Published online Apr 21, 2018. doi: 10.3748/wjg.v24.i15.1641
Peer-review started: January 4, 2018
First decision: January 16, 2018
Revised: March 10, 2018
Accepted: March 25, 2018
Article in press: March 25, 2018
Published online: April 21, 2018
Processing time: 105 Days and 13.1 Hours
To investigate novel predictors of survival in hepatocellular carcinoma (HCC) patients following transarterial chemoembolization (TACE).
One hundred sixty seven patients with un-resectable HCC were retrospectively analyzed to identify factors that might contribute to their HCC biology and aggressiveness. We correlated routine laboratory results (total bilirubin, AST, ALKP, GGTP, albumin etc.) to maximum tumor diameter, number of tumor nodules, portal vein thrombosis and blood alpha-fetoprotein levels. These 4 parameters were previously combined to form an aggressiveness index (AgI). We used The Wilcoxon rank-sum (Mann-Whitney), to test the correlation between the AgI categories and liver function parameters. The Cox proportional hazards model was applied to evaluate the categories of AgI associated with overall survival.
The AgI was strongly correlated with survival in this novel patient population. Three year survival probability for AgI > or < 4 was 42.4% vs 61.8%; P < 0.0863 respectively. Several factors independently correlated with AgI using univariate multiple logistic regression of AgI with 8 laboratory parameters. Lower albumin levels had an OR of 2.56 (95%CI: 1.120-5.863 P < 0.026), elevated Alkaline phosphatase and gamma glutamyl transpeptidase (GGTP) had ORs of 1.01 (95%CI: 1.003-1.026, P < 0.017) and 0.99 (95%CI: 0.99-1.00, P < 0.053) respectively. In a Cox proportional hazard model combining mortality for AgI score and liver function parameters, only GGTP levels and the AgI were independently associated with survival. An AgI > 4 had HR for mortality of 2.18 (95%CI: 1.108-4.310, P < 0.024). GGTP’s single unit change had a HR for mortality of 1.003 (95%CI: 1.001-1.006, P < 0.016). These were considered in the final multivariate model with the total cohort. An AgI > 4 had a HR for mortality of 2.26 (95%CI: 1.184-4.327, P < 0.016). GGTP had a HR of 1.003 (95%CI: 1.001-1.004, P < 0.001).
Our study validates the AgI in a new population with un-resectable HCC patients undergoing TACE. The analysis establishes a correlation between GGTP and the AgI.
Core tip: Our cohort’s population included patients with multiple underlying liver diseases and can be widely generalized. The aggressiveness index (AgI) was correlated with survival. AgI > 4 was associated with decreased survival. Combining the AgI with elevated GGTP and ALKP levels improved its prognostic yield in our patient population. We validated the AgI as a prognostic tool to predict overall survival in a novel population of hepatocellular carcinoma patients undergoing transarterial chemoembolization.
- Citation: Ventura Y, Carr BI, Kori I, Guerra V, Shibolet O. Analysis of aggressiveness factors in hepatocellular carcinoma patients undergoing transarterial chemoembolization. World J Gastroenterol 2018; 24(15): 1641-1649
- URL: https://www.wjgnet.com/1007-9327/full/v24/i15/1641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i15.1641
Hepatocellular carcinoma (HCC) is the fourth most common cancer and the third leading cause of cancer-related deaths in the world[1]. In the last several decades the incidence of HCC in developed countries has been rising, secondary to an increased incidence of HCV and non alcoholic steatohepatitis (NASH) associated cirrhosis[2]. The annual Nation Cancer Report of the United States published in 2016, noted that between 2003 and 2012, in contrast to the general decline in cancer incidence rates, HCC incidence continues to rise. Most cases of HCC arise on the background of chronic liver disease. Patients are usually asymptomatic until late in their disease, when symptoms and signs related to their cirrhosis are manifested. Early detection of HCC can be accomplished by screening populations at risk. The recommended surveillance of cirrhotic patients is abdominal ultrasound every six-months[3]. Measuring levels of alpha fetoprotein (AFP) a serum marker for HCC can be used together with ultrasonography. However, due to its low sensitivity and specificity it was recently omitted from clinical practice guidelines[4]. A staging system introduced by the Barcelona Clinic Liver Cancer (BCLC), is currently recommended as the best method for HCC staging and treatment allocation. The system incorporates the dimensions of the primary lesion, vascular invasion, extra-hepatic spread, performance status, general symptoms and the degree of severity of the underlying liver disease according to the Child-Pugh-Turcot score[5].
Trans-arterial chemoembolization (TACE) is performed by catheterization of tumor feeding branches of the hepatic artery and injecting chemotherapy with Lipidol. After the injection, the artery is embolized by particles. The TACE procedure is the treatment of choice for non-operable, intermediate stage HCC according to the BCLC classification[6]. Survival after the procedure varies and ranges between 12 to 34 mo[7]. Given the complexity of TACE and the variability in response, there is an urgent need to identify prognostic indices to predict overall survival in HCC patients undergoing the procedure[8]. Current prognostic indices include different inflammation scores such as the Glasgow prognostic score (GPS), neutrophil to lymphocyte ratio (NLR) and staging systems such as Barcelona Clinic Liver Cancer (BCLC), and Cancer of the Liver Italian Program (CLIP) scores. The GPS score was demonstrated as an independent marker of poor prognosis in patients with HCC and as a prognostic score predicting survival for patients with HBV related HCC after TACE[9-14]. All these indices have their shortcomings with some lacking strong prognostic power (even when combined), and others lacking validation and or limited to specific populations.
An “HCC Aggressiveness” scoring system was recently described, which incorporates 4 tumor-related parameters: maximum tumor diameter (MTD), number of tumor nodules, portal vein thrombosis (PVT) and serum AFP levels. The score was shown to predict survival in HCC patients[15-17].
We retrospectively analyzed laboratory and clinical data from 167 patients with HCC that underwent TACE in Tel-Aviv medical Center in order to identify novel biomarkers to predict survival following TACE. These, 167 patients, were diagnosed predominantly through screening and thus at an earlier stage in their disease and some underwent the procedure as bridging for transplantation.
We retrospectively analyzed prospectively-collected data from manual and computerized medical records of 167 HCC patients at Tel-Aviv Medical center, a tertiary center with a liver transplantation service, who underwent the TACE procedure between the years 2000 and 2015. We excluded patients with fibrolamellar HCC, mixed cholangio-hepatocellular carcinoma and sarcomatous type HCC.
Data was collected for 161 patients (6 patients were excluded because of missing data) during the 3 mo period before the first TACE procedure. Baseline tumor parameters including: maximum tumor diameter, number of tumor nodules and presence of PVT - were gathered from imaging reports carried out at Tel-Aviv medical center. Labs including: blood count; routine liver function tests, (total bilirubin, AST, ALKP, GGTP, albumin) and plasma AFP levels; demographics and overall survival information. The Tel-Aviv medical center database management conforms to Israeli legislation on privacy and this study was approved by the institutional research committee in Tel-Aviv Medical Center (Approval number: 0528-16-TLV) in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. We collected data to conform to the previously described aggressiveness index (AgI): including the following four parameters: Maximum Tumor dimension, AFP, presence or absence of PVT, and the number of tumor nodules. The AgI score was calculated as follows: MTD (in tertiles): MTD < 4.5; 4.5 ≤ MTD ≤ 9.6; MTD > 9.6; scores 1, 2, 3 respectively. AFP (cut-off): AFP < 100; 100 ≤ AFP ≤ 1000; AFP > 1000 ng/mL; scores 1, 2, 3 respectively. PVT (No/Yes): PVT (No); PVT (Yes); scores 1, 3 respectively. Tumor Nodule (number): Nodules ≤ 3; Nodules > 3; scores 1, 3 respectively. The AgI score was divided into three categories for Cox analysis (Table 1): a, score - < 4; b, 4 < score ≤ 7; and c, score ≥ 8.
| All models in total cohort | OR | se(OR) | P value | 95%CI |
| Variables included together in the model | ||||
| Total Bilirubin (mg/dL) | 2.044 | 0.875 | 0.095 | 0.883 to 4.729 |
| ALKP (IU/mL) | 1.013 | 0.006 | 0.046 | 1.000 to 1.025 |
| GGTP (IU/mL) | 0.995 | 0.002 | 0.045 | 0.990 to 0.999 |
| AST (IU/L) | 1.002 | 0.003 | 0.442 | 0.996 to 1.009 |
| Albumin (g/dL) | 3.197 | 1.74 | 0.033 | 0.101 to 9.288 |
| Platelets (× 109/L) | 1.014 | 0.008 | 0.056 | 1.000 to 1.029 |
| WBC (× 109/L) | 0.811 | 0.121 | 0.158 | 0.606 to 1.085 |
| Lymphocyte (× 109/L) | 1.311 | 0.354 | 0.315 | 0.773 to 2.227 |
| Final model from stepwise method in backward | ||||
| ALKP (IU/mL) | 1.014 | 0.006 | 0.017 | 1.003 to 1.026 |
| GGTP (IU/mL) | 0.996 | 0.002 | 0.053 | 0.991 to 1.000 |
| Albumin (g/dL) | 2.562 | 1.082 | 0.026 | 1.120 to 5.863 |
The TACE procedure was first introduced in 1974 by Doyon et al[18] and was performed in our institute with the following modifications. In brief: Classical Seldinger catheterization with an end-hole angiographic catheter was used. Arteriography of the celiac trunk or the superior mesenteric artery was obtained to visualize the arterial vascularization of the liver. The same catheter was used for both drug injection and embolization. Selective injection was performed unless technical difficulties prevented selective catheterization. If the hepatic artery was occluded, an attempt was made to catheterize extrahepatic collaterals supplying the liver such as the inferior diaphragmatic, gastro-duodenal and left gastric arteries. The therapeutic emulsion contained Adriamycin and Lipiodol. The emulsion was injected into the hepatic artery distal to the gastroduodenal artery origin. Gelatin sponge particles, 1-2 mm in diameter, were then utilized to embolize the feeding vessels until a markedly reduced flow was observed. Particle size and arterial slow-down intensity) as evaluated fluoroscopically) were adapted to the status of the hepatic portal perfusion, being less aggressive (larger particles and lesser degree of arterial slowdown) in cases of poor hepatic portal perfusion. Patients received 1.5 L/d of intravenous fluid from 24 h before to 48 h after treatment. Cefamezin (1 g) and Dexamethasone (10-20 mg) was given 1 h before the procedure. Patients underwent repeated TACE procedures according to tumor viability and clinical condition as assessed by a multi-disciplinary team.
Mean and SD for continuous variables were used as indices of centrality and dispersion of the distribution. For non-normally distributed values it was necessary to use a non-parametric methods, The Wilcoxon rank-sum (Mann-Whitney) test, was used for continuous variables, to test the comparisons between the AgI categories of liver function parameters. The Cox proportional hazards model was applied to evaluate the predictive factors as categories of AgI score associated with overall survival. The results were presented as HR with 95%CI. Unconditional multiple logistic regression model was used to evaluate the Odds-Ratio of the AgI score (≥ 4) on the dichotomized Gamma Glutamyl-Transpeptidase (GGTP). All variables were included together in the model. The results were presented as OR with 95%CI. In all models, Cox regression and Logistic regression, the HR and the OR respectively, represent the risk for one-unit variation of the predictor variable considered as dummy variables. Patient survival between the two categories of AgI score was estimated with the Kaplan-Meier method and comparison of survival was made with the Breslow (generalized Wilcoxon) test. The log rank test was used, due to the small proportion of patients who died early. When testing the hypothesis of significant association, P-value was < 0.05, two tailed for all analyses. Statistical analysis was performed with State Corp 2007 State Statistical Software: release 10. College Station, TX: StataCorp LP.
A total of 167 patients were included in this study. Six patients were omitted because of missing data, leaving 161 patients in the final analysis. Sixty seven patients were under surveillance for their underlying liver disease. The median age was 64.24 ± 10.35 years, the majority were males (n = 124, 74.25%) and 80.24% had cirrhosis with complications, including ascites (n = 40), varices (n = 67), encephalopathy (n = 20), and abdominal pain (n = 34) at diagnosis. Etiologies of the underlying liver disease included: HCV (n = 91); HBV (n = 35); NASH (n = 17); cryptogenic cirrhosis (n = 17); HCV and HBV (n = 3); ASH and HCV (n = 2); Autoimmune hepatitis (n = 1), Alcoholic steatohepatitis (n = 1). The mean AFP levels were 53.8 (range: 1-66000). Mean number of tumor nodules was 1.95 + 1.39 and the MTD was 4.45 ± 2.65 cm. Twenty four patients (14.37%) had PVT. Mean serum ALKP, GGTP, bilirubin and albumin levels were 104.68 ± 110.73 IU, 152.26 ± 152.8 IU, 1.23 ± 0.80 (mg/dL) and 3.63 ± 1.93 (g/dL) respectively (Table 2).
| Parameter1 | Value |
| Age (yr) | 64.24 ± 10.35 |
| Sex (M) (%) | 124 (74.25) |
| Cirrhosis (yes) (%) | 134 (80.24) |
| AFP (ng/dL) | 1769.49 ± 7297.65 |
| AFP (median, range) | 53.80 (1-66000) |
| AFP > 100 (%) | 54 (40.60) |
| Number nodules | 1.95 ± 1.39 |
| MTD (cm) | 4.45 ± 2.64 |
| PVT (yes) (%) | 24 (14.37) |
| Aggressiveness index score (%) | |
| Score > 4 | 75 (63.56) |
| Total bilirubin (mg/dL) | 1.23 ± 0.80 |
| ALKP (IU/mL) | 133.29 ± 74.74 |
| GGTP (IU/mL) | 152.26 ± 152.80 |
| AST (IU/L) | 104.68 ± 110.73 |
| ALT (IU/L) | 77.85 ± 88.25 |
| Albumin (g/dL) | 3.63 ± 1.93 |
| Platelet count (× 109/L) | 113.78 ± 82.70 |
| WBC (× 109/L) | 5.46 ± 2.59 |
| Lymphocyte (× 109/L) | 1.41 ± 0.98 |
| Survival time (median, range) | 38 (3-175) |
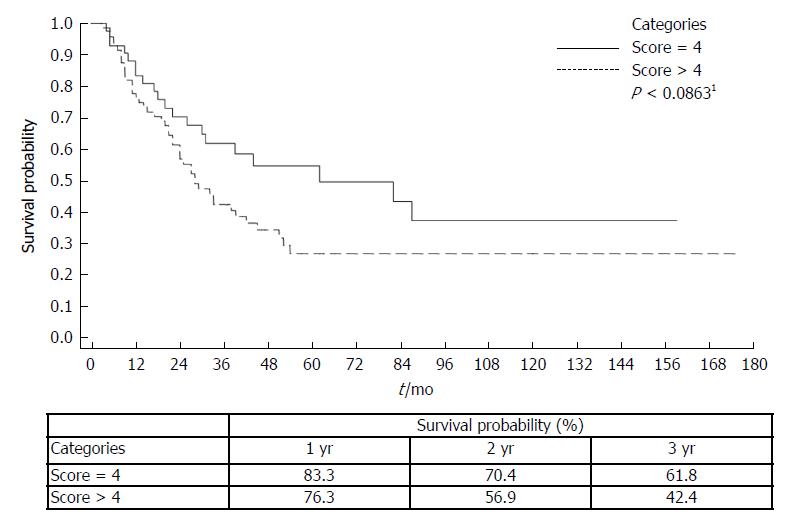
Median survival from the time of HCC diagnosis to death or transplantation was 38 mo (range 3-175 mo) (Table 2). Seventy five (63.5%) patients had a score of > 4 on the AgI (Table 2). The AgI was correlated with survival. The 3-year survival probability for AgI of > 4 vs < 4 was 42.4% vs 61.8%; P < 0.0863, from the time of diagnosis by Kaplan-Meier plot (Figure 1). Moreover, according to the univariate Cox proportional hazard model for mortality with AgI score of > 4, there was a HR of 2.18 (95%CI: 1.108-4.310, P < 0.024) (Table 3).
| All models in total cohort | HR | se(HR) | P value | 95%CI |
| Variables included together in the model | ||||
| Aggressiveness Index | ||||
| Score = 4 [Ref. category] | 1 | - | - | - |
| Score > 4 | 2.185 | 0.757 | 0.024 | 1.108 to 4.310 |
| Total bilirubin (mg/dL) | 0.985 | 0.154 | 0.925 | 0.725 to 1.339 |
| ALKP (IU/mL) | 0.999 | 0.003 | 0.793 | 0.993 to 1.005 |
| GGTP (IU/mL) | 1.003 | 0.001 | 0.016 | 1.001 to 1.006 |
| AST (IU/L) | 0.999 | 0.002 | 0.511 | 0.995 to 1.003 |
| Albumin (g/dL) | 0.793 | 0.250 | 0.462 | 0.427 to 1.472 |
| Platelets (×109/L) | 1.002 | 0.002 | 0.161 | 0.999 to 1.006 |
| Final model from stepwise method in backward | ||||
| Aggressiveness Index | ||||
| Score = 4 [Ref. category] | 1 | - | - | - |
| Score > 4 | 2.263 | 0.748 | 0.013 | 1.184 to 4.327 |
| GGTP (IU/mL) | 1.003 | 0.001 | 0.001 | 1.001 to 1.004 |
A univariate multiple logistic regression of AgI with 8 laboratory parameters was obtained and two baseline laboratory parameters were found to independently correlate with the AgI score (Table 1). Albumin’s single unit change was associated with an OR of 3.19 (95%CI: 0.101-9.299), followed by ALKP with an OR of 1.01 (95%CI: 1.000-1.025) (Table 1). These two parameters were then assessed in a multivariate multiple logistic regression to a final model which showed the following correlation: Albumin with an OR of 2.56 (95%CI: 1.120-5.863) followed by ALKP with an OR of 1.01 (95%CI: 1.003-1.026) (Table 1).
A univariate Cox proportional hazard model for mortality with AgI score and liver function parameters was performed. We found that only GGTP levels and the AgI were independently associated with survival of the HCC patients following TACE (Table 3). We used our cohort to validate the previously described AgI. An AgI with the score > 4 had HR for mortality of 2.18 (95%CI: 1.108-4.310, P < 0.024). GGTP’s single unit change had an HR for mortality of 1.003 (95%CI: 1.001-1.006, P < 0.016). We considered them in the final multivariate model with the total cohort. An AgI with the score > 4 had an HR for mortality of 2.26 (95%CI: 1.184-4.327, P < 0.016). GGTP had an HR of 1.003 (95%CI: 1.001-1.004, P < 0.001) (Table 3).
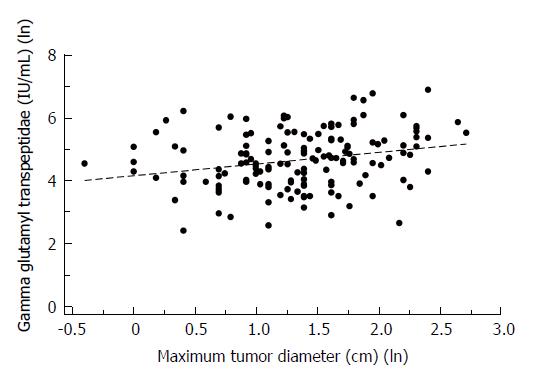
We then compared the HCC patients (Table 4) dichotomized by GGTP levels of 100IU/L (< 100/> 100) based on a previous finding that there is a marked difference in survival of patients with HCC between these values[19]. The GGTP < 100 group had 81 patients and the GGTP > 100 had 80 patients. The GGTP > 100 group had higher liver enzyme levels: ALKP = 160.92 + 89.79 vs 104.55 + 42.06; respectively (P < 0.0001) (Table 4). The 1 and 3 year survival was 17% higher in the GGTP (< or =) 100 group (87.18% vs 70.1% and 50% vs 32.47%, P = 0.01 and P = 0.03, respectively) (Table 4). We also assessed correlation between laboratory parameters and MTD. Gamma-glutamyl-transpeptidase levels (IU/mL) and the Maximum Tumor Diameter (cm) showed a low positive correlation with a Spearman’s coefficient of r = 0.2604 and a P-value of 0.0012 with a linear regression line (Figure 2).
| GGTP (IU/L) | |||
| Parameter1 | ≤ 100 (n = 81) (50.31%) | > 100 (n = 80) (49.69%) | P2 value |
| Total bilirubin (mg/dL) | 1.25 ± 0.75 | 1.19 ± 0.87 | 0.17 |
| ALKP (IU/mL) | 104.55 ± 42.06 | 160.92 ± 89.79 | < 0.0001 |
| GGTP (IU/mL) | 55.44 ± 24.46 | 250.29 ± 165.35 | < 0.0001 |
| AST (IU/L) | 93.61 ± 79.10 | 115.45 ± 137.27 | 0.06 |
| Albumin (g/dL) | 3.70 ± 2.68 | 3.56 ± 0.57 | 0.13 |
| Platelets (× 109/L) | 100.14 ± 61.45 | 129.60 ± 100.06 | 0.02 |
| Aggressiveness index (%) | |||
| Score > 4 | 33 (56.90) | 42 (75.00) | 0.043 |
| Survival at time (%) | |||
| 1 yr | 68 (87.18) | 54 (70.13) | 0.013 |
| 2 yr | 52 (66.67) | 37 (48.05) | 0.023 |
| 3 yr | 39 (50.00) | 25 (32.47) | 0.033 |
Tumor factors and liver function parameters were shown to have prognostic value in predicting survival of HCC patients. Our goal was to validate the usefulness of the recently-described HCC AgI, in a novel HCC cohort and to assess its usefulness in patients that underwent TACE while trying to identify additional laboratory serum parameters to improve its accuracy. We excluded patients with fibrolamellar HCC, mixed cholangio-hepatocellular carcinoma and sarcomatous type HCC, because of their rarity and their variant clinical course which may be different then “regular” HCC.
The AgI was recently reported and includes the following tumor parameters: maximum tumor diameter, number of tumor nodules and presence of PVT and plasma AFP levels.
Our cohort included patients with multiple underlying liver diseases and can be widely generalized, in contrast to the original cohort, where the underlying etiology of the liver disease was not specified.
HCC tumor parameters were previously shown to have prognostic value and were used in various staging systems[20]. In a 2011 paper by Hu et al[7], analyzing data from 362 patients undergoing TACE, all 4 of the AgI parameters were found to be independently significant predictors of patient’s survival. Maximal tumor size (HR = 1.66, P < 0.002), Portal vein invasion (HR = 2.39, P < 0.001), Tumor nodule number (HR = 1.92, P < 0.001), and AFP value (HR = 1.54, P < 0. 003). Portal vein invasion was associated with a marked decrease in patients’ survival, while the other parameters had only a modest effect on survival. However, combining them in the AgI increased their predictive power considerably. Furthermore, in their original paper the authors used single categories for each parameter, whereas the AgI divides each parameter into tertiles refining the scoring. We similarly show that combining these parameters in our specific population of patients that underwent TACE increases their predictive power.
Our patients had smaller tumors and longer survival time for both of the < 4 and > 4 score groups compared to previously reported groups[21-23]. The fact that most of the patients had an underlying liver disease and 40% were under a strict surveillance program made it possible to detect HCC at an early stage.
We found a significant increase in HR for death in patients with an AgI > 4, thus expanding the original observation to our patient population (Table 3).
Our second goal was to examine possible correlation between the AgI and other laboratory parameters of liver function (Table 1). We focused on 8 parameters that were previously shown to be associated with prognosis in HCC patients. Included were albumin and bilirubin which are part of the CPT score. Also included were markers of liver damage such as AST, ALK and GGTP[15]. Finally we looked at the hematologic parameters; platelets that were previously shown to be associated with tumor aggressiveness[16] and WBC and Lymphocytes, that were considered because Neutrophils to Lymphocytes ratio predicted overall survival in HBV-related HCC patients after TACE[13].
