001 | 0.003 | 66 |
| Mortality | 16 | 2523 | 0.96 | 0.56-1.65 | 0.89 | 0.99 | 0 |
| Readmission | 13 | 2598 | 1.09 | 0.84-1.43 | 0.52 | 0.82 | 0 |
| Unintended reoperation | 11 | 1787 | 0.96 | 0.65-1.41 | 0.83 | 0.94 | 0 |
We aimed to investigate the influence of a single study on the overall results by omitting one study in each turn. This analysis revealed that no single study generated an especially strong influence on the results, with estimates ranging from an OR of 0.54 to 0.62 (Table 4).
| Studies | OR | 95%CI | P-value |
| Omitting Vanounou et al[15] | 0.56 | 0.44-0.72 | < 0.00001 |
| Omitting Kennedy et al[14] | 0.56 | 0.44-0.71 | < 0.00001 |
| Omitting Balzano et al[16] | 0.56 | 0.43-0.73 | < 0.0001 |
| Omitting Kennedy et al[17] | 0.58 | 0.46-0.74 | < 0.00001 |
| Omitting Abu Hilal et al[18] | 0.58 | 0.45-0.73 | < 0.00001 |
| Omitting Yui et al[23] | 0.56 | 0.44-0.72 | < 0.00001 |
| Omitting Kobayashi et al[25] | 0.58 | 0.45-0.74 | < 0.00001 |
| Omitting Coolsen et al[21] | 0.54 | 0.43-0.69 | < 0.00001 |
| Omitting Braga et al[19] | 0.55 | 0.43-0.71 | < 0.00001 |
| Omitting Pillai et al[20] | 0.57 | 0.45-0.73 | < 0.00001 |
| Omitting Joliat et al[27] | 0.57 | 0.45-0.73 | < 0.0001 |
| Omitting Partelli et al[28] | 0.55 | 0.44-0.68 | < 0.00001 |
| Omitting Williamsson et al[29] | 0.56 | 0.44-0.71 | < 0.00001 |
| Omitting Morales Soriano et al[30] | 0.58 | 0.46-0.74 | < 0.00001 |
| Omitting Shao et al[26] | 0.57 | 0.44-0.74 | < 0.0001 |
| Omitting Zouros et al[32] | 0.57 | 0.44-0.73 | < 0.00001 |
| Omitting Bai et al[31] | 0.56 | 0.44-0.71 | < 0.00001 |
| Omitting Dai et al[33] | 0.62 | 0.52-0.71 | < 0.00001 |
| Overall effect | 0.57 | 0.45-0.72 | < 0.00001 |
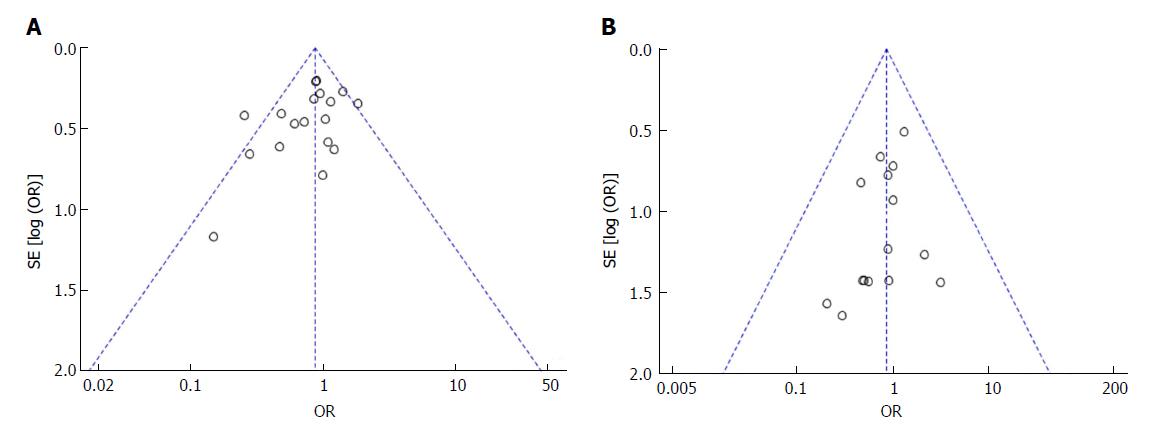
Funnel plots based on the incidence of POPF and mortality were used to evaluate potential publication bias in this study (Figure 11). There was no evidence of publication bias of POPF, mortality or other outcomes of this study (other figures not shown).
ERAS requires surgical, nursing, anesthesia, nutritionist and other specialties to work together and uses a series of optimal and evidence-based management measures to lessen perioperative surgical stress while promoting the recovery of organ function in the early postoperative period[34,35]. ERAS programs were initially implemented in colorectal surgery, with recommendations for each step to achieve optimal perioperative care[36]. Subsequently, ERAS programs had been launched in numerous fields of surgery, such as orthopedics, urology, esophageal and gynecology.
The literature from these disciplines has suggested that standardizing ERAS measures could reduce the incidence of complications, accelerate recovery for patients, reduce hospitalization costs and save medical resources in perioperative care[3,4,7,8]. Pancreatic surgery is an effective treatment of pancreatic tumors, periampullary tumors, duodenal tumors and distal bile duct tumors. Currently, despite surgical techniques, anesthesia, and preoperative imaging assessment making great progress and the mortality of the procedure dropping to approximately 2% in high-volume medical centers, it is still considered a complicated and high-risk abdominal surgery[37].
Coolsen et al[38] analyzed 8 studies, which related to pancreatic surgery, and suggested that the ERAS group had shorter PLOS and lower postoperative complication rates; however, there were no significant differences in rates of DGE, POPF, readmission, and mortality. Kagedan et al[39] analyzed 10 studies suggesting that the ERAS group had only shorter PLOS and no differences in other complications. As mentioned above, we may reasonably conclude that the influence of ERAS programs on the postoperative complications of pancreatic surgery is controversial. Hence, the application of ERAS programs in the perioperative period of pancreatic surgery is still being explored in our practices.
The main measures of the ERAS programs include no bowel preparation and clear fluids until 2-3 h before surgery, multimodal analgesia of postoperative, clear fluids or food intakes, enhanced mobilization and removal of the drainage tube in early period. The ERAS group has reduced time of fasting in the preoperative period, which can decrease the insulin resistance in the postoperative period. We adopted multimodal analgesia in the postoperative period, which was able to reduce the stress caused by pain. The programs, such as, no bowel preparation before surgery, clear fluids or food intakes, enhanced mobilization in the early postoperative period which may promote rehabilitation of gastrointestinal function[40].
The ERAS programs aimed to reduce the incidence of complications and accelerate recovery for patients. Among them, gastrointestinal function rehabilitation is an important part of the rapid recovery in abdominal surgery. In addition, the early postoperative oral feeding, which may play an important role in the gastrointestinal function rehabilitation in the postoperative period. This is because early postoperative oral feeding is more in line with human physiology of the digestive tract, and which may have a beneficial effect on immunological, inflammatory and nutritional status. In addition, early postoperative oral feeding can promote the recovery of gastrointestinal motility, protect the gastrointestinal mucosal barrier, shorten time to gas and stools passage, and reduce the incidence of complications.
A total of 20 studies and 3694 patients were included in our meta-analysis. Compared with the control group, the ERAS group had lower rates of DGE, lower postoperative complication rates, particularly lower mild postoperative complication rates, lower abdominal infection rates, and shorter PLOS. However, no significant differences existed in POPF, moderate to severe complications, mortality, readmission or unintended reoperation in both groups.
Many factors, such as age, nutritional status, and serious comorbidity, can influence patients’ postoperative complication rates and the process of postoperative recovery[41,42]. The patients’ demographic data in the included studies was basically identical, so these influences may be eliminated for the outcomes in this study. In addition, all of the included studies described the diagnostic criteria for postoperative complications.
Despite our careful work on this meta-analysis of currently available evidence, some limitations should be acknowledged. First, the diagnostic criteria of some postoperative complications were not uniformly defined, though all the included studies gave a description of the diagnostic criteria. Therefore, to a certain extent, information bias was possible, because some complications did not have national criteria. Second, only retrospective case control studies were included in this analysis. Therefore, to a certain extent, the outcomes of this study may be influenced by the selection bias. Third, the degree of implementation of ERAS programs and the compliance of patients may be different between studies. Finally, there was no evidence to indicate that major publication bias existed in these studies, and potential publication bias is impossible to completely rule out in small studies. Hence, these factors had some influence on our results.
In summary, the results from our present study demonstrate that the implementation of ERAS programs could reduce overall complication rates, especially of mild complications, DGE, rates of abdominal infection, and PLOS, while not affecting the rates of POPF, reoperation, readmission, and mortality during the perioperative period for pancreatic surgery. The perioperative period for pancreatic surgery is safe and effective to implement ERAS programs that can decrease postoperative complication rates and promote recovery. However, in the future, we need to include more high-quality and strict prospective studies to assess the contributions of individual program components.
Enhanced recovery after surgery (ERAS) is a multidisciplinary and evidence-based framework, developed to decrease perioperative surgical stress, accelerate postoperative recovery and significantly reduce the postoperative length of hospital stay (PLOS). ERAS programs have been launched in a variety of other fields of surgery, such as colorectal, orthopedics, urology, esophageal, and gynecology, and have demonstrated favorable outcomes. The implementation of ERAS programs has lagged surrounding pancreatic surgeries because of the anatomical location of the pancreas and the high rate of postoperative complications (30%-60%). It is very important to promote the postoperative recovery for this high-risk abdominal surgery via implementing ERAS programs during the perioperational period.
ERAS requires surgical, nursing, anesthesia and other specialties to work together and uses a series of optimal or evidence-based management measures to lessen perioperative surgical stress while promoting the recovery of organ function in the early postoperative period. The implementation of ERAS programs may play a very important role in the perioperational period for pancreatic surgery.
This study evaluated the impact of ERAS programs on postoperative complications and PLOS of pancreatic surgery.
Computer searches were performed in databases (including PubMed, Cochrane Library, and Embase) for randomized controlled trials or case-control studies describing ERAS programs in patients undergoing pancreatic surgery published between January 1995 and August 2017. Two researchers independently evaluated the quality of the studies’ extracted data that met inclusion criteria and performed a meta-analysis using RevMan5.3.5 software. Forest plots, demonstrating the outcomes of the ERAS group versus the control group after pancreatic surgery, and funnel plots were used to evaluate potential publication bias.
Twenty case-control studies, published between January 1995 and August 2017, including 3694 patients, were selected for the meta-analysis. They included the ERAS group (n = 1886) and control group (n = 1808), which adopted the traditional perioperative management. Compared to the control group, the ERAS group had lower delayed gastric emptying (DGE) rates (odds ratio (OR) = 0.58, 95% confidence interval (CI): 0.48-0.72, P < 0.00001), lower postoperative complication rates (OR = 0.57, 95%CI: 0.45-0.72, P < 0.00001), particularly for mild postoperative complications (Clavien-Dindo I- II) (OR = 0.71, 95%CI: 0.58-0.88, P = 0.002), lower abdominal infection rates (OR = 0.70, 95%CI: 0.54-0.90, P = 0.006) and shorter PLOS (weighted mean difference (WMD) = -4.45, 95%CI: -5.99 to -2.91, P < 0.00001). However, there were no significant differences in postoperative pancreatic fistulas (POPF), moderate to severe complications (Clavien-Dindo III- IV), mortality, readmission and unintended reoperation in both groups.
The results from our present study demonstrate that the implementation of ERAS programs could reduce overall complication rates, especially of mild complications, DGE, rate of abdominal infection and PLOS, while not affecting the rates of POPF, reoperation, readmission and mortality during the perioperative period for pancreatic surgery. The perioperative period for pancreatic surgery is safe and effective to implement ERAS programs that can decrease postoperative complication rates and promote recovery
We need to include more high-quality and strict prospective studies to assess the contributions of individual program components, such as clear fluids or food intakes in the early period, and removal of the drainage tube.
Manuscript source: Unsolicited manuscript.
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
PRISMA 2009 Checklist: The authors have read and revised according to the PRISMA 2009 Checklist.
P- Reviewer: Cesaretti M, Mastoraki A, Negoi I, Tang Y, Wani IA S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [PubMed] |
| 2. | Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbølle P, Hendel HW, Rosenberg J, Kehlet H. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Barbieri A, Vanhaecht K, Van Herck P, Sermeus W, Faggiano F, Marchisio S, Panella M. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Azhar RA, Bochner B, Catto J, Goh AC, Kelly J, Patel HD, Pruthi RS, Thalmann GN, Desai M. Enhanced recovery after urological surgery: a contemporary systematic review of outcomes, key elements, and research needs. Eur Urol. 2016;70:176-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 5. | Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB (Oxford). 2014;16:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Pisarska M, Małczak P, Major P, Wysocki M, Budzyński A, Pędziwiatr M. Enhanced recovery after surgery protocol in oesophageal cancer surgery: systematic review and meta-analysis. PLoS One. 2017;12:e0174382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | de Groot JJ, Ament SM, Maessen JM, Dejong CH, Kleijnen JM, Slangen BF. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2016;95:382-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 11. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2327] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 13. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6888] [Article Influence: 344.4] [Reference Citation Analysis (0)] |
| 14. | Kennedy EP, Rosato EL, Sauter PK, Rosenberg LM, Doria C, Marino IR, Chojnacki KA, Berger AC, Yeo CJ. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution--the first step in multidisciplinary team building. J Am Coll Surg. 2007;204:917-23; discussion 923-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Vanounou T, Pratt W, Fischer JE, Vollmer CM Jr, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg. 2007;204:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Kennedy EP, Grenda TR, Sauter PK, Rosato EL, Chojnacki KA, Rosato FE Jr, Profeta BC, Doria C, Berger AC, Yeo CJ. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg. 2009;13:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Abu Hilal M, Di Fabio F, Badran A, Alsaati H, Clarke H, Fecher I, Armstrong TH, Johnson CD, Pearce NW. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology. 2013;13:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Braga M, Pecorelli N, Ariotti R, Capretti G, Greco M, Balzano G, Castoldi R, Beretta L. Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg. 2014;38:2960-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Pillai SA, Palaniappan R, Pichaimuthu A, Rajendran KK, Sathyanesan J, Govindhan M. Feasibility of implementing fast-track surgery in pancreaticoduodenectomy with pancreaticogastrostomy for reconstruction--a prospective cohort study with historical control. Int J Surg. 2014;12:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Coolsen MM, van Dam RM, Chigharoe A, Olde Damink SW, Dejong CH. Improving outcome after pancreaticoduodenectomy: experiences with implementing an enhanced recovery after surgery (ERAS) program. Dig Surg. 2014;31:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Nussbaum DP, Penne K, Stinnett SS, Speicher PJ, Cocieru A, Blazer DG 3rd, Zani S, Clary BM, Tyler DS, White RR. A standardized care plan is associated with shorter hospital length of stay in patients undergoing pancreaticoduodenectomy. J Surg Res. 2015;193:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Yui R, Satoi S, Toyokawa H, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Ryota H, Michiura T, Inoue K. Less morbidity after introduction of a new departmental policy for patients who undergo open distal pancreatectomy. J Hepatobiliary Pancreat Sci. 2014;21:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Nussbaum DP, Penne K, Speicher PJ, Stinnett SS, Perez A, White RR, Clary BM, Tyler DS, Blazer DG 3rd. The role of clinical care pathways: an experience with distal pancreatectomy. J Surg Res. 2014;190:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kobayashi S, Ooshima R, Koizumi S, Katayama M, Sakurai J, Watanabe T, Nakano H, Imaizumi T, Otsubo T. Perioperative care with fast-track management in patients undergoing pancreaticoduodenectomy. World J Surg. 2014;38:2430-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Shao Z, Jin G, Ji W, Shen L, Hu X. The role of fast-track surgery in pancreaticoduodenectomy: a retrospective cohort study of 635 consecutive resections. Int J Surg. 2015;15:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Joliat GR, Labgaa I, Petermann D, Hübner M, Griesser AC, Demartines N, Schäfer M. Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg. 2015;102:1676-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Partelli S, Crippa S, Castagnani R, Ruffo G, Marmorale C, Franconi AM, De Angelis C, Falconi M. Evaluation of an enhanced recovery protocol after pancreaticoduodenectomy in elderly patients. HPB (Oxford). 2016;18:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Williamsson C, Karlsson N, Sturesson C, Lindell G, Andersson R, Tingstedt B. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg. 2015;102:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Morales Soriano R, Esteve Pérez N, Tejada Gavela S, Cuadrado García Á, Rodríguez Pino JC, Morón Canis JM, Molina Romero X, Muñoz Pérez J, González Argente X. Outcomes of an enhanced recovery after surgery programme for pancreaticoduodenectomy. Cir Esp. 2015;93:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Bai X, Zhang X, Lu F, Li G, Gao S, Lou J, Zhang Y, Ma T, Wang J, Chen W. The implementation of an enhanced recovery after surgery (ERAS) program following pancreatic surgery in an academic medical center of China. Pancreatology. 2016;16:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Zouros E, Liakakos T, Machairas A, Patapis P, Agalianos C, Dervenis C. Improvement of gastric emptying by enhanced recovery after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int. 2016;15:198-208. [PubMed] |
| 33. | Dai J, Jiang Y, Fu D. Reducing postoperative complications and improving clinical outcome: Enhanced recovery after surgery in pancreaticoduodenectomy - a retrospective cohort study. Int J Surg. 2017;39:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473-476. [PubMed] |
| 35. | Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641. [PubMed] |
| 36. | Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 799] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 37. | Barton JG. Enhanced recovery pathways in pancreatic surgery. Surg Clin North Am. 2016;96:1301-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Coolsen MM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg. 2013;37:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Kagedan DJ, Ahmed M, Devitt KS, Wei AC. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Martos-Benítez FD, Gutiérrez-Noyola A, Soto-García A, González-Martínez I, Betancourt-Plaza I. Program of gastrointestinal rehabilitation and early postoperative enteral nutrition: a prospective study. Updates Surg. 2018;70:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Kobayashi S, Segami K, Hoshino H, Nakahara K, Katayama M, Koizumi S, Otsubo T. Risk factors for failure of early recovery from pancreatoduodenectomy despite the use of enhanced recovery after surgery protocols and a physical aging score to predict postoperative risks. J Hepatobiliary Pancreat Sci. 2018;25:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |