Published online Apr 14, 2018. doi: 10.3748/wjg.v24.i14.1521
Peer-review started: March 9, 2018
First decision: March 14, 2018
Revised: March 27, 2018
Accepted: March 31, 2018
Article in press: March 31, 2018
Published online: April 14, 2018
Processing time: 34 Days and 0.1 Hours
Non celiac gluten sensitivity (NCGS) is a syndrome characterized by a cohort of symptoms related to the ingestion of gluten-containing food in subjects who are not affected by celiac disease (CD) or wheat allergy. The possibility of systemic manifestations in this condition has been suggested by some reports. In most cases they are characterized by vague symptoms such as ‘foggy mind’, headache, fatigue, joint and muscle pain, leg or arm numbness even if more specific complaints have been described. NCGS has an immune-related background. Indeed there is a strong evidence that a selective activation of innate immunity may be the trigger for NCGS inflammatory response. The most commonly autoimmune disorders associated to NCGS are Hashimoto thyroiditis, dermatitis herpetiformis, psoriasis and rheumatologic diseases. The predominance of Hashimoto thyroiditis represents an interesting finding, since it has been indirectly confirmed by an Italian study, showing that autoimmune thyroid disease is a risk factor for the evolution towards NCGS in a group of patients with minimal duodenal inflammation. On these bases, an autoimmune stigma in NCGS is strongly supported; it could be a characteristic feature that could help the diagnosis and be simultaneously managed. A possible neurological involvement has been underlined by NCGS association with gluten ataxia, gluten neuropathy and gluten encephalopathy. NCGS patients may show even psychiatric diseases such as depression, anxiety and psychosis. Finally, a link with functional disorders (irritable bowel syndrome and fibromyalgia) is a topic under discussion. In conclusion, the novelty of this matter has generated an expansion of literature data with the unavoidable consequence that some reports are often based on low levels of evidence. Therefore, only studies performed on large samples with the inclusion of control groups will be able to clearly establish whether the large information from the literature regarding extra-intestinal NCGS manifestations could be supported by evidence-based agreements.
Core tip: Non celiac gluten sensitivity is an expanding field of investigation within gluten-related disorders. Similarly to celiac disease, it shows a systemic involvement, therefore several extra-intestinal manifestations have been hypothesized and investigated in many studies. They may involve many districts and have neurological/psychiatric, dermatological, rheumatologic and nutritional implications. Moreover, the possibility of association with other autoimmune diseases should not be underestimated. However, the large data amount from the literature often requires to be supported by evidence-based agreements.
- Citation: Losurdo G, Principi M, Iannone A, Amoruso A, Ierardi E, Di Leo A, Barone M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J Gastroenterol 2018; 24(14): 1521-1530
- URL: https://www.wjgnet.com/1007-9327/full/v24/i14/1521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i14.1521
Non celiac gluten sensitivity (NCGS) is a syndrome characterized by a set of symptoms related to the ingestion of gluten-containing food in subjects who are not affected by celiac disease (CD) or wheat allergy[1]. Despite it has been included in the spectrum of gluten related disorders, it shows a peculiar picture with some elements resembling CD, i.e., immunological involvement and response to gluten free diet, and some features close to irritable bowel syndrome[2].
In detail, NCGS is distinguished by symptoms that typically take place soon after gluten ingestion, withdraw with gluten exclusion, and relapse following gluten challenge within hours or days. The “classical” clinical picture of NCGS is a combination of irritable bowel syndrome-like manifestations, such as abdominal pain, bloating, diarrhea or alterations in bowel habit with alternation of constipation and loose stools.
However, the possibility of systemic manifestations in this condition has been suggested by some reports. In most cases they are characterized by vague symptoms such as ‘foggy mind’, headache, fatigue, joint and muscle pain, leg or arm numbness even if more specific complaints have been described, such as dermatitis, (eczema or skin rash), depression, neurological symptoms and anemia[3-8]. Moreover, the possibility of association with other autoimmune diseases has been hypothesized. Indeed, similarly to CD, NCGS can be considered as an immune system-related disease and this aspect should be of relevance.
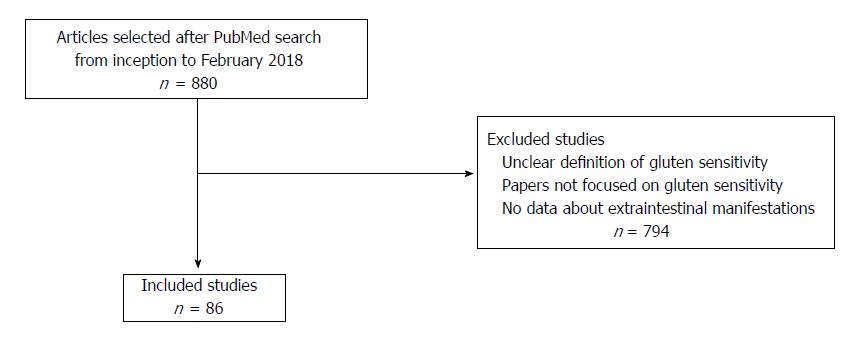
In conclusion, the spectrum of NCGS extra-intestinal manifestations is constantly expanding with new reports. Therefore, we aimed to summarize the main extra-intestinal manifestations of NCGS in a narrative review. In particular, in this review we focused on the associations supported by an evidence-based link more than single case reports, where it is difficult to differentiate a casual association from a real relationship. For this reason we searched in PubMed database in February 2018 using the following terms: gluten sensitivity, extra-intestinal, autoimmune, thyroid, neurology, psychiatry, rheumatology, skin, dermatology, nutrition, irritable bowel syndrome and fibromyalgia. In this way, 880 articles were found, and, as reported in the flow chart in Figure 1, we selected 86 studies for this review. Other studies which were not focused on NCGS or reporting an unclear definition of NCGS, or in which results about extra-intestinal manifestation were not listed have been excluded. Additionally, we graded the level of evidence on the association between NCGS and systemic manifestations using the Oxford consensus[9].
On the base of convincing evidence, NCGS has an immune-related background. Indeed it has been demonstrated that a selective activation of innate immunity may be the trigger for NCGS inflammatory response[10,11]. It is unclear whether gliadin is the real responsible for the autoimmune event onset, since some other components of wheat, such as amylase-trypsin inhibitors or fermentable oligo-di-mono-saccharides and polyols (FODMAPs) have been invoked[12-14]. For this reason some Authors consider the term “non celiac wheat sensitivity” more appropriate than the current one[15].
CD, which is the most common and studied gluten-related disorder, is often associated to several other autoimmune diseases, such as type 1 diabetes, autoimmune thyroiditis or dermatitis herpetiformis[16]. For this reason it is conceivable that also patients with NCGS could show autoimmune disorders. In a cohort of 131 NCGS patients[17], the prevalence of autoimmune disease (29%) was found to be higher than in control group (4%, P < 0.001). Moreover, anti-nucleus antibody (ANA) positivity, a well-known marker of autoimmune setting, was present in the 46% of NCGS subjects, compared to the 2% of controls, and ANA positivity correlated with DQ2/8 haplotypes. In detail, the most frequently reported NCGS-associated autoimmune disorder was Hashimoto thyroiditis (29 patients). Other diseases were psoriasis (4 cases), type 1 diabetes (4 cases), mixed connective tissue disease (1 case) and ankylosing spondylitis (1 case). The predominance of autoimmune thyroiditis represents an interesting finding, since it was indirectly confirmed by an Italian experience[18], showing that autoimmune thyroiditis is a risk factor for the evolution towards NCGS in a group of patients with minimal duodenal inflammation[19].
On these bases, an autoimmune stigma in NCGS is strongly supported; it could be a characteristic feature that could help the diagnosis and be simultaneously managed.
Recently, many studies explored the bond between the ingestion of gluten-containing food and the onset of neurologic and psychiatric disorders or symptoms such as ataxia, peripheral neuropathy, schizophrenia, autism, depression, anxiety, and hallucinations[20].
In patients with CD, a neurological involvement could be the only clinical manifestation of the disease. The production of autoantibodies directed against the tissue transglutaminase isoform 6 (expressed selectively in brain tissue) has been found in up to the 85% of these patients[21,22]. Anti-gliadin antibodies (AGA) frequently occur in such cases[21,22]. It is unclear whether the production of these antibodies takes place in the brain or in the gut mucosa, but these antibodies are considered to be the etiologic agent of neurological manifestations of CD. Finally, an inflammatory infiltrate of T lymphocytes resembling IELs in the white matter or in perivascular cuff of nerves is an important finding suggesting a specific pathogenetic mechanism of gluten-induced neuropathies[23].
Three main diseases have been described in the spectrum of gluten-related neurologic manifestations: gluten ataxia, gluten neuropathy and gluten encephalopathy[23].
Gluten ataxia has the strongest relationship with gluten-related disorders. It encompasses about the 20% of all causes of ataxia. This is mainly characterized by pure cerebellar ataxia and, rarely, by ataxia combined with myoclonus, palatal tremor, opsoclonus, or chorea. Gaze-evoked nystagmus and other ocular marks of cerebellar dysfunction are observed in about the 80%. All subjects show gait ataxia and most of them have limb ataxia[24]. A frequent finding at magnetic resonance imaging is cerebellar atrophy, secondary to necrosis of Purkinje cells[25]. Less than 10% of patients with gluten ataxia complain of gastrointestinal symptoms. A gluten free diet is able to reverse symptoms, however an early diagnosis significantly improves the prognosis, since gluten free diet may stop the loss of Purkinje cells. Therefore, a late diagnosis may be associated with an irreversible damage[26].
Gluten neuropathy is a form of peripheral neuronal damage, in which there is a serological evidence of CD positivity in the absence of alternative aetiologies. The most common type is a symmetrical sensorimotor axonal peripheral neuropathy, but other types have also been described (asymmetrical neuropathy, pure motor neuropathy or autonomic neuropathy)[27]. Gluten neuropathy occurs in the sixth decade and slowly progresses with a 9 year mean latency time between the diagnosis of neuropathy and that of CD. A third of patients shows duodenal inflammation on biopsy, however the presence or absence of enteropathy does not influence the effect of a gluten-free diet[28]. The most common histopathological feature of gluten neuropathy is lymphocyte infiltration of peri-neural vessels[29].
Gluten encephalopathy is a central nervous system disease characterized by focal abnormalities of the white matter (usually area of low perfusion) in presence of AGA or anti-transglutaminase 2 antibodies[30]. The most common symptom is migraine. It has been demonstrated that a gluten free diet improves the headaches and stops the progression of cerebral alterations detected at magnetic resonance imaging[31].
Some reports about the direct relationship between the above cited diseases and NCGS have been published in the last years. Hadjivassiliou et al[32] have retrospectively evaluated 562 patients with gluten-related disorders (228 CD and 334 NCGS) and concomitant neurological involvement. In NCGS the most frequent disorder was peripheral neuropathy (54%) followed by ataxia (46%) and encephalopathy, while in CD, ataxia was the most frequent one (41%). In all cases a deep linkage with AGA positivity was recorded. Additionally, the severity of ataxia was similar in both conditions (CD and NCGS), while patients with CD exhibited more frequently severe forms of neuropathy. Rodrigo et al[33] found, in a cohort of 31 subjects with gluten ataxia, AGA positivity rate of 100%; this value was more similar to NCGS (89%) than CD (48%) and was associated to Marsh 1 duodenal histological picture. On the bases of such results, they concluded that gluten ataxia shows a strict affinity to NCGS more than CD.
Headache is a very frequent finding in NCGS. However, no study has so far analyzed in depth the nature of this association. The available data relies mainly on observational studies aiming to elucidate the prevalence of this condition, which ranges around the 25%[3-8,34,35]. However, the lack of case-control studies is a serious limitation to ascertain the reliability of the association. Moreover there are no studies investigating possible pathogenetic mechanisms.
The association with other neurologic diseases such as epilepsy[36], miopathy[37] and demyelinating disease[38], is anecdotal or based on a non conventional diagnosis of NCGS, therefore it is not possible to draw solid conclusions.
Among the psychiatric diseases, depression and anxiety have been hypothesized as systemic manifestations of NCGS. In an Australian study[39], a group of patients with established diagnosis of NCGS underwent a double blind crossover study with a placebo versus oral gluten supplementation after a gluten free diet. Results showed that gluten induced depression scale worsening when compared to placebo, while other symptoms (anxiety, curiosity and anger) were not influenced by the diet. However, the mechanism by which gluten may induce these changes is not yet clear. Depression is indeed a frequent finding in Western society, and it could be a distinctive mood tract of personality rather than an extra-intestinal manifestation of NCGS. However, in another study NCGS patients did not exhibit a tendency for general somatization. Additionally, personality and quality of life did not differ between NCGS and CD patients and were mostly similar to healthy controls[40].
Some authors have invoked a role of gluten for some psychiatric diseases like schizophrenia or bipolar disorder[41], but there are no studies exploring these entities in NCGS. On the other hand, some cases of “gluten psychosis” in patients with NCGS have been described[42]. In these patients, hallucinations, crying spells, relevant confusion, ataxia, severe anxiety and paranoid delirium occurred shortly after gluten ingestion and disappeared within one week of gluten free diet.
Finally, the relationship between autism and gluten is an hot topic. It has been shown that children with autism have more frequently IgG-AGA positivity than healthy children (24% vs 7%)[43], but currently there are no studies in which a solid diagnosis of NCGS has been achieved in autistic subjects. A gluten free diet is often proposed to these children in an empiric setting, since it has been demonstrated that it improves behavioral scores[44,45]. However, at present there are no evidence-based reasons to look for gluten sensitivity in autism and to advise an exclusion diet[46].
The association between CD and skin diseases, in particular dermatitis herpetiformis, is well known[47]. Similarly to CD, the possibility of a skin involvement in the 18% of NCGS has been reported[4]. In the published case series[3-8], undefined dermatitis, rash and eczema were the most common skin manifestations in NCGS. The possibility of an association with skin autoimmune diseases such as psoriasis has been above mentioned[17]. A case report has shown that even dermatitis herpetiformis may occur[48].
Some reports have been mainly focused on the characteristics of skin lesions in NCGS from a dermatological point of view. In a series of 17 NCGS patients with skin lesions, the most common ones were very similar to dermatitis herpetiformis or subacute eczema (erythematous, excoriated papular-vesicular and extremely itchy)[49]. Some patients had also hyperkeratotic scaly lesions resembling psoriasis. The most common skin location was the extensor surfaces of upper limbs, in the 94%, alike dermatitis herpetiformis. The histological analysis showed complement C3 deposits at dermoepidermal junction in the 82%. Finally, in all patients a gluten free diet was able to lead to lesions disappearance within one month, much faster than in dermatitis herpetiformis.
Some Authors have claimed that an allergic sensitivity to food allergens other than gluten could underlie NCGS[50]. Indeed, an Italian study found that the 10% of NCGS patients suffered from nickel allergy with contact dermatitis and this prevalence was higher than in control group (5%, P = 0.04). However, NCGS subjects referred onset of dermatitis after wheat ingestion[51].
As we already mentioned, NCGS shows the tendency to cluster autoimmune diseases. Some reports about its coexistence with rheumatologic diseases are available. The first evidence demonstrated that in a group of 30 subjects with ankylosing spondylitis, 11 had AGA positivity, while no patient in a control group exhibited this finding[52]. Isasi et al[53] reported 4 cases of axial spondyloarthritis (2 ankylosing spondylitis and 1 psoriatic spondyloarthritis) with a microscopic enteritis picture at duodenal biopsy. They all underwent a gluten free diet, and in all cases an improvement or remission of back pain was reported, with a recrudescence after wheat challenge. The same result was recorded in another group of patients with systemic sclerosis, Raynaud’s phenomenon, symmetric polyarthritis and Sjogren’s syndrome[53].
However, despite such reports, the evidence for NCGS/rheumatologic association is weak, since case reports represent only a low level of evidence and case-control studies are necessary.
Fibromyalgia is a disease characterized by widespread pain, often accompanied by fatigue, memory problems, sleep disturbances, depression or irritable bowel syndrome[54]. In many case series, several NCGS patients complain of chronic muscle or joint pain, leg numbness, fatigue and headache[3-8], therefore it is possible that an underlying undiagnosed fibromyalgia could be present. Indeed, starting from some case reports demonstrating this association[55], further studies have analyzed in depth this relationship. In a Spanish series[56] of 246 fibromyalgia patients undergoing gluten free diet, 90 showed clinical symptom improvement. Additionally, Authors described the features of 20 out of such 90 patients. They had a mean duration of fibromyalgia of 12 years, and 17 had also gastrointestinal symptoms. Eighteen had a DQ2/8 haplotype and all showed an increase in duodenal IELs. After a mean gluten free diet period of 16.4 mo, 15 of them (75%) experienced a full remission of pain and in 8 of them gluten challenge led to symptom re-appearance. In another trial, gluten free diet was able to induce a decrease in some scales evaluating fibromyalgia symptoms[57]. On these bases, it is possible to hypothesize that the link between these two disorders is quite strong, but the role of microscopic enteritis in this setting should be tested in other controlled trials.
Fibromyalgia is frequently recognized as a functional disease. In this regard, NCGS has a tight bond with irritable bowel syndrome (IBS)[58]. Many patients with IBS often identify some foods that they believe to be more offending, and wheat is often invoked. Furthermore, a certain symptom overlap between NCGS and IBS-type symptoms exists[4,59]. For this reason, many patients tend to exclude gluten from their diet on their own, without medical advice, as summarized in Table 1[7,8,59-65]. The basic difference between the two conditions is that patients with NCGS assert that symptoms take place when they eat wheat so that they believe to have identified gluten as the culprit. Some experimental investigations have shown that gliadin can alter the integrity of the small intestinal mucosa, as shown by the appearance of epithelial leaks/gaps and widened inter-villous spaces detected by using confocal laser endomicroscopy[66]. Based on these assumptions, some clinical trials have demonstrated that a gluten free diet may lead to improvement of gastrointestinal symptoms in IBS, as reported in Table 2[5,67-73]. However it is not clear whether gluten is really the responsible for such symptoms. Indeed wheat contains FODMAPs as well, which are considered as a possible trigger for IBS itself, and FODMAP restriction demonstrated an improvement in IBS symptoms in up to the 74%[74]. Additionally, one trial underlined that subjects with self-reported NCGS (and IBS-like symptoms) had benefits by a low FODMAP diet despite they were still consuming a gluten free diet[75]. Based on these evidences, the link between IBS and NCGS seems to be strict even if quite nebulous. Is it possible that IBS and NCGS should be considered as the two sides of the same coin? Such fascinating question needs to be answered by well designed studies for this purpose.
| Ref. | Country | Population | Sample size | Avoidance rate of gluten-based products |
| Tanpowpong et al[60], 2012 | New Zealand | Pediatric | 916 | 5.2% |
| Rubio-Tapia et al[61], 2013 | United States | Pediatric | 7798 | 0.7% |
| DiGiacomo et al[62], 2013 | United States | National Health and Nutrition Examination Survey | 7762 | 0.6% |
| Lis et al[63], 2014 | Australia | Adults | 910 | 41.2% |
| Golley et al[64], 2015 | Australia | Adults | 1184 | 10.6% |
| Mardini et al[65], 2015 | United States | Pediatric | 14701 | 1% |
| Aziz et al[59], 2014 | United Kingdom | Adults | 1002 | 3.7% |
| Van Gils et al[8], 2016 | The Netherlands | Adults | 785 | 6.2% |
| Carroccio et al[7], 2017 | Italy | Adolescents | 548 | 2.9% |
| Ref. | Country | Population | Outcome |
| Wahnschaffe et al[67], 2001 | Germany | 102 IBS-D | Stool frequency/bowel movement improved in DQ2-8 positive subjects |
| Aziz et al[68], 2016 | United Kingdom | 40 IBS-D | A 6-wk GFD reduced symptoms in 70% |
| Vazquez-Roque et al[69], 2013 | United States | 45 IBS-D | Stool frequency/bowel movement reduced in patients under GFD |
| Di Sabatino et al[5], 2015 | Italy | 59 IBS with self-diagnosis of NCGS | A challenge with 4 g/d of gluten worsened symptoms compared to placebo |
| Shahbazkhani et al[70], 2015 | Iran | 72 IBS | Worsening of intestinal symptoms with gluten compared to placebo |
| Zanwar et al[71], 2016 | India | 60 IBS | A 4-wk GFD improved a visual-analogue scale of symptoms |
| Elli et al[72], 2016 | Italy | 140 IBS with self-diagnosis of NCGS | Only the 14% showed a response to GFD as well as challenge test |
| Barmeyer et al[73], 2017 | Germany | 34 IBS | The 34% showed clinical improvement to GFD and continued for one year |
CD is often disclosed by nutritional impairments, such as vitamin D or iron deficiency, anemia or alterations in bone mineralization[76,77].
Anemia prevalence value ranges between 15% and 23% in NCGS[3,4]. Nevertheless, studies enclosing a control group are lacking, therefore it is not possible to establish which is the real relationship between anemia and NCGS. Additionally, folate deficiency has been reported in NCGS with solid evidence and it has been even described as a predictive factor for its development[18].
An Italian study illustrated that NCGS carries a risk of osteopenia similar to CD[78]. Low bone mineral density measured by Dual-energy X-ray absorptiometry was found in 28% of NCGS subjects, vs 6% of IBS as well as an influence of body mass index on mineralization was observed. This result has been explained by a lower calcium dietary intake (only 615 mg/d, while recommended dose is 1000 mg/d).
This last observation may suggest that NCGS patients could experience an alteration in macro- and micronutrients intake due to dietary self-restrictions. Indeed Zingone et al[79] evaluated diet habits of 29 NCGS subjects and discovered that they ingested lower mean amounts of carbohydrates, proteins, fiber, and polyunsaturated fatty acids. Patients with NCGS reported avoiding fruit, vegetables, milk, and dairy products as well as snacks and mixed spices when compared to a control population.
NCGS is characterized by absent or minimal duodenal inflammation and, therefore, cannot be associated to nutrient deficiencies linked to malabsorption. However, an inflammatory status of duodenal mucosa, witnessed by increased expression of interferon gamma, may not be overlooked[33,80-82]. Finally, alterations in dietary pattern should not be underestimated. Gluten free diet itself can lead to an inadequate balance in macronutrients assumption[83-85].
Data from literature about extra-intestinal manifestations of NCGS strongly suggests that this condition could have a systemic involvement, similarly to CD. However, the novelty of this topic has generated an expansion of literature data with the unavoidable consequence that some reports are often based on low levels of evidence, as summarized in Table 3, with a grading of evidence according to the Oxford classification[9]. Therefore, only studies performed on large samples with the addition of control groups will be able to clearly establish whether the large information from the literature regarding extra-intestinal NCGS manifestations could be supported by evidence-based agreements.
| Manifestations | Extra-intestinal manifestations | Level of evidence | Associated disorders | Level of evidence |
| General symptoms | Tiredness | 4 | Aphthous stomatitis | 4 |
| Lack of wellbeing | 4 | |||
| Foggy mind | 4 | |||
| Joint or muscle pain | 4 | |||
| Arm/leg numbness | 4 | |||
| Neurologic manifestations | Ataxia | 3b | ||
| Neuropathy | 3b | |||
| Encephalopathy | 3b | |||
| Epilepsy | 4 | |||
| Miopathy | 4 | |||
| Myelopathy | 4 | |||
| Demyelinating disease | 4 | |||
| Psychiatric manifestations | Depression | 1c | Bipolar disorder | 4 |
| Anxiety | 1c | Gluten psychosis | 4 | |
| Autism | 2b | |||
| Schizophrenia | 4 | |||
| Other autoimmune diseases and rheumatologic diseases | Psoriasis | 2b | ||
| Autoimmune thyroiditis | 2b | |||
| Rheumatoid arthritis | 4 | |||
| Scleroderma | 4 | |||
| Sjogren syndrome | 4 | |||
| Raynaud phenomenon | 4 | |||
| Skin diseases | Dermatitis herpetiformis | 2b | ||
| Contact dermatitis | 2b | |||
| Rash and undetermined dermatitis | 2b | |||
| Functional disorders | Fibromyalgia | 1c | ||
| Irritable bowel syndrome | 1c | |||
| Nutritional imbalance | Anemia | 4 | ||
| Osteoporosis | 2b | |||
| Other | Interstitial cystitis | 4 | ||
| Ingrown hairs | 4 | |||
| Rhinitis, asthma | 4 | |||
| Postural tachycardia syndrome | 2b | |||
| Oligo- or polymenorrhea | 4 |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jadallah KA, Rostami-Nejad M, Sergi CM S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 676] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 3. | Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, Fiorini E, Caio G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol. 2012;46:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Volta U, Bardella MT, Calabrò A, Troncone R, Corazza GR; Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014;12:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, Di Stefano M, Corazza GR. Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin Gastroenterol Hepatol. 2015;13:1604-1612.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898-1906; quiz 1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Carroccio A, Giambalvo O, Blasca F, Iacobucci R, D’Alcamo A, Mansueto P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients. 2017;9:pii: E771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | van Gils T, Nijeboer P, IJssennagger CE, Sanders DS, Mulder CJ, Bouma G. Prevalence and Characterization of Self-Reported Gluten Sensitivity in The Netherlands. Nutrients. 2016;8:pii: E714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Centre for Evidence-Based Medicine. Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009). Accessed March 17. 2018; Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. |
| 10. | Caio G, Riegler G, Patturelli M, Facchiano A, DE Magistris L, Sapone A. Pathophysiology of non-celiac gluten sensitivity: where are we now? Minerva Gastroenterol Dietol. 2017;63:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 12. | Zevallos VF, Raker V, Tenzer S, Jimenez-Calvente C, Ashfaq-Khan M, Rüssel N, Pickert G, Schild H, Steinbrink K, Schuppan D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology. 2017;152:1100-1113.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 13. | Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, Veierød MB, Henriksen C, Lundin KEA. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology. 2018;154:529-539.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 14. | De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Carroccio A, Rini G, Mansueto P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology. 2014;146:320-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 16. | Leffler DA, Green PH, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 17. | Carroccio A, D’Alcamo A, Cavataio F, Soresi M, Seidita A, Sciumè C, Geraci G, Iacono G, Mansueto P. High Proportions of People With Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology. 2015;149:596-603.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Losurdo G, Piscitelli D, Giangaspero A, Principi M, Buffelli F, Giorgio F, Montenegro L, Sorrentino C, Amoruso A, Ierardi E. Evolution of nonspecific duodenal lymphocytosis over 2 years of follow-up. World J Gastroenterol. 2015;21:7545-7552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ierardi E, Losurdo G, Iannone A, Piscitelli D, Amoruso A, Barone M, Principi M, Pisani A, Di Leo A. Lymphocytic duodenitis or microscopic enteritis and gluten-related conditions: what needs to be explored? Ann Gastroenterol. 2017;30:380-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr Q. 2012;83:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 21. | Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol. 2008;64:332-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Zis P, Rao DG, Sarrigiannis PG, Aeschlimann P, Aeschlimann DP, Sanders D, Grünewald RA, Hadjivassiliou M. Transglutaminase 6 antibodies in gluten neuropathy. Dig Liver Dis. 2017;49:1196-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Hadjivassiliou M, Sanders DS, Grünewald RA, Woodroofe N, Boscolo S, Aeschlimann D. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 24. | Hadjivassiliou M, Sanders DS, Woodroofe N, Williamson C, Grünewald RA. Gluten ataxia. Cerebellum. 2008;7:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Wilkinson ID, Hadjivassiliou M, Dickson JM, Wallis L, Grünewald RA, Coley SC, Widjaja E, Griffiths PD. Cerebellar abnormalities on proton MR spectroscopy in gluten ataxia. J Neurol Neurosurg Psychiatry. 2005;76:1011-1013. [PubMed] |
| 26. | Pellecchia MT, Scala R, Perretti A, De Michele G, Santoro L, Filla A, Ciacci C, Barone P. Cerebellar ataxia associated with subclinical celiac disease responding to gluten-free diet. Neurology. 1999;53:1606-1608. [PubMed] |
| 27. | Chin RL, Sander HW, Brannagan TH, Green PH, Hays AP, Alaedini A, Latov N. Celiac neuropathy. Neurology. 2003;60:1581-1585. [PubMed] |
| 28. | Hadjivassiliou M, Kandler RH, Chattopadhyay AK, Davies-Jones AG, Jarratt JA, Sanders DS, Sharrack B, Grünewald RA. Dietary treatment of gluten neuropathy. Muscle Nerve. 2006;34:762-766. [PubMed] |
| 29. | Hadjivassiliou M, Grünewald RA, Kandler RH, Chattopadhyay AK, Jarratt JA, Sanders DS, Sharrack B, Wharton SB, Davies-Jones GA. Neuropathy associated with gluten sensitivity. J Neurol Neurosurg Psychiatry. 2006;77:1262-1266. [PubMed] |
| 30. | Hadjivassiliou M, Grünewald RA, Lawden M, Davies-Jones GA, Powell T, Smith CM. Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology. 2001;56:385-388. [PubMed] |
| 31. | Serratrice J, Disdier P, de Roux C, Christides C, Weiller PJ. Migraine and coeliac disease. Headache. 1998;38:627-628. [PubMed] |
| 32. | Hadjivassiliou M, Rao DG, Grìnewald RA, Aeschlimann DP, Sarrigiannis PG, Hoggard N, Aeschlimann P, Mooney PD, Sanders DS. Neurological Dysfunction in Coeliac Disease and Non-Coeliac Gluten Sensitivity. Am J Gastroenterol. 2016;111:561-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Rodrigo L, Hernández-Lahoz C, Lauret E, Rodriguez-Peláez M, Soucek M, Ciccocioppo R, Kruzliak P. Gluten ataxia is better classified as non-celiac gluten sensitivity than as celiac disease: a comparative clinical study. Immunol Res. 2016;64:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Losurdo G, Giorgio F, Piscitelli D, Montenegro L, Covelli C, Fiore MG, Giangaspero A, Iannone A, Principi M, Amoruso A. May the assessment of baseline mucosal molecular pattern predict the development of gluten related disorders among microscopic enteritis? World J Gastroenterol. 2016;22:8017-8025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 35. | Losurdo G, Piscitelli D, Pezzuto F, Fortarezza F, Covelli C, Marra A, Iannone A, Amoruso A, Principi M, Ierardi E. T Helper Lymphocyte and Mast Cell Immunohistochemical Pattern in Nonceliac Gluten Sensitivity. Gastroenterol Res Pract. 2017;2017:5023680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Bruni O, Dosi C, Luchetti A, Della Corte M, Riccioni A, Battaglia D, Ferri R. An unusual case of drug-resistant epilepsy in a child with non-celiac gluten sensitivity. Seizure. 2014;23:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Hadjivassiliou M, Chattopadhyay AK, Grünewald RA, Jarratt JA, Kandler RH, Rao DG, Sanders DS, Wharton SB, Davies-Jones GA. Myopathy associated with gluten sensitivity. Muscle Nerve. 2007;35:443-450. [PubMed] |
| 38. | Jacob S, Zarei M, Kenton A, Allroggen H. Gluten sensitivity and neuromyelitis optica: two case reports. J Neurol Neurosurg Psychiatry. 2005;76:1028-1030. [PubMed] |
| 39. | Peters SL, Biesiekierski JR, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity - an exploratory clinical study. Aliment Pharmacol Ther. 2014;39:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Brottveit M, Vandvik PO, Wojniusz S, Løvik A, Lundin KE, Boye B. Absence of somatization in non-coeliac gluten sensitivity. Scand J Gastroenterol. 2012;47:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Casella G, Pozzi R, Cigognetti M, Bachetti F, Torti G, Cadei M, Villanacci V, Baldini V, Bassotti G. Mood disorders and non-celiac gluten sensitivity. Minerva Gastroenterol Dietol. 2017;63:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Lionetti E, Leonardi S, Franzonello C, Mancardi M, Ruggieri M, Catassi C. Gluten Psychosis: Confirmation of a New Clinical Entity. Nutrients. 2015;7:5532-5539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Lau NM, Green PH, Taylor AK, Hellberg D, Ajamian M, Tan CZ, Kosofsky BE, Higgins JJ, Rajadhyaksha AM, Alaedini A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS One. 2013;8:e66155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr. 2016;12:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. 2017;32:1935-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Cruchet S, Lucero Y, Cornejo V. Truths, Myths and Needs of Special Diets: Attention-Deficit/Hyperactivity Disorder, Autism, Non-Celiac Gluten Sensitivity, and Vegetarianism. Ann Nutr Metab. 2016;68 Suppl 1:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Collin P, Salmi TT, Hervonen K, Kaukinen K, Reunala T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med. 2017;49:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Philips C, Paramaguru R, Indiran DA, Augustine P. Dermatitis Herpetiformis as the Initial Presentation of Primary Biliary Cholangitis in a Male with Gluten Sensitivity. Cureus. 2017;9:e1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Bonciolini V, Bianchi B, Del Bianco E, Verdelli A, Caproni M. Cutaneous Manifestations of Non-Celiac Gluten Sensitivity: Clinical Histological and Immunopathological Features. Nutrients. 2015;7:7798-7805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 50. | Mansueto P, D’Alcamo A, Seidita A, Carroccio A. Food allergy in irritable bowel syndrome: The case of non-celiac wheat sensitivity. World J Gastroenterol. 2015;21:7089-7109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | D’Alcamo A, Mansueto P, Soresi M, Iacobucci R, Blasca F, Geraci G, Cavataio F, Fayer F, Arini A, Di Stefano L. Contact Dermatitis Due to Nickel Allergy in Patients Suffering from Non-Celiac Wheat Sensitivity. Nutrients. 2017;9:pii: E103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Toğrol RE, Nalbant S, Solmazgül E, Ozyurt M, Kaplan M, Kiralp MZ, Dinçer U, Sahan B. The significance of coeliac disease antibodies in patients with ankylosing spondylitis: a case-controlled study. J Int Med Res. 2009;37:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Isasi C, Tejerina E, Morán LM. Non-celiac gluten sensitivity and rheumatic diseases. Reumatol Clin. 2016;12:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1060] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 55. | Isasi C, Tejerina E, Fernandez-Puga N, Serrano-Vela JI. Fibromyalgia and chronic fatigue syndrome caused by non-celiac gluten sensitivity. Reumatol Clin. 2015;11:56-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Isasi C, Colmenero I, Casco F, Tejerina E, Fernandez N, Serrano-Vela JI, Castro MJ, Villa LF. Fibromyalgia and non-celiac gluten sensitivity: a description with remission of fibromyalgia. Rheumatol Int. 2014;34:1607-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Slim M, Calandre EP, Garcia-Leiva JM, Rico-Villademoros F, Molina-Barea R, Rodriguez-Lopez CM, Morillas-Arques P. The Effects of a Gluten-free Diet Versus a Hypocaloric Diet Among Patients With Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J Clin Gastroenterol. 2017;51:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 59. | Aziz I, Lewis NR, Hadjivassiliou M, Winfield SN, Rugg N, Kelsall A, Newrick L, Sanders DS. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J Gastroenterol Hepatol. 2014;26:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 60. | Tanpowpong P, Ingham TR, Lampshire PK, Kirchberg FF, Epton MJ, Crane J, Camargo CA Jr; New Zealand Asthma and Allergy Cohort Study Group. Coeliac disease and gluten avoidance in New Zealand children. Arch Dis Child. 2012;97:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-44; quiz 1537, 1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 531] [Article Influence: 40.8] [Reference Citation Analysis (2)] |
| 62. | DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009-2010. Scand J Gastroenterol. 2013;48:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 63. | Lis DM, Stellingwerff T, Shing CM, Ahuja KD, Fell JW. Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int J Sport Nutr Exerc Metab. 2015;25:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Golley S, Corsini N, Topping D, Morell M, Mohr P. Motivations for avoiding wheat consumption in Australia: results from a population survey. Public Health Nutr. 2015;18:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Mardini HE, Westgate P, Grigorian AY. Racial Differences in the Prevalence of Celiac Disease in the US Population: National Health and Nutrition Examination Survey (NHANES) 2009-2012. Dig Dis Sci. 2015;60:1738-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, Bethge J, Böttner M, Klose J, Milla PJ. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147:1012-1020.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 67. | Wahnschaffe U, Ullrich R, Riecken EO, Schulzke JD. Celiac disease-like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology. 2001;121:1329-1338. [PubMed] |
| 68. | Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a Gluten-Free Diet in Subjects With Irritable Bowel Syndrome-Diarrhea Unaware of Their HLA-DQ2/8 Genotype. Clin Gastroenterol Hepatol. 2016;14:696-703.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 69. | Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903-911.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 70. | Shahbazkhani B, Sadeghi A, Malekzadeh R, Khatavi F, Etemadi M, Kalantri E, Rostami-Nejad M, Rostami K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients. 2015;7:4542-4554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Zanwar VG, Pawar SV, Gambhire PA, Jain SS, Surude RG, Shah VB, Contractor QQ, Rathi PM. Symptomatic improvement with gluten restriction in irritable bowel syndrome: a prospective, randomized, double blinded placebo controlled trial. Intest Res. 2016;14:343-350. [PubMed] |
| 72. | Elli L, Tomba C, Branchi F, Roncoroni L, Lombardo V, Bardella MT, Ferretti F, Conte D, Valiante F, Fini L. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients. 2016;8:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 73. | Barmeyer C, Schumann M, Meyer T, Zielinski C, Zuberbier T, Siegmund B, Schulzke JD, Daum S, Ullrich R. Long-term response to gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome. Int J Colorectal Dis. 2017;32:29-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 75. | Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320-8.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 76. | Abenavoli L, Delibasic M, Peta V, Turkulov V, De Lorenzo A, Medić-Stojanoska M. Nutritional profile of adult patients with celiac disease. Eur Rev Med Pharmacol Sci. 2015;19:4285-4292. [PubMed] |
| 77. | García-Manzanares A, Lucendo AJ. Nutritional and dietary aspects of celiac disease. Nutr Clin Pract. 2011;26:163-173. [PubMed] [DOI] [Full Text] |
| 78. | Carroccio A, Soresi M, D’Alcamo A, Sciumè C, Iacono G, Geraci G, Brusca I, Seidita A, Adragna F, Carta M. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: a prospective observation study. BMC Med. 2014;12:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Zingone F, Bartalini C, Siniscalchi M, Ruotolo M, Bucci C, Morra I, Iovino P, Ciacci C. Alterations in Diets of Patients With Nonceliac Gluten Sensitivity Compared With Healthy Individuals. Clin Gastroenterol Hepatol. 2017;15:63-68.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Ierardi E, Amoruso A, Giorgio F, Principi M, Losurdo G, Piscitelli D, Buffelli F, Fiore MG, Mongelli A, Castellaneta NM. Mucosal molecular pattern of tissue transglutaminase and interferon gamma in suspected seronegative celiac disease at marsh 1 and 0 stages. Saudi J Gastroenterol. 2015;21:379-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Dinu M, Macchia D, Pagliai G, Gori AM, Cesari F, Marcucci R, Sofi F, Casini A. Symptomatic efficacy of buckwheat products in Non-Celiac Gluten Sensitivity (NCGS). Asia Pac J Clin Nutr. 2017;26:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 82. | Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, Sollid LM, Lundin KE. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: A review. Clin Nutr. 2016;35:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 84. | Barone M, Della Valle N, Rosania R, Facciorusso A, Trotta A, Cantatore FP, Falco S, Pignatiello S, Viggiani MT, Amoruso A. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur J Clin Nutr. 2016;70:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 2013;26:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (2)] |