Other parameters that may better predict liver function or tumor aggressiveness such as Indocyanine Green (ICG) clearance[24] and Des-Gamma carboxyprothrombin (DCP) were excluded because they were not routinely utilized in our center and are not in wide clinical use[25,26].
In contrast to previous reports[10,11,27], and although albumin was correlated to AgI, we were unable to show that albumin is an independent predictor of survival in our cohort. We attribute this discrepancy to the fact that in a large portion of our cohort the tumor was discovered early when tumor parameters were favorable and liver function relatively preserved. Therefore, most of the patients (n = 110) had Albumin levels within the normal range.
Similar to previous reports[15,16] we also found elevated GGTP, to be an independent risk factor for mortality (HR = 1.003; 95%CI: 1.001-1.004; P < 0.001). In earlier publications, elevated levels of GGTP were found to be a poor prognostic factor after liver transplantation and before liver resection as they were associated with advanced tumor stage and aggressive tumor behaviors[28,29]. This factor can now be used as a predictor of prognosis in the population of TACE treated HCC patients.
We divided GGTP into two groups with a cutoff of 100IU/L (< 100/> 100). The group with the higher GGTP (> 100) had higher AgI score (52.5% compared with 40.7%) and lower survival at 1 and 3 years. (87.18% vs 70.1% and 50% vs 32.47%, P = 0.01, 0.03 respectively). In a recently published paper by Barman et al[30], focusing on patients undergoing TACE, it was noted that survival was higher among those patients with well-preserved liver synthetic function.
We were unable to show that other laboratory parameters associated with CPT (Bilirubin and INR-data not shown) were independently associated with prognosis. Other studies assessing these factors, show conflicting results. A previously published study found that elevated bilirubin (HR = 4.2; 95%CI: 2.2-7.9; P < 0.001) was a significant independent risk factor for mortality in 84 consecutive patients with HCC treated with TACE as first-line or second-line treatment that were enrolled between 2004 to 2009[31]. In contrast, a study of 109 patients who underwent TACE from 2006 to 2012 in a veterans hospital in the United States, did not show any single component of Child-Pugh score to be a predictor of survival[30]. The authors explain their results stating that their population was mostly males with HCV and dissimilar to previously published studies in which the population was mostly Asian with HBV and preserved liver function. These discrepancies suggest that different factors may predict tumor aggressiveness in different patient populations.
We concluded that the AgI is a validated tool to predict overall survival in unresectable HCC patients undergoing the TACE procedure. We further suggest that it can be combined with elevated GGTP levels, elevated levels of ALKP and decreased levels of albumin to improve its prognostic yield in this patient population.
Hepatocellular carcinoma (HCC) is a common and deadly cancer. Transterial chemoembolization (TACE) is the treatment of choice for non-operable, intermediate stage HCC.
There is a need to identify prognostic indices in HCC patients undergoing TACE. An “HCC aggressiveness index (AgI)” incorporates 4 tumor-related parameters: maximum tumor diameter (MTD), number of tumor nodules, portal vein thrombosis (PVT) and serum alpha fetoprotein (AFP) levels. This score predicts survival in HCC patients.
To identify novel biomarkers to predict survival following TACE and combine them with the AgI.
We retrospectively analyzed data from 167 patients with HCC that underwent TACE at Tel-Aviv Medical center from 2000 to 2015. Baseline tumor parameters including: maximum tumor diameter, number of tumor nodules and presence of PVT; labs including: blood count; routine liver function tests and plasma AFP levels; demographics and overall survival information were all collected. The Cox proportional hazards model was applied to identify the correlation of AgI with overall survival and analyze laboratory factors’ associated with the AgI.
The AgI was correlated with survival. The 3-year survival probability for AgI of > 4 vs < 4 was 42.4% vs 61.8%; P < 0.0863, from the time of diagnosis by Kaplan-Meier plot. Moreover, According to the univariate Cox proportional hazard model for mortality with AgI score of > 4, there was a HR of 2.18 (95%CI: 1.108-4.310, P < 0.024). We found that only GGTP levels and the AgI were independently associated with survival of the HCC patients following TACE.
AgI was validated as a useful predictor of survival in HCC patients undergoing TACE. Combining the AgI with liver function parameters may improve its prognostic yield in this patient population.
This novel score can be used to assess prognosis in HCC undergoing TACE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hashimoto N, Kang KJ S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Mlynarsky L, Menachem Y, Shibolet O. Treatment of hepatocellular carcinoma: Steps forward but still a long way to go. World J Hepatol. 2015;7:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 3. | Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10:153-161. [PubMed] |
| 5. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 6. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Hu HT, Kim JH, Lee LS, Kim KA, Ko GY, Yoon HK, Sung KB, Gwon DI, Shin JH, Song HY. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol. 2011;22:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Kadalayil L, Benini R, Pallan L, O’Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 9. | Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, Koike K, Nishino H, Tajiri H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, Kubota K, Sharma R. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol. 2011;103:801-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Zhou DS, Xu L, Luo YL, He FY, Huang JT, Zhang YJ, Chen MS. Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization. World J Gastroenterol. 2015;21:5582-5590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Li X, Chen ZH, Xing YF, Wang TT, Wu DH, Wen JY, Chen J, Lin Q, Dong M, Wei L. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2015;36:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Carr BI, Guerra V. A Hepatocellular Carcinoma Aggressiveness Index and Its Relationship to Liver Enzyme Levels. Oncology. 2016;90:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M, Borzio F. A Liver Index and its Relationship to Indices of HCC Aggressiveness. J Integr Oncol. 2016;5:pii: 178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Ludovico Rapaccini G, Di Marco M, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group. Association of abnormal plasma bilirubin with aggressive hepatocellular carcinoma phenotype. Semin Oncol. 2014;41:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. [Hepatic, arterial embolization in patients with malignant liver tumours (author’s transl)]. Ann Radiol (Paris). 1974;17:593-603. [PubMed] |
| 19. | Carr BI, Guerra V, Pancoska P. Thrombocytopenia in relation to tumor size in patients with hepatocellular carcinoma. Oncology. 2012;83:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 21. | Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, Zajko A. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Carr BI, Irish W, Federle MP. Chemoembolization for unresectable hepatocellular carcinoma in patients with or without portal vein thrombosis. Hepatogastroenterology. 2010;57:1375-1381. [PubMed] |
| 24. | Sheng QS, Lang R, He Q, Yang YJ, Zhao DF, Chen DZ. Indocyanine green clearance test and model for end-stage liver disease score of patients with liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2009;8:46-49. [PubMed] |
| 25. | Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res. 2013;4:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (23)] |
| 26. | Okuda H, Nakanishi T, Takatsu K, Saito A, Hayashi N, Takasaki K, Takenami K, Yamamoto M, Nakano M. Serum levels of des-gamma-carboxy prothrombin measured using the revised enzyme immunoassay kit with increased sensitivity in relation to clinicopathologic features of solitary hepatocellular carcinoma. Cancer. 2000;88:544-549. [PubMed] |
| 27. | Brown DB, Fundakowski CE, Lisker-Melman M, Crippin JS, Pilgram TK, Chapman W, Darcy MD. Comparison of MELD and Child-Pugh scores to predict survival after chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2004;15:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Fu SJ, Zhao Q, Ji F, Chen MG, Wu LW, Ren QQ, Guo ZY, He XS. Elevated Preoperative Serum Gamma-glutamyltranspeptidase Predicts Poor Prognosis for Hepatocellular Carcinoma after Liver Transplantation. Sci Rep. 2016;6:28835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Barman PM, Sharma P, Krishnamurthy V, Willatt J, McCurdy H, Moseley RH, Su GL. Predictors of mortality in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Dig Dis Sci. 2014;59:2821-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Cabibbo G, Genco C, Di Marco V, Barbara M, Enea M, Parisi P, Brancatelli G, Romano P, Craxì A, Cammà C. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |