Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1361
Peer-review started: December 22, 2017
First decision: January 4, 2018
Revised: February 9, 2018
Accepted: February 26, 2018
Article in press: February 26, 2018
Published online: March 28, 2018
Processing time: 93 Days and 22 Hours
To assess daclatasvir plus asunaprevir (DUAL) in treatment-naïve patients from mainland China, Russia and South Korea with hepatitis C virus (HCV) genotype 1b infection.
Patients were randomly assigned (3:1) to receive 24 wk of treatment with DUAL (daclatasvir 60 mg once daily and asunaprevir 100 mg twice daily) beginning on day 1 of the treatment period (immediate treatment arm) or following 12 wk of matching placebo (placebo-deferred treatment arm). The primary endpoint was a comparison of sustained virologic response at posttreatment week 12 (SVR12) compared with the historical SVR rate for peg-interferon plus ribavirin (70%) among patients in the immediate treatment arm. The first 12 wk of the study were blinded. Safety was assessed in DUAL-treated patients compared with placebo patients during the first 12 wk (double-blind phase), and during 24 wk of DUAL in both arms combined.
In total, 207 patients were randomly assigned to immediate (n = 155) or placebo-deferred (n = 52) treatment. Most patients were Asian (86%), female (59%) and aged < 65 years (90%). Among them, 13% had cirrhosis, 32% had IL28B non-CC genotypes and 53% had baseline HCV RNA levels of ≥ 6 million IU/mL. Among patients in the immediate treatment arm, SVR12 was achieved by 92% (95% confidence interval: 87.2-96.0), which was significantly higher than the historical comparator rate (70%). SVR12 was largely unaffected by cirrhosis (89%), age ≥ 65 years (92%), male sex (90%), baseline HCV RNA ≥ 6 million (89%) or IL28B non-CC genotypes (96%), although SVR12 was higher among patients without (96%) than among those with (53%) baseline NS5A resistance-associated polymorphisms (at L31 or Y93H). During the double-blind phase, aminotransferase elevations were more common among placebo recipients than among patients receiving DUAL. During 24 wk of DUAL therapy (combined arms), the most common adverse events (≥ 10%) were elevated alanine aminotransferase and upper respiratory tract infection; emergent grade 3-4 laboratory abnormalities were infrequently observed, and all grade 3-4 aminotransferase abnormalities (alanine aminotransferase, n = 9; aspartate transaminase, n = 6) reversed within 8-11 d. Two patients discontinued DUAL treatment; one due to aminotransferase elevations, nausea, and jaundice and the other due to a fatal adverse event unrelated to treatment. There were no treatment-related deaths.
DUAL was well-tolerated during this phase 3 study, and SVR12 with DUAL treatment (92%) exceeded the historical SVR rate for peg-interferon plus ribavirin of 70%.
Core tip: This phase 3, placebo-controlled study assessed the efficacy and safety of daclatasvir (NS5A inhibitor) plus asunaprevir (NS3/4A protease inhibitor) in treatment-naïve patients from mainland China, Russia and South Korea with hepatitis C virus (HCV) genotype 1b infection. The rate of sustained virologic response at posttreatment week 12 among patients in the immediate treatment arm was 92%, which was significantly higher than the historical comparator rate (70%). The combination was well tolerated during 24 wk of treatment. These results demonstrate that for countries such as China, where interferon-based combinations are still widely used for the treatment of HCV genotype 1b, daclatasvir/asunaprevir offers a more efficacious and tolerable alternative with a shorter treatment duration.
- Citation: Wei L, Wang FS, Zhang MX, Jia JD, Yakovlev AA, Xie W, Burnevich E, Niu JQ, Jung YJ, Jiang XJ, Xu M, Chen XY, Xie Q, Li J, Hou JL, Tang H, Dou XG, Gandhi Y, Hu WH, McPhee F, Noviello S, Treitel M, Mo L, Deng J. Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection. World J Gastroenterol 2018; 24(12): 1361-1372
- URL: https://www.wjgnet.com/1007-9327/full/v24/i12/1361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i12.1361
Chronic hepatitis C virus (HCV) infection is a significant health burden across Asia[1], and affects 5-7 million people in China alone[2]. Without effective treatment, patients can develop severe complications, such as hepatocellular carcinoma (HCC)[3,4], for which HCV infection has become one of the most common causes in Asian and Western countries[5,6].
DUAL is an all-oral combination of daclatasvir (pangenotypic NS5A inhibitor with in vitro activity against genotypes 1-6)[7,8] and asunaprevir (NS3 protease inhibitor with in vitro activity against genotypes 1 and 4-6)[9]. This regimen has demonstrated efficacy in several phase 3 studies of patients infected with HCV genotype 1b[10-13], the predominant genotype in East Asia[14-16], including those with characteristics known to attenuate response to interferon (IFN)-based treatment[17-19]. DUAL also has a superior safety profile compared with IFN-based combinations[20] and in April 2017 became the first all-oral, nonribavirin-containing combination for chronic HCV infection to gain approval in China[21].
In this study, we evaluated the efficacy and safety of DUAL in treatment-naïve patients from mainland China, South Korea and Russia with HCV genotype 1b infection.
This was a phase 3, double-blind, placebo-controlled study (ClinicalTrials.gov number, NCT02496078) of DUAL, conducted between August 2015 and February 2017 in treatment-naïve patients from mainland China, South Korea and Russia with chronic HCV genotype 1b infection. Patients were randomly assigned (3:1) to receive DUAL (daclatasvir 60 mg tablet once daily and asunaprevir 100 mg soft capsule twice daily) for 24 wk either immediately (immediate treatment arm) or after 12 wk of matching placebo (placebo-deferred treatment arm) via an interactive voice-response system, and stratified according to the presence or absence of cirrhosis. Treatment was blinded to patients, investigators and the sponsor until week 12, and was open label thereafter.
The study was conducted according to local laws and regulatory requirements, and in accordance with Good Clinical Practice, as defined by the International Conference on Harmonization and the principles of the Declaration of Helsinki. Written informed consent was gained prior to study initiation.
The study population comprised male and female patients aged ≥ 18 years (body mass index: 18-35 kg/m2) with chronic HCV genotype 1b infection (HCV RNA of ≥ 10000 IU/mL at screening) and no prior exposure to any IFN formulation, ribavirin or direct-acting antiviral agent for HCV. Patients with compensated cirrhosis were included (enrollment capped at approximately 25%). Cirrhosis status was defined by a hierarchical algorithm based on available biopsy, Fibroscan® or Fibrotest® (BioPredictive, Paris, France) and aspartate transaminase (AST):platelet ratio index (APRI) data. Patients were considered noncirrhotic if they met one of the following criteria: liver biopsy within 36 mo of screening showing absence of cirrhosis; Fibroscan® result of ≤ 9.6 kPa within 1 year of baseline/day 1; or FibroTest® score of ≤ 0.48 with APRI of ≤ 1 (performed during screening). Patients were considered cirrhotic if they met one of the following criteria: liver biopsy showing cirrhosis any time prior to screening; Fibroscan® showing cirrhosis or results of > 14.6 kPa within 1 year of baseline; or FibroTest® score of > 0.75 and an APRI of > 2 (at screening). Both sets of criteria are listed in decreasing hierarchical order.
Key exclusion criteria included: HCV infection other than genotype 1b; evidence of a medical condition contributing to chronic liver disease other than HCV, or of decompensated liver disease (e.g., history or presence of ascites, bleeding varices, or hepatic encephalopathy); diagnosed or suspected HCC or other malignancies; uncontrolled diabetes or hypertension; moderate to severe depression (well-controlled mild depression was permitted); total bilirubin ≥ 34 μmol/L (or ≥ 2 mg/dL) unless the patient had a documented history of Gilbert’s disease; alanine aminotransferase (ALT) ≥ 5 × the upper limit of normal; albumin < 3.5 g/dL; alpha-fetoprotein > 100 ng/mL (patients with alpha-fetoprotein 50-100 ng/mL required a liver ultrasound, and those with findings suspicious of HCC were excluded); hemoglobin < 8.5 g/dL; absolute neutrophil count < 0.5 × 109 cells/L; and, platelet count < 50 × 109 cells/L.
HCV RNA was quantified using the COBAS®TaqMan® assay v2.0 (Roche Molecular Diagnostics, Pleasanton, CA, United States) with a lower limit of quantitation (LLOQ) of 25 IU/mL. HCV genotype and subtype were determined using the RealTime HCV Genotype II assay (Abbott Molecular, Des Plaines, IL, United States); if the results were inconclusive, the Versant HCV Genotype 2.0 assay (Siemens, Erlangen, Germany) or population-based sequencing of the NS5A region was employed. IL28B rs12979860 single-nucleotide polymorphisms were identified using PCR amplification and sequencing (TaqMan assay; Applied Biosystems, Waltham, MA, United States).
Treatment failure comprised: virologic breakthrough, defined as any confirmed > 1 log10 increase in HCV RNA from nadir, or increase in HCV RNA ≥ LLOQ after confirmed HCV RNA < LLOQ target detected or not detected (TD or TND) during treatment; HCV RNA < LLOQ but still detectable at end of treatment (EOT); or, relapse, defined as HCV RNA ≥ LLOQ in any posttreatment window following HCV RNA < LLOQ TND at EOT.
Resistance testing was performed using population-based sequencing (threshold ≥ 20% of a viral population) of the NS5A and NS3 regions on all available plasma samples at baseline, and on the samples of patients experiencing treatment failure with HCV RNA ≥ 1000 IU/mL.
Safety was monitored based on incidence of adverse events (AEs) and abnormalities in clinical laboratory assessments, vital signs and physical examinations.
The primary efficacy outcome was the proportion of patients, randomly assigned to the immediate treatment arm, achieving a sustained virologic response (HCV RNA < LLOQ, TD or TND) at posttreatment week 12 (SVR12), and the primary endpoint was comparison of this outcome against a historical SVR rate of 70% associated with peg-IFN plus ribavirin treatment.
SVR12 in the placebo-deferred treatment arm was a secondary endpoint. Safety-related secondary endpoints included the incidence of AEs, serious (S)AEs, discontinuations due to AEs, deaths, and grade 3-4 laboratory abnormalities observed during the 12-wk double-blind phase (DUAL vs placebo), and in both arms during 24 wk of treatment with DUAL. Efficacy-related secondary endpoints included SVR12 according to rs12979860 single-nucleotide polymorphisms in the IL28B gene; the proportion of patients achieving HCV RNA < LLOQ, TD or TND and TND only, in each treatment arm at on-treatment weeks 1, 2, 4, 6, 8, and 12, both on-treatment weeks 4 and 12, EOT, and post-treatment weeks 4 and 24.
The statistical methods used in this study were reviewed by the biometrics group at Bristol-Myers Squibb. The primary objective was to determine whether SVR12 among patients in the immediate treatment arm would be significantly higher than the historical 70% SVR rate associated with peg-IFN plus ribavirin. The lower bound of a two-sided 95% confidence interval (CI) for SVR12 was used to compare to the historical SVR rate; if it exceeded 70%, it was concluded that the primary objective was met and SVR12 for patients in the immediate treatment arm was significantly higher than the SVR rate associated with peg-IFN plus ribavirin. A sample size of approximately 150 patients would have provided a 95%CI with a lower bound exceeding 70% for a corresponding SVR12 rate of approximately 77.3% or higher, while an SVR12 rate of 90% would have provided a lower bound not less than 85%. Missing HCV RNA data at posttreatment week 12 were imputed using the next value carried backwards approach, where the next and closest available HCV RNA measurement after posttreatment week 12 was utilized instead.
In total, 229 patients were enrolled, of whom 207 were randomly assigned to the immediate (n = 155) or placebo-deferred (n = 52) treatment arms.
Of 155 patients assigned to the immediate treatment arm, all completed the 12-wk double-blind phase, 148 completed 24 wk of treatment with DUAL, and 151 completed 24 wk of follow-up; seven discontinued treatment with DUAL due to lack of efficacy (n = 6) or AEs (n = 1), and four discontinued follow-up after posttreatment week 12 due to withdrawal of consent (n = 3) or inability to attend the visit due to an accident (n = 1).
Of 52 patients randomly assigned to placebo-deferred treatment, 51 completed the 12-wk double-blind phase, 44 completed 24 wk of treatment with DUAL, and 48 completed 24 wk of follow-up; one discontinued placebo due to an SAE (hepatitis E), seven discontinued treatment with DUAL due to lack of efficacy (n = 6) or AEs (n = 1), and two discontinued follow-up after posttreatment week 12 due to withdrawal of consent (n = 1) or initiation of alternative HCV therapy (n = 1).
The majority of patients were Chinese (77.8%) and female (60.6%); among them, 12.6% had compensated cirrhosis, 31.9% had IL28B non-CC genotypes, 53.1% had baseline HCV RNA ≥ 6 million IU/mL and 9.7% were aged 65 years or older (Table 1). These data include six patients who were found not to meet the study enrollment criteria after treatment initiation; one of these patients, from mainland China, was reclassified as having genotype 1a infection, and five had received prior treatment with ribavirin and/or IFN regimens.
| Characteristic | Immediate treatment, n = 1552 | Placebo-deferred treatment, n = 52 | Overall, n = 2072 |
| Age, median (range) years | 49 (18-73) | 49 (23-69) | 49 (18-73) |
| < 65 yr | 142 (92) | 45 (87) | 187 (90) |
| ≥ 65 yr | 13 (8) | 7 (14) | 20 (10) |
| Male | 61 (39) | 23 (44) | 84 (41) |
| Race | |||
| Asian | 132 (85) | 45 (87) | 177 (86) |
| White | 23 (15) | 7 (14) | 30 (15) |
| Country | |||
| Mainland China | 119 (77) | 42 (81) | 161 (78) |
| Russia | 23 (15) | 7 (14) | 30 (15) |
| South Korea | 13 (8) | 3 (6) | 16 (8) |
| HCV RNA, median (range) log10 IU/mL | 6.78 (3.1-7.6) | 6.86 (5.6-7.6) | 6.79 (3.1-7.6) |
| ≥ 6 million IU/mL | 79 (51) | 31 (60) | 110 (53) |
| IL28B genotype | |||
| CC | 107 (69) | 34 (65) | 141 (68) |
| CT | 43 (28) | 17 (33) | 60 (29) |
| TT | 5 (3) | 1 (2) | 6 (3) |
| Cirrhosis | 19 (12) | 7 (14) | 26 (13) |
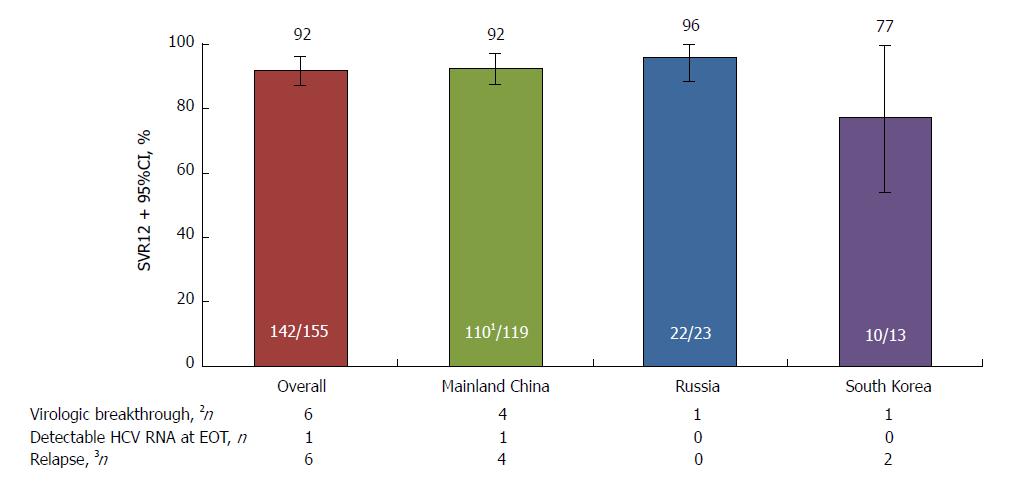
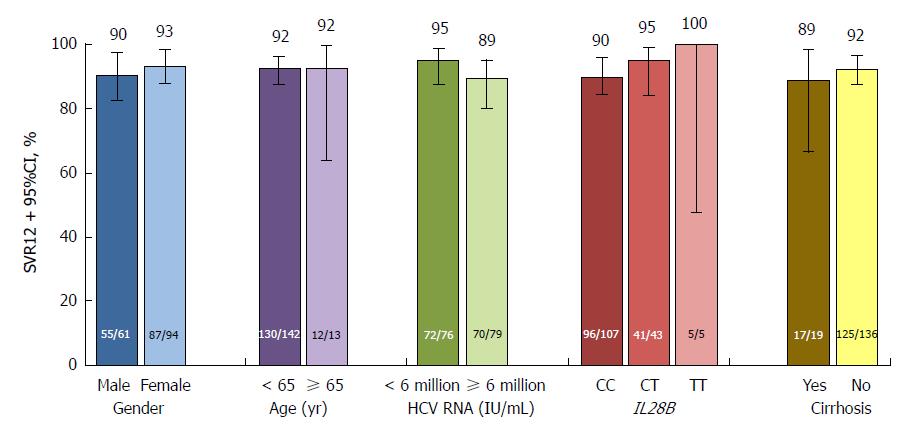
The study met its primary endpoint, with SVR12 achieved by 142 (91.6%, 95%CI: 87.2-96.0) patients in the immediate treatment arm (including the patient with HCV genotype 1a infection), significantly above the 70% historical comparator (Figure 1). SVR12 was comparable between patients from mainland China (110/119, 92.4%) and Russia (22/23, 95.7%), although lower among the smaller cohort of patients from South Korea (10/13, 76.9%). SVR12 in this arm was also comparable between patients with (17/19, 89.5%) and without (125/136, 91.9%) cirrhosis, with IL28B CC (96/107, 89.7%) and non-CC genotypes (46/48, 95.8%), aged < 65 (130/142, 91.5%) and ≥ 65 (12/13, 92.3%) years, with baseline HCV RNA < 6 million (72/76, 94.7%) and ≥ 6 million (70/79, 88.6%) IU/mL, and between male (55/61, 90.2%) and female (87/94, 92.6%) patients (Figure 2). HCV RNA declined rapidly from baseline, and by week 4 was undetectable in 140 (90.3%) patients.
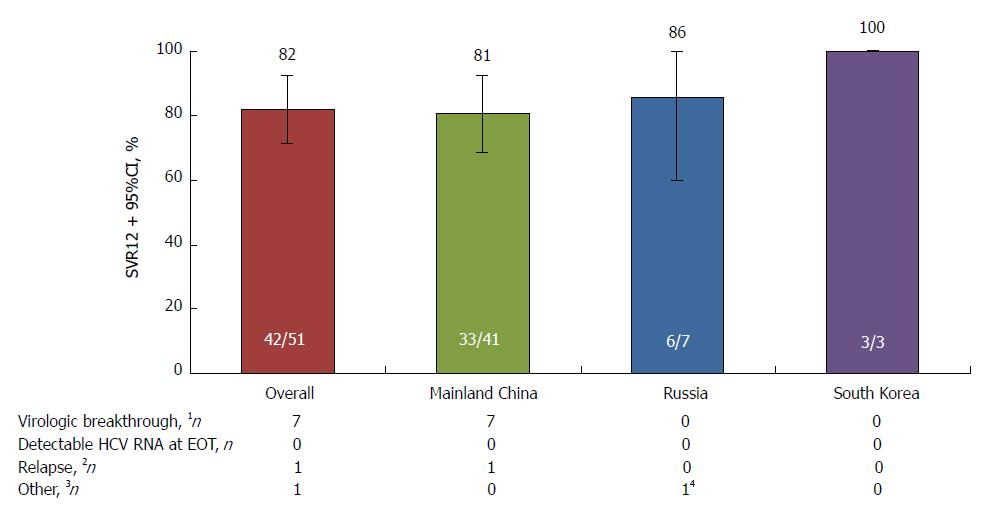
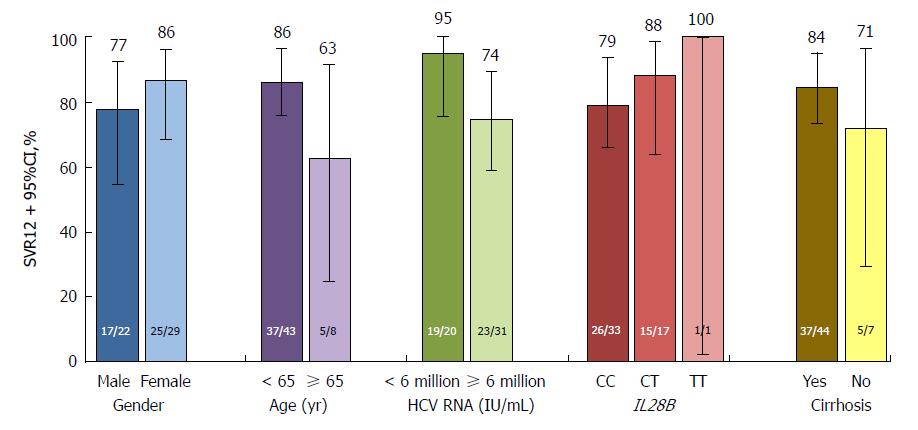
SVR12 rates in the placebo-deferred treatment arm, overall and according to selected baseline characteristics, are provided in Figures 3 and 4.
Thirteen (8.4%) patients in the immediate treatment arm failed to achieve SVR12. Six patients experienced virologic breakthrough [mainland China (n = 4), South Korea (n = 1), and Russia (n = 1)], one patient from mainland China had detectable HCV RNA at EOT, and six patients relapsed [mainland China (n = 4) and South Korea (n = 2)] (Figure 1).
Treatment failure in the placebo-deferred treatment arm is described in Figure 3.
Resistance analyses were conducted at baseline for 154 patients in the immediate treatment arm (excluding the patient with HCV genotype 1a infection) (Tables 2 and 3). Daclatasvir resistance-associated polymorphisms at NS5A amino acid positions L31 or Y93H preexisted in 17 (11.0%) patients, 9 of whom (52.9%) achieved SVR12. By contrast, SVR12 was achieved by 132 of 137 (96.4%) patients without baseline NS5A-L31 or NS5A-Y93H, and was comparably high among patients with (17/19, 89.5%) and without (115/118, 97.5%) cirrhosis who did not have baseline resistance-associated polymorphisms.
| All patients - immediate treatment arm | ||||||||
| With RAPs at baseline | Without RAPs at baseline | |||||||
| Mainland China | Russia | South Korea | Overall | Mainland China | Russia | South Korea | Overall | |
| NS5A-L31M/V | 1/1 (100) | 1/1(100) | 0 | 2/2 (100) | 108/117 (92.3) | 21/22 (95.5) | 10/13 (76.9) | 139/152 (91.4) |
| Y93H | 7/13(53.8) | 0 | 0/2 (0) | 7/15 (46.7) | 102/105 (97.1) | 22/23 (95.7) | 10/11(90.9) | 134/139 (96.4) |
| L31M/V or Y93H | 8/14 (57.1) | 1/1 (100) | 0/2 (0) | 9/17 (52.9) | 101/104 (97.1) | 21/22 (95.5) | 10/11(90.9) | 132/137 (96.4) |
| NS3-D168E | 0/1 (0) | 0 | 0 | 0/1 (0) | 109/117 (93.2) | 22/23 (95.7) | 10/13 (76.9) | 141/153 (92.2) |
| Patients with cirrhosis - immediate treatment arm | ||||||||
| With RAPs at baseline | Without RAPs at baseline | |||||||
| Mainland China | Russia | South Korea | Overall | Mainland China | Russia | South Korea | Overall | |
| Patients with cirrhosis | ||||||||
| NS5A-L31M/V | 0 | 0 | 0 | 0 | 15/16 (93.8) | 0 | 2/3 (66.7) | 17/19 (89.5) |
| Y93H | 0 | 0 | 0 | 0 | 15/16 (93.8) | 0 | 2/3 (66.7) | 17/19 (89.5) |
| L31M/V or Y93H | 0 | 0 | 0 | 0 | 15/16 (93.8) | 0 | 2/3 (66.7) | 17/19 (89.5) |
| NS3-D168E | 0 | 0 | 0 | 0 | 15/16 (93.8) | 0 | 2/3 (66.7) | 17/19 (89.5) |
| Patients without cirrhosis | ||||||||
| NS5A-L31M/V | 1/1 (100) | 1/1 (100) | 0 | 2/2 (100) | 93/101 (92.1) | 21/22 (95.5) | 8/10 (80.0) | 122/133 (91.7) |
| Y93H | 7/13 (53.8) | 0 | 0/2 (0) | 7/15 (46.7) | 87/89 (97.8) | 22/23 (95.7) | 8/8 (100) | 117/120 (97.5) |
| L31M/V or Y93H | 8/14 (57.1) | 1/1 (100) | 0/2 (0) | 9/17 (52.9) | 86/88 (97.7) | 21/22 (95.5) | 8/8 (100) | 115/118 (97.5) |
| NS3-D168E | 0/1 (0) | 0 | 0 | 0/1 (0) | 94/101 (93.1) | 22/23 (95.7) | 8/10 (80.0) | 124/134 (92.5) |
The asunaprevir resistance-associated polymorphism NS3-D168E preexisted in one (0.6%) patient who did not achieve SVR12; this patient also had NS5A-Y93H at baseline. Of the 13 patients in the immediate treatment arm who failed to achieve SVR12, 8 (61.5%) had the NS5A-Y93H polymorphism at baseline, including the patient who also had baseline NS3-D168E. At treatment failure, all 13 patients had emergent NS5A-L31 and/or NS5A-Y93H substitutions, while 10 of these patients also had emergent NS3-D168 substitutions (A/E/H/V/Y).
The impact of baseline resistance-associated polymorphisms on SVR12 in the placebo-deferred arm is shown in Tables 4 and 5.
| All patients - placebo-deferred treatment arm | |||||||||
| With RAPs at baseline | Without RAPs at baseline | ||||||||
| Mainland China | Russia | South Korea | Overall | Mainland China | Russia | South Korea | Overall | ||
| NS5A-L31M/V | 0 | 0 | 0 | 0 | 33/41 (80.5) | 6/6 (100) | 3/3 (100) | 42/50 (84.0) | |
| Y93H | 2/8 (25.0) | 0 | 0 | 2/8 (25.0) | 31/33 (93.9) | 6/6 (100) | 3/3 (100) | 40/42 (95.2) | |
| L31M/V or Y93H | 2/8 (25.0) | 0 | 0 | 2/8 (25.0) | 31/33 (93.9) | 6/6 (100) | 3/3 (100) | 40/42 (95.2) | |
| NS3-D168E | 0 | 0 | 0 | 0 | 33/41 (80.5) | 6/6 (100) | 3/3 (100) | 42/50 (84.0) | |
| Patients with cirrhosis - placebo-deferred treatment arm | ||||||||
| With RAPs at baseline | Without RAPs at baseline | |||||||
| Mainland China | Russia | South Korea | Overall | Mainland China | Russia | South Korea | Overall | |
| Patients with cirrhosis | ||||||||
| NS5A-L31M/V | 0 | 0 | 0 | 0 | 3/5 (60.0) | 1/1 (100) | 1/1 (100) | 5/7 (71.4) |
| Y93H | 1/3 (33.3) | 0 | 0 | 1/3 (33.3) | 2/2 (100) | 1/1 (100) | 1/1 (100) | 4/4 (100) |
| L31M/V or Y93H | 1/3 (33.3) | 0 | 0 | 1/3 (33.3) | 2/2 (100) | 1/1 (100) | 1/1 (100) | 4/4 (100) |
| NS3-D168E | 0 | 0 | 0 | 0 | 3/5 (60.0) | 1/1 (100) | 1/1 (100) | 5/7 (71.4) |
| Patients without cirrhosis | ||||||||
| NS5A-L31M/V | 0 | 0 | 0 | 0 | 30/36 (83.3) | 5/5 (100) | 2/2 (100) | 37/43 (86.0) |
| Y93H | 1/5 (20.0) | 0 | 0 | 1/5 (20.0) | 29/31 (93.5) | 5/5 (100) | 2/2 (100) | 36/38 (94.7) |
| L31M/V or Y93H | 1/5 (20.0) | 0 | 0 | 1/5 (20.0) | 29/31 (93.5) | 5/5 (100) | 2/2 (100) | 36/38 (94.7) |
| NS3-D168E | 0 | 0 | 0 | 0 | 30/36 (83.3) | 5/5 (100) | 2/2 (100) | 37/43 (86.0) |
The safety outcomes observed during the 12-wk double-blind phase are summarized in Table 6. Five (3.2%) patients in the immediate-treatment arm had SAEs considered related [study drug overdose (n = 2)] or unrelated to treatment [ventricular extra-systoles (n = 1), acute cholecystitis (n = 1) and intervertebral disc protrusion (n = 1)], and three (5.8%) patients in the placebo-deferred treatment arm had SAEs [ALT elevation (n = 1), coronary artery disease (n = 1), and hepatitis E virus infection plus liver injury (n = 1; leading to study discontinuation)] while receiving placebo. No treatment-related deaths were observed during the study.
| Parameter | Immediate treatment, n = 155 | Placebo-deferred treatment, n = 52 |
| AEs leading to discontinuation | 0 (0) | 1 (2)1 |
| Serious AEs | 5 (3)2 | 3 (6)13 |
| AEs (any grade), ≥ 5% | ||
| ALT elevation | 5 (3) | 12 (23) |
| AST elevation | 2 (1) | 8 (15) |
| Hypertension | 11 (7) | 4 (8) |
| Upper respiratory tract infection | 10 (6) | 3 (6) |
| Platelet count decrease | 3 (2) | 4 (8) |
| Pyrexia | 1 (1) | 3 (6) |
| On-treatment grade 3-4 laboratory abnormalities | ||
| ALT | 1 (1) | 5 (10) |
| AST | 1 (1) | 3 (6) |
| Total bilirubin | 1 (1) | 0 (0) |
| Hemoglobin | 3 (2) | 0 (0) |
The most common AEs (any grade) occurring in > 5% of patients in either arm during the initial 12-weeks of treatment with DUAL (immediate treatment arm) compared with placebo (placebo-deferred arm) were elevated ALT (3.2% vs 23.1%), elevated AST (1.3% vs 15.4%), hypertension (7.1% vs 7.7%), upper respiratory tract infection (6.5% vs 5.8%), platelet count decrease (1.9% vs 7.7%) and pyrexia (0.6% vs 5.8%). The most common grade 3-4 laboratory abnormalities during this period (DUAL vs placebo) were related to ALT (0.6% vs 9.6%), AST (0.6% vs 5.8%), total bilirubin (0.6% vs 0%) and hemoglobin (1.9% vs 0%).
The safety outcomes observed during 24 wk of DUAL treatment in either arm are summarized in Table 7. Two (1.3%) patients in the immediate treatment arm had SAEs deemed unrelated to treatment [appendicitis (n = 1) and retinal detachment (n = 1)] in addition to the five patients with SAEs during the 12-wk double-blind phase. One (2.0%) patient in the placebo-deferred treatment arm (excluding the patient who discontinued during the 12-wk double-blind phase) discontinued due to fatality unrelated to treatment (stab wound). One patient in the immediate treatment arm discontinued after twice meeting the biochemical criteria for Hy’s law. On day 118, treatment was interrupted for this patient until day 124 due to grade 3 ALT (320 U/L) and AST (237 U/L), grade 2 bilirubin (36.3 μmol/L), and grade 1 alkaline phosphatase (201 U/L). By day 133, the patient’s AST level had improved to 195 U/L (grade 3), but levels of ALT (223 U/L) and blood bilirubin (37.6 μmol/L) remained elevated. On day 141, the patient’s blood bilirubin and ALT levels had improved to 32.5 μmol/L (grade 2) and 155 U/L (grade 2), respectively; however, he was diagnosed with grade 2 AST (152 U/L) and grade 2 AEs of jaundice and nausea. Given this patient’s already elevated levels of ALT, AST and alkaline phosphatase, he met the biochemical criteria for Hy’s law for a second time and discontinued treatment the next day. All events resolved by day 152 and the patient achieved SVR12.
| Parameter | Immediate treatment, n = 155 | Placebo-deferred treatment, n = 511 | Overall, n = 206 |
| AEs leading to discontinuation | 1 (1)2 | 1 (2)3 | 2 (1) |
| Serious AEs | 7 (5)45 | 1 (2)3 | 8 (4) |
| Deaths | 0 (0) | 1 (2)3 | 1 (< 1) |
| AEs (any grade), ≥ 5% | |||
| ALT elevation | 17 (11) | 5 (10) | 22 (11) |
| Upper respiratory tract infection | 13(8) | 8(16) | 21(10) |
| Hypertension | 11 (7) | 6 (12) | 17 (8) |
| AST elevation | 13 (8) | 3 (6) | 16 (8) |
| INR elevation6 | 11 (7) | 2 (4) | 13 (6) |
| Blood bilirubin elevation | 12 (8) | 0 (0) | 12 (6) |
| Fatigue | 5 (3) | 6 (12) | 11 (5) |
| On-treatment grade 3-4 laboratory abnormalities | |||
| ALT | 7 (5)2 | 2 (4)7 | 9 (4) |
| AST | 5 (3)2 | 1 (2)7 | 6 (3) |
| Total bilirubin | 1 (1) | 0 (0) | 1 (< 1) |
| Hemoglobin | 3 (2) | 0 (0) | 3 (1) |
| Platelets | 1 (1) | 0 (0) | 1 (< 1) |
| Absolute lymphocyte count | 0 (0) | 1 (2) | 1 (< 1) |
| Absolute neutrophil count | 1 (1) | 0 (0) | 1 (< 1) |
| Lipase | 3 (2) | 0 (0) | 3 (1) |
The most common AEs (any grade) occurring in > 5% of patients during 24 wk of treatment with DUAL in either treatment arm were elevated ALT (11%), upper respiratory tract infection (10%), hypertension (8%), elevated AST (8%), elevated international normalized ratio (6%), elevated blood bilirubin (6%) and fatigue (5%). The most common grade 3-4 laboratory abnormalities were related to ALT (4%), AST (3%), hemoglobin (1%) or lipase (1%) (Table 7).
In this study, SVR12 was achieved by 91.6% of patients with HCV genotype 1b infection who were randomly assigned to receive immediate treatment with DUAL. With the lower bound of the corresponding 95%CI (87.2%) greater than the prespecified 70% threshold, the primary endpoint was met, confirming that DUAL is more efficacious than peg-IFN plus ribavirin in patients with HCV genotype 1b infection.
SVR12 was comparable between patients from mainland China (92.4%) and Russia (95.7%). By contrast, SVR12 was lower among patients from South Korea (76.9%); however, this was a small cohort and two of the three patients experiencing virologic failure had the NS5A-Y93H polymorphism at baseline, which has been shown to reduce SVR in patients with HCV genotype 1b infection receiving DUAL[18,22,23]. SVR12 was also lower among patients in the placebo-deferred arm following treatment with DUAL (42/51, 82.4%); however, again this was a small cohort and six of the eight patients with virologic failure had the NS5A-Y93H polymorphism at baseline. Nonetheless, consistent with the results of other phase 3 studies, SVR12 was high overall and largely unaffected by characteristics known to attenuate response to IFN, namely cirrhosis, IL28B non-CC genotypes, male sex, advanced age, and high baseline HCV RNA[10-13]. Virologic failure in the immediate treatment arm tended to coincide with the presence of baseline NS5A polymorphisms at L31M or Y93H, consistent with previous observations[18]. Although the prevalence of NS5A-L31 or NS5A-Y93H was relatively low in this study (11.0%), the observed SVR12 rates were, consistent with previous reports, higher among patients without these baseline polymorphisms (132/137, 96.4%), including those with cirrhosis (17/19, 89.5%), compared with cirrhotic patients with these baseline polymorphisms (9/17, 52.9%).
During the 12-wk double-blind phase, SAEs and AEs leading to discontinuation were infrequently observed in the immediate (5/155, 3.2% and none) and placebo-deferred (3/52, 5.8% and 1/52, 1.9%) treatment arms. However, although the AE profiles were broadly comparable between the two arms, elevations of ALT and AST were more common among patients receiving placebo (12/52, 23.1% and 8/52, 15.4%) compared with those receiving DUAL (5/155, 3.2% and 2/155, 1.3%). Consistent with this, grade 3-4 ALT and AST laboratory abnormalities during the blinded phase were more common among patients receiving placebo compared with those receiving DUAL. These elevations most likely reflected ongoing inflammation from untreated HCV infection; indeed, ALT and AST levels in most of these patients had begun to decrease by week 2 of open-label treatment with DUAL. One patient in the immediate treatment arm met the criteria for Hy’s law during treatment with DUAL; however, following treatment discontinuation, the events resolved and the patient achieved SVR12.
DUAL was well tolerated during 24 wk of treatment in both arms, consistent with findings from other phase 3 studies[10-12,19]. SAEs (8/206, 3.9%) and AEs leading to discontinuation (2/206, 1.0%) were infrequently observed and, except for two cases of study drug overdose, no SAEs were deemed treatment related. Emergent grade 3-4 laboratory abnormalities were similarly uncommon. The most common grade 3-4 laboratory abnormalities were related to ALT (9/206, 4.4%) and AST (6/206, 2.9%), however these reversed rapidly (median reversal times: 11.0 and 8.5 d for ALT and AST abnormalities, respectively) during or after treatment, and their incidences were comparable with those observed in other studies[10,24-26].
A limitation of this study was the absence of a direct IFN-based comparator for the primary efficacy endpoint. However, despite the continuing importance of IFN-based treatment across much of Asia, it was felt that including an IFN-based treatment arm in the study design would have been unethical. Peg-IFN is associated with a high burden of systemic AEs that include “flu-like” symptoms, neutropenia and thrombocytopenia[27], while ribavirin is associated with hemolytic anemia, birth defects, nausea, rash, itching, coughing and hyperuricemia[28,29]. The result is a combination with poor treatment adherence and a high rate of study discontinuations due to AEs[30]. Comparing DUAL, an all-oral combination with superior efficacy and safety profiles, to peg-IFN plus ribavirin, a combination containing an injectable drug with inferior efficacy and safety profiles, would therefore have lacked clinical equipoise. We also acknowledge that some patients were denied access to DUAL for 12 wk during the double-blind phase; however, as liver disease progresses slowly in patients with HCV infection, we do not believe that giving placebo instead of active treatment for 12 wk in compensated, treatment-naïve patients posed any ethical concerns.
In conclusion, the findings of this study showed that the all-oral DUAL combination of daclatasvir plus asunaprevir was highly effective and well tolerated in treatment-naïve patients from mainland China, Russia and South Korea with HCV genotype 1b infection. For patients in China, where IFN-based combinations have been considered the standard of care for HCV infection, DUAL was the first all-oral, nonribavirin-containing combination to gain approval, providing patients with access to a more efficacious and tolerable alternative for the treatment of HCV genotype 1b infection, with an easier route of administration and shorter treatment duration. DUAL is also predicted to be a cost-effective treatment alternative for HCV genotype 1b in China[31]. In addition, in countries such as Japan, where all-oral regimens are considered the standard of care for the treatment of HCV genotype 1b infection, DUAL is expected to be cost-saving compared with sofosbuvir/ledipasvir, with similar health outcomes[32].
Chronic hepatitis C virus (HCV) infection is a significant health burden across Asia, and affects 5-7 million people in China alone. Without effective treatment, patients can develop severe complications, such as cirrhosis or hepatocellular carcinoma. Previous therapies for the treatment of chronic HCV infection have been based on a combination of peg-interferon and ribavirin, both of which are associated with a high burden of adverse events (AEs) that contribute to poor treatment adherence and high rates of treatment discontinuations.
Daclatasvir plus asunaprevir (DUAL) is an all-oral combination of daclatasvir, an HCV NS5A inhibitor, and asunaprevir, an NS3 protease inhibitor. This regimen has previously demonstrated efficacy in several phase 3 studies of patients infected with HCV genotype 1b, including those characteristics known to attenuate response to interferon-based therapies. In this study, we sought to evaluate the efficacy and safety of DUAL in treatment-naïve patients from mainland China, South Korea and Russia.
The primary efficacy objective of the study was to measure the rate of sustained virologic response at posttreatment week 12 (SVR12) and to determine if this rate was significantly higher than the historical rate of 70% associated with peg-interferon plus ribavirin. Safety was monitored based on incidence of AEs and abnormalities in clinical laboratory assessments, vital signs and physical examinations.
This was a phase 3, double-blind, placebo-controlled study of DUAL in treatment-naïve patients from mainland China, South Korea and Russia with chronic HCV genotype 1b infection. Patients were randomly assigned (3:1) to receive DUAL (daclatasvir 60 mg tablet once daily and asunaprevir 100 mg soft capsule twice daily) for 24 wk either immediately (immediate treatment arm) or after 12 wk of matching placebo (placebo-deferred treatment arm).
An SVR12 rate of 91.6% (95% confidence interval: 87.2-96.0) was observed among patients in the immediate treatment arm, which was significantly higher than the historical comparator rate (70%). SVR12 was largely unaffected by cirrhosis (89%), age ≥ 65 years (92%), male sex (90%), baseline HCV RNA ≥ 6 million (89%), or IL28B non-CC genotypes (96%), although SVR12 was higher among patients without (96%) than among those with (53%) baseline NS5A resistance-associated polymorphisms (at L31 or Y93H). DUAL was well tolerated during 24 wk of therapy in this study; the most common AEs (≥ 10% in the combined arms) were elevated alanine aminotransferase and upper respiratory tract infection. Two patients discontinued DUAL treatment; one due to aminotransferase elevations, nausea and jaundice and the other due to a fatality unrelated to treatment. There were no treatment-related deaths.
This study demonstrates that the all-oral DUAL combination of daclatasvir plus asunaprevir was highly effective and well tolerated in treatment-naïve patients with HCV genotype 1b infection from mainland China, Russia and South Korea.
These findings suggest that for patients in many Asian countries, such as China, where interferon-based combinations have been considered the standard of care for HCV infection, DUAL offers a more efficacious and tolerable alternative for the treatment of HCV genotype 1b infection, with an easier route of administration and shorter treatment duration.
The authors would like to thank Phil Yin for support with the study. Editorial support was provided by Matthew Young of Articulate Science and was funded by Bristol-Myers Squibb.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Köksal AS, Takahashi T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Bennett H, Waser N, Johnston K, Kao JH, Lim YS, Duan ZP, Lee YJ, Wei L, Chen CJ, Sievert W. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol Int. 2015;9:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31 Suppl 2:61-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 396] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 3. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 4. | Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Chinese Society of Hepatology, Chinese Medical Association, Wei L; Chinese Society of Infectious Diseases, Chinese Medical Association, Hou JL. [The guideline of prevention and treatment for hepatitis C: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:906-923. [PubMed] |
| 6. | Tsoulfas G, Goulis I, Giakoustidis D, Akriviadis E, Agorastou P, Imvrios G, Papanikolaou V. Hepatitis C and liver transplantation. Hippokratia. 2009;13:211-215. [PubMed] |
| 7. | Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O’Boyle DR 2nd, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 762] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 9. | McPhee F, Sheaffer AK, Friborg J, Hernandez D, Falk P, Zhai G, Levine S, Chaniewski S, Yu F, Barry D. Preclinical Profile and Characterization of the Hepatitis C Virus NS3 Protease Inhibitor Asunaprevir (BMS-650032). Antimicrob Agents Chemother. 2012;56:5387-5396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 11. | Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, Everson GT, Heo J, Gerken G. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 12. | Wei L, Zhang M, Xu M, Chuang WL, Lu W, Xie W, Jia Z, Gong G, Li Y, Bae SH. A phase 3, open-label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J Gastroenterol Hepatol. 2016;31:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Kumada H, Suzuki F, Suzuki Y, Toyota J, Karino Y, Chayama K, Kawakami Y, Fujiyama S, Ito T, Itoh Y. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese hepatitis C virus patients. J Gastroenterol Hepatol. 2016;31:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1363] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Chen LM, He M. Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution. Virol J. 2017;14:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Dan YY, Lim SG. Hepatitis C: An Eastern Perspective. Gastroenterol Clin North Am. 2015;44:793-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Kao JH, Lee YJ, Heo J, Ahn SH, Lim YS, Peng CY, Chang TT, Torbeyns A, Hughes E, Bhore R. All-oral daclatasvir plus asunaprevir for chronic hepatitis C virus (HCV) genotype 1b infection: a sub-analysis in Asian patients from the HALLMARK DUAL study. Liver Int. 2016;36:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | McPhee F, Suzuki Y, Toyota J, Karino Y, Chayama K, Kawakami Y, Yu ML, Ahn SH, Ishikawa H, Bhore R. High Sustained Virologic Response to Daclatasvir Plus Asunaprevir in Elderly and Cirrhotic Patients with Hepatitis C Virus Genotype 1b Without Baseline NS5A Polymorphisms. Adv Ther. 2015;32:637-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Kao JH, Jensen DM, Manns MP, Jacobson I, Kumada H, Toyota J, Heo J, Yoffe B, Sievert W, Bessone F. Daclatasvir plus asunaprevir for HCV genotype 1b infection in patients with or without compensated cirrhosis: a pooled analysis. Liver Int. 2016;36:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Signorovitch JE, Betts KA, Song Y, Sorg RA, Li J, Behl AS, Kalsekar A. Comparative efficacy and safety of daclatasvir/asunaprevir versus IFN-based regimens in genotype 1b hepatitis C virus infection. J Comp Eff Res. 2015;4:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Bristol-Myers Squibb. China FDA approves country’s first all-oral regimen for chronic hepatitis C, Daklinza® (daclatasvir) in combination with Sunvepra® (asunaprevir) (press release). [Internet]. [cited. 2017;Jun 16] Available from: https://News.bms.com/press-release/bms/china-fda-approves-countrys-first-all-oral-regimen-chronic-hepatitis-c-daklinza-da. |
| 22. | Hernandez D, Yu F, Huang X, Kirov S, Pant S, McPhee F. Impact of Pre-existing NS5A-L31 or -Y93H Minor Variants on Response Rates in Patients Infected with HCV Genotype-1b Treated with Daclatasvir/Asunaprevir. Adv Ther. 2016;33:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | McPhee F, Hernandez D, Zhou N, Yu F, Ueland J, Monikowski A, Chayama K, Toyota J, Izumi N, Yokosuka O. Virological escape in HCV genotype-1-infected patients receiving daclatasvir plus ribavirin and peginterferon alfa-2a or alfa-2b. Antivir Ther. 2014;19:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 25. | Lok AS, Gardiner DF, Hézode C, Lawitz EJ, Bourlière M, Everson GT, Marcellin P, Rodriguez-Torres M, Pol S, Serfaty L. Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders. J Hepatol. 2014;60:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Bronowicki JP, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, Morris DW, Younes Z, Fried MW, Bourlière M. Randomized study of asunaprevir plus pegylated interferon-α and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antivir Ther. 2013;18:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Ferenci P. Safety and efficacy of treatment for chronic hepatitis C with a focus on pegylated interferons: the backbone of therapy today and in the future. Expert Opin Drug Saf. 2011;10:529-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Feld JJ, Jacobson IM, Sulkowski MS, Poordad F, Tatsch F, Pawlotsky JM. Ribavirin revisited in the era of direct-acting antiviral therapy for hepatitis C virus infection. Liver Int. 2017;37:5-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Dusheiko G, Main J, Thomas H, Reichard O, Lee C, Dhillon A, Rassam S, Fryden A, Reesink H, Bassendine M. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591-598. [PubMed] |
| 30. | Younossi ZM, Stepanova M, Henry L, Nader F, Younossi Y, Hunt S. Adherence to treatment of chronic hepatitis C: from interferon containing regimens to interferon and ribavirin free regimens. Medicine (Baltimore). 2016;95:e4151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Ward T, Gordon J, Wygant G, Yan J, Wang F, McEwan P. Assessing the economic impact of the introduction of daclatasvir in combination with asunaprevir for the treatment of chronic hepatitis C in China. ISPOR 20th Annual European Congress. Abstract/Poster 247. Accessed January 20. 2018; Available from: https://www.ispor.org/ScientificPresentationsDatabase/Presentation/78290?pdfid=51391. |
| 32. | Ward T, Webster S, Mishina S, McEwan P, Wygant G, Wang F. Assessing the Budget Impact and Economic Outcomes of the Introduction of Daclatasvir + Asunaprevir and Sofosbuvir/Ledipasvir for the Treatment of Chronic Hepatitis C Virus Infection in Japan. Value Health Reg Issues. 2017;12:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |