Published online Mar 28, 2018. doi: 10.3748/wjg.v24.i12.1332
Peer-review started: February 13, 2018
First decision: February 24, 2018
Revised: March 5, 2018
Accepted: March 7, 2018
Article in press: March 7, 2018
Published online: March 28, 2018
Processing time: 41 Days and 5.6 Hours
To evaluate the usefulness of frozen section diagnosis (FSD) of bile duct margins during surgery for extrahepatic cholangiocarcinoma (CCA).
We retrospectively analyzed 74 consecutive patients who underwent surgery for extrahepatic CCA from 2012 to 2017, during which FSD of bile duct margins was performed. They consisted of 40 distant and 34 perihilar CCAs (45 and 55 bile duct margins, respectively). The diagnosis was classified into three categories: negative, borderline (biliary intraepithelial neoplasia-1 and 2, and indefinite for neoplasia), or positive. FSD in the epithelial layer, subepithelial layer, and total layer was compared with corresponding permanent section diagnosis (PSD) postoperatively. Then, association between FSD and local recurrence was analyzed with special reference to borderline.
Analysis of 100 duct margins revealed that concordance rate between FSD and PSD was 68.0% in the total layer, 69.0% in the epithelial layer, and 98.0% in the subepithelial layer. The extent of remaining biliary epithelium was comparable between FSD and PSD, and more than half of the margins lost > 50% of the entire epithelium, suggesting low quality of the samples. In FSD, the rate of negative margins decreased and that of borderline and positive margins increased according to the extent of the remaining epithelium. Diagnostic discordance between FSD and PSD was observed in 31 epithelial layers and two subepithelial layers. Alteration from borderline to negative was the most frequent (20 of the 31 epithelial layers). Patients with positive margin in the total and epithelial layers by FSD demonstrated a significantly worse local recurrence-free survival (RFS) compared with patients with borderline and negative margins, which revealed comparable local RFS. Patients with borderline and negative margins in the epithelial layer by PSD also revealed comparable local RFS. These results suggested that epithelial borderline might be regarded substantially as negative. When classifying the status of the epithelial layer either as negative or positive, concordance rates between FSD and PSD in the total, epithelial, and subepithelial layers were 95.0%, 93.0%, and 98.0%, respectively.
During intraoperative assessment of bile duct margin, borderline in the epithelial layer can be substantially regarded as negative, under which condition FSD is comparable to PSD.
Core tip: Usefulness of intraoperative frozen section diagnosis (FSD) of bile duct margin for extrahepatic cholangiocarcinoma was investigated. The diagnosis was classified into negative, borderline (biliary intraepithelial neoplasia-1 and 2, and indefinite for neoplasia), or positive, and FSD was compared with permanent section diagnosis postoperatively. In contrast to previous studies, positive FSD in the epithelial layer was significantly associated with local recurrence. Furthermore, borderline FSD in the epithelial layer could be substantially regarded as negative, which could aid surgeons to determine the resection range of the bile duct. Finally, we demonstrated that FSD was reliable enough for pathological diagnosis.
- Citation: Shiraki T, Kuroda H, Takada A, Nakazato Y, Kubota K, Imai Y. Intraoperative frozen section diagnosis of bile duct margin for extrahepatic cholangiocarcinoma. World J Gastroenterol 2018; 24(12): 1332-1342
- URL: https://www.wjgnet.com/1007-9327/full/v24/i12/1332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i12.1332
Bile duct cancer (cholangiocarcinoma: CCA) is a rare malignancy (incidence < 6 cases per 100000 people) in most countries[1], and approximately 8000 people in the United States are diagnosed with CCA annually[2]. It develops in any part of the bile duct system and it is classified into three types based on location: intrahepatic CCA, perihilar CCA (pCCA), and distal CCA (dCCA). The latter two types are grouped as extrahepatic CCA (eCCA). Taken together, CCAs represent the second most frequent liver cancer and up to 3% of all gastrointestinal cancers[1,3]. CCA is generally asymptomatic in the early stages, and a late diagnosis and anatomical complexity of the cancer location result in poor prognosis: Five-year survival rate of eCCA with American Joint Committee on Cancer tumor node metastasis (TNM) stage I is 30%, stages II and III 24%, and stage IV 2%[2].
Most TNM stage 0, I, and II CCAs and some stage III CCAs are potentially resectable, and complete surgical resection is the only treatment with the potential for cure. The status of the final ductal margin is strongly associated with prognosis of patients with resectable CCA[2,4]. Intraoperative frozen section diagnosis (FSD) of the bile duct margins has traditionally been used to guide the extent of operative resection, but the usefulness of FSD has been controversial until now[5-9]. Because of the rarity and locoregional anatomical complexity of CCA, few centers have substantial clinical experience of managing this disease, and few pathologists have expertise in characterizing resected specimens accurately. In addition, the greatest difficulty of FSD is the low quality of samples because of tissue degeneration and/or destruction during freezing and sectioning. Therefore, production of formalin-fixed and paraffin-embedded samples that reuses frozen samples, and comparison between FSD and permanent section diagnosis (PSD) are mandatory. As a result, alteration of diagnosis often occurs. The primary purpose of this study was to examine reliability of intraoperative FSD to evaluate the margin status. The secondary purpose was to clarify clinical relevance of borderline lesions that could not be definitely determined whether malignant or benign. Borderline in the present study included such lesions as low-grade and intermediate-grade dysplasia (biliary intraepithelial neoplasia (BilIN)-1 and BilIN-2)[10] and lesions indefinite for neoplasia that could not be determined as reactive or neoplastic. For these purposes, we analyzed postoperative local recurrence of eCCA according to the margin status of FSD.
We analyzed 74 consecutive patients who underwent hemihepatectomy and/or pancreaticoduodenectomy for eCCA at the Department of Gastroenterological Surgery, Dokkyo Medical University from December 2012 to February 2017. There were 40 cases of dCCA (45 bile duct margins) and 34 of pCCA (55 bile duct margins). The histopathological diagnosis was reviewed by two experienced pathologists (HK and YI), and diagnostic inconsistency between the two pathologists was resolved by discussion. The clinicopathological information was retrospectively retrieved on the electronic medical chart system of the Dokkyo Medical University Hospital (Table 1).
| pCCA (n = 34) | dCCA (n = 40) | |
| Age (yr), median (range) | 71.5 (44-82) | 72.5 (39-85) |
| Gender | ||
| Male | 23 | 6 |
| Female | 11 | 34 |
| Preoperative biliary drainage | ||
| Yes | 33 | 38 |
| No | 1 | 2 |
| Procedure | ||
| PD | 1 | 38 |
| HH | 27 | 1 |
| PD + HH | 5 | 0 |
| Bile duct resection | 0 | 1 |
| Others | 1 | 0 |
| Total number of duct margins for frozen section | 55 | 45 |
| Number of duct margins for frozen section | ||
| 1 | 15 | 35 |
| 2 | 17 | 5 |
| 3 | 2 | 0 |
| pT | ||
| pT1/pT2 | 27 | 20 |
| pT3/pT4 | 6 | 19 |
| Unknown | 1 | 1 |
FSD of the resected bile duct margin was performed during the operation. The margin tissue was mounted in WHITE TISSUE-COAT (U.I. Kasei, Amagasaki, Hyogo, Japan), frozen in liquid nitrogen, and thin sections were cut from the frozen blocks using a cryostat. The sections were stained by hematoxylin and eosin and subjected to microscopic diagnosis. At least two, three or more as needed, pieces of frozen sections were examined for each margin. When FSD was positive for malignancy (positive) in the first submitted specimen, additional resection of the margin was performed to the maximal extent possible. Results of the last submitted specimens were analyzed in the present study. After FSD, the tissues were thawed, fixed in formalin, and embedded in paraffin. Thin sections were cut from paraffin-embedded blocks, stained, and observed with microscopy. FSD and PSD were compared with each other.
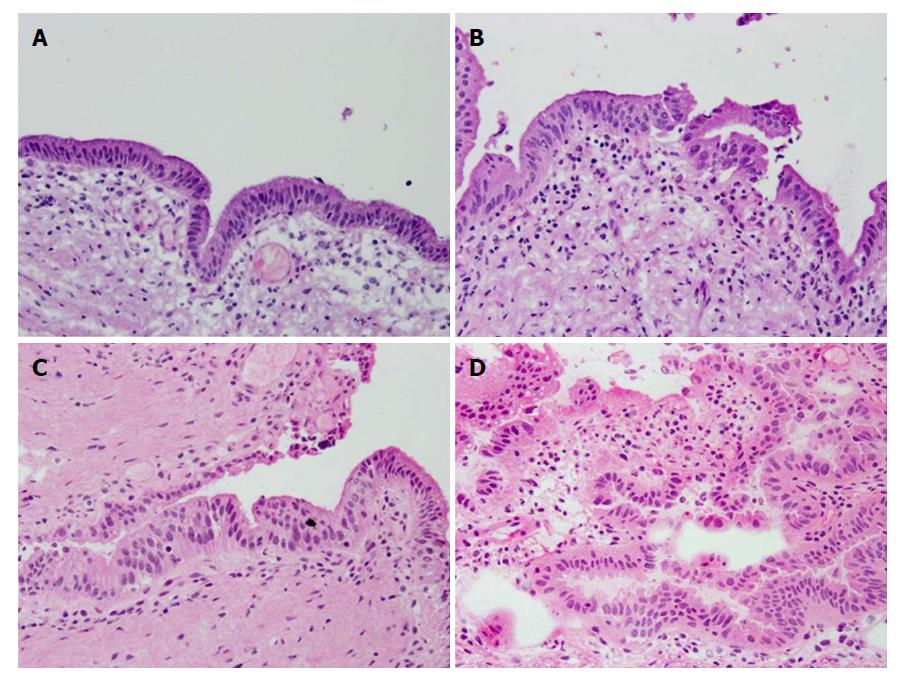
The surgical margins were diagnosed as either negative for malignancy (negative), borderline, or positive (Figure 1). Borderline included BilIN-1 and 2, and indefinite for neoplasia. We separately assessed the epithelial and subepithelial layers, and made a diagnosis based on both results. The epithelial layer tends to detach from the basement membrane during sample preparation for FSD. In relation to the entire circumference, we defined E1 as 0%-24% remaining epithelium, E2 as 25%-49% remaining epithelium, and E3 as 50%-100% remaining epithelium.
The concordance rate between FSD and PSD was investigated at the margin and patient levels, but survival analysis was performed solely at the patient level; for example, a patient with two negative margins and one positive margin was assigned to the positive group.
The presence or absence of postoperative local recurrence was detected by imaging studies including ultrasonography and computed tomography. The criteria for the local recurrence were defined as mass lesions within the resection field with or without clinical manifestation and/or elevated tumor markers. The diagnosis of local recurrence was made by the surgeons in charge of each patient.
Comparison of categorical data sets between FSD and PSD was performed by the χ2 test. Local recurrence-free survival (RFS) curves were depicted using the Kaplan-Meier method and analyzed by the log-rank test. P < 0.05 was considered significant. Statistical analysis was performed using IBM SPSS Statistics 24 (IBM, Armonk, NY, United States).
FSD revealed 41 (41.0%) negative, 20 (20.0%) positive, and 39 (39.0%) borderline out of 100 bile duct margins, while PSD revealed 56 (56.0%) negative, 21 (21.0%) positive, and 23 (23.0%) borderline margins (Table 2). The number of positive margins was similar between FSD and PSD, but the number of negative margins increased and that of borderline decreased significantly in PSD (P = 0.039) (Table 2). The concordance rate between FSD and PSD is summarized in Table 3 as original diagnostic results.
| Negative (%) | Borderline (%) | Positive (%) | Total (%) | P value | |
| Margin level | |||||
| Total layer | |||||
| FSD | 41 (41.0) | 39 (39.0) | 20 (20.0) | 100 (100) | 0.039 |
| PSD | 56 (56.0) | 23 (23.0) | 21 (21.0) | 100 (100) | |
| Epithelial layer | |||||
| FSD | 44 (44.0) | 41 (41.0) | 15 (15.0) | 100 (100) | 0.078 |
| PSD | 59 (59.0) | 27 (27.0) | 14 (14.0) | 100 (100) | |
| Subepithelial layer | |||||
| FSD | 87 (87.0) | 1 (1.0) | 12 (11.0) | 100 (100) | 0.560 |
| PSD | 86 (86.0) | 0 (0.0) | 14 (14.0) | 100 (100) | |
| Patient level | |||||
| Total layer | |||||
| FSD | 26 (35.1) | 31 (41.9) | 17 (23.0) | 74 (100) | 0.134 |
| PSD | 36 (48.7) | 20 (27.0) | 18 (24.3) | 74 (100) |
| Concordance rate (%) | Sensitivity (%) | Specificity (%) | Positive-predictive value (%) | Negative-predictive value (%) | |
| Original diagnostic results | |||||
| Total layer | 68.0 | 85.7 | 66.1 | 90.0 | 90.2 |
| Epithelial layer | 69.0 | 78.6 | 64.6 | 73.3 | 86.4 |
| Subepithelial layer | 98.0 | 85.7 | 100.0 | 100.0 | 98.9 |
| Revised diagnostic results | |||||
| Total layer | 95.0 | 85.7 | 97.5 | 90.0 | 97.5 |
| Epithelial layer | 93.0 | 70.0 | 95.6 | 73.3 | 96.5 |
| Subepithelial layer | 98.0 | 85.7 | 100.0 | 100.0 | 98.9 |
We separately analyzed the status of the surgical margin in the epithelial and subepithelial layers. FSD in the epithelial layer revealed 44 (44.0%) negative, 15 (15.0%) positive, and 41 (41.0%) borderline margins, while PSD revealed 59 (59.0%) negative, 14 (14.0%) positive, and 27 (27.0%) borderline margins. The number of positive margins was similar between FSD and PSD, but the number of negative margins increased and that of borderline decreased in PSD with marginal significance (P = 0.078) (Table 2) (Figure 2A and B).
The extent of the remaining biliary epithelium lining the resected margin might represent the quality of samples especially in evaluating the epithelial layer. A total of 33 samples were E1, 21 were E2, and 46 were E3 in FSD, while a total of 32 samples were E1, 21 were E2, and 47 were E3 in PSD (Table 4). The rate of the remaining epithelium was almost identical between FSD and PSD. More than half of the total margins lacked > 50% of the entire biliary epithelium in FSD and PSD. The rate of negative margins decreased and the rate of borderline and positive margins increased in FSD according to the rate of the remaining epithelium. This suggested proportional sensitivity to the remaining rate and intrinsic difficulty in the assessment of the epithelial layer. The concordance rate between FSD and PSD in the evaluation of the epithelial layer is summarized in Table 3 as original diagnostic results.
| Extent of the remaining epithelium | Negative (%) | Borderline (%) | Positive (%) | Total (%) | |
| FSD | |||||
| E1 (0%-24%) | 22 (66.6) | 9 (27.3) | 2 (6.1) | 33 (100) | |
| E2 (25%-49%) | 8 (38.1) | 11 (52.4) | 2 (9.5) | 21 (100) | |
| E3 (50%-100%) | 14 (30.4) | 21 (45.7) | 11 (23.9) | 46 (100) | |
| PSD | |||||
| E1 (0%-24%) | 22 (68.7) | 7 (21.9) | 3 (9.4) | 32 (100) | |
| E2 (25%-49%) | 9 (42.9) | 9 (42.9) | 3 (14.3) | 21 (100) | |
| E3 (50%-100%) | 28 (59.6) | 11 (23.4) | 8 (17.0) | 47 (100) |
In the subepithelial layer, FSD revealed 87 (87.0%) negative, 12 (12.0%) positive, and 1 (1.0%) borderline margins, while PSD revealed 86 (86.0%) negative and 14 (14.0%) positive margins. There was a nearly complete consistency between FSD and PSD (P = 0.560) (Table 2). The concordance rate between FSD and PSD in the evaluation of the subepithelial layer is summarized in Table 3 as original diagnostic results.
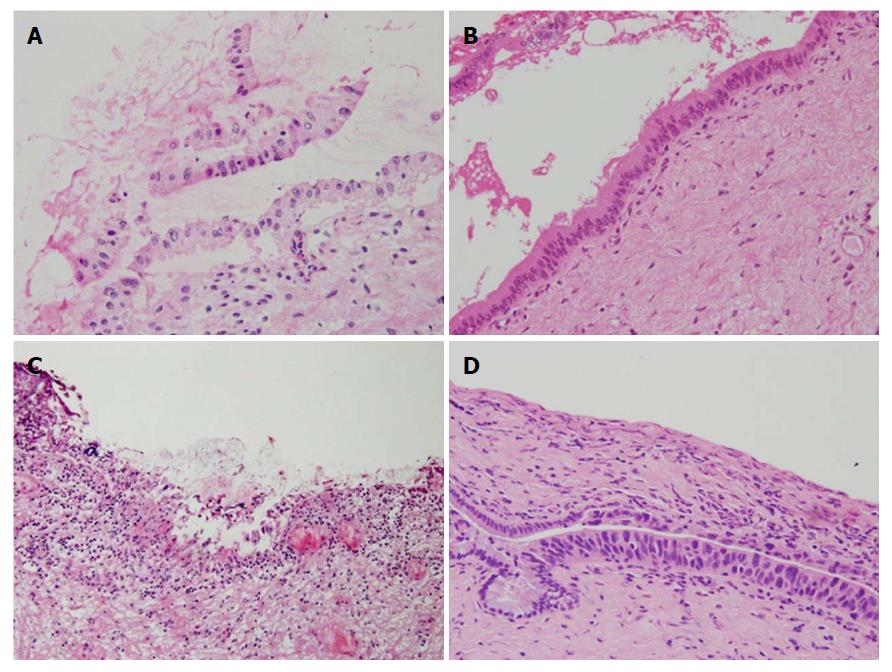
Diagnostic discordance between FSD and PSD was observed in 31 epithelial layers and two subepithelial layers (Table 5). The discordance rate in the epithelial layer was considerably high, while that in the subepithelial layer was very low. The discordance rate in the epithelial layer was somewhat higher in pCCA than dCCA, but there was no significant difference in the discordance rate between pCCA and dCCA in the epithelial layer and subepithelial layer (P = 0.128 and 1.000, respectively). Alteration from borderline to negative in the epithelial layer was the most frequent (20 margins). Less frequently, alterations from negative to borderline (4 margins) and positive to borderline (4 margins) were observed in the epithelial layer (Table 6). Regrettably, alteration from negative to positive was also noted in two margins (Figure 2C and D).
| Diagnostic discordance | Total (%) | ||
| Yes (%) | No (%) | ||
| Epithelial layer | 31 (31.0) | 69 (69.0) | 100 (100) |
| pCCA | 21 (38.2) | 34 (61.8) | 55 (100) |
| dCCA | 10 (22.2) | 35 (77.8) | 45 (100) |
| Subepithelial layer | 2 (2.0) | 98 (98.0) | 100 (100) |
| pCCA | 1 (1.8) | 54 (98.2) | 55 (100) |
| dCCA | 1 (2.2) | 44 (97.8) | 45 (100) |
| Total layer | 28 (28.0) | 72 (72.0) | 100 (100) |
| pCCA | 18 (32.7) | 37 (67.3) | 55 (100) |
| dCCA | 10 (22.2) | 35 (77.8) | 45 (100) |
| Epithelial layer (pCCA:dCCA) | Subepithelial layer (pCCA:dCCA) | |
| From negative to borderline | 4 (2:2) | 0 (0:0) |
| From negative to positive | 1 (0:1) | 1 (1:0) |
| From borderline to negative | 20 (13:7) | 0 (0:0) |
| From borderline to positive | 2 (2:0) | 1 (0:1) |
| From positive to borderline | 4 (4:0) | 0 (0:0) |
| Total | 31 (21:10) | 2 (1:1) |
FSD revealed 26 (35.1%) negative, 17 (23.0%) positive, and 31 (41.9%) borderline margins in 74 patients with eCCA, while PSD revealed 36 (48.7%) negative, 18 (24.3%) positive, and 20 (27.0%) borderline margins. The number of positive margins was similar between FSD and PSD, but the number of negative margins increased and that of borderline decreased slightly in PSD (P = 0.134) (Table 2).
The overall follow-up period of the 74 patients from surgery to disease-related death or censoring were 4 to 2343 days (Median, 623 d).
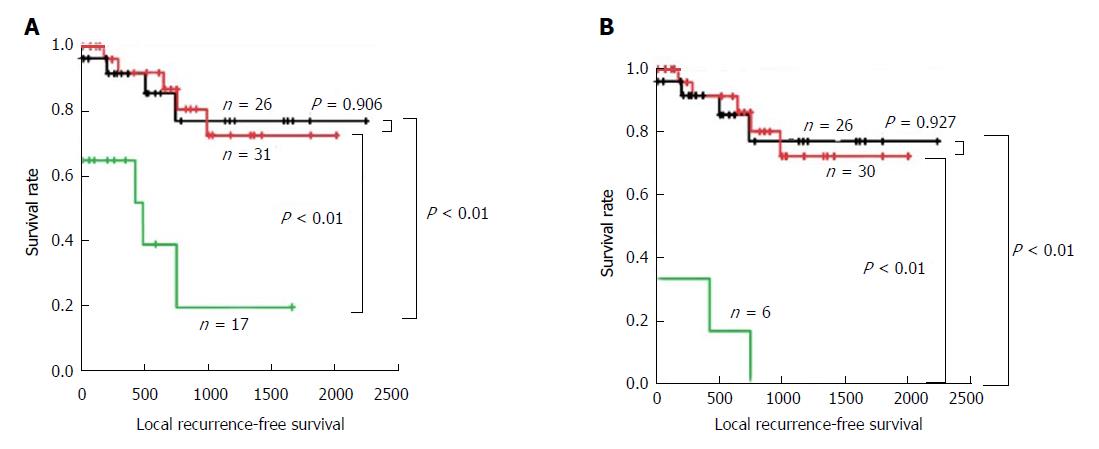
We first performed local RFS analysis based on FSD of the bile duct margin in the total layer. Local RFS rates for 1, 3, and 5 years are listed in Table 7. Patients with positive margins demonstrated a significantly worse survival compared with those with negative or borderline margins (both P < 0.01). In contrast, patients with negative and borderline margins showed comparable prognoses (P = 0.906) (Figure 3A).
| Duration (yr) | Negative | Borderline | Positive | |
| Total layer | ||||
| Number of cases | 26 | 31 | 17 | |
| 1 | 0.916 | 0.918 | 0.518 | |
| 3 | 0.769 | 0.725 | 0.194 | |
| 5 | 0.769 | 0.725 | 0.194 | |
| 1Epithelial layer | ||||
| Number of cases | 26 | 30 | 6 | |
| 1 | 0.916 | 0.915 | 0.333 | |
| 3 | 0.769 | 0.722 | 0.000 | |
| 5 | 0.769 | 0.722 | 0.000 |
We then focused on the status of the epithelial layer, since we thought that diagnosis as borderline was the greatest issue for surgeons in deciding whether to perform additional resection. Patients with borderline and positive margins in the subepithelial layer were excluded from this analysis in order to investigate the pure effect of the status of the epithelial layer. Local RFS rates for 1, 3, and 5 years are listed in Table 7. Patients with positive margins demonstrated a significantly worse survival compared with those with negative or borderline margins (both P < 0.01). In contrast, patients with negative and borderline margins showed comparable prognoses (P = 0.927) (Figure 3B).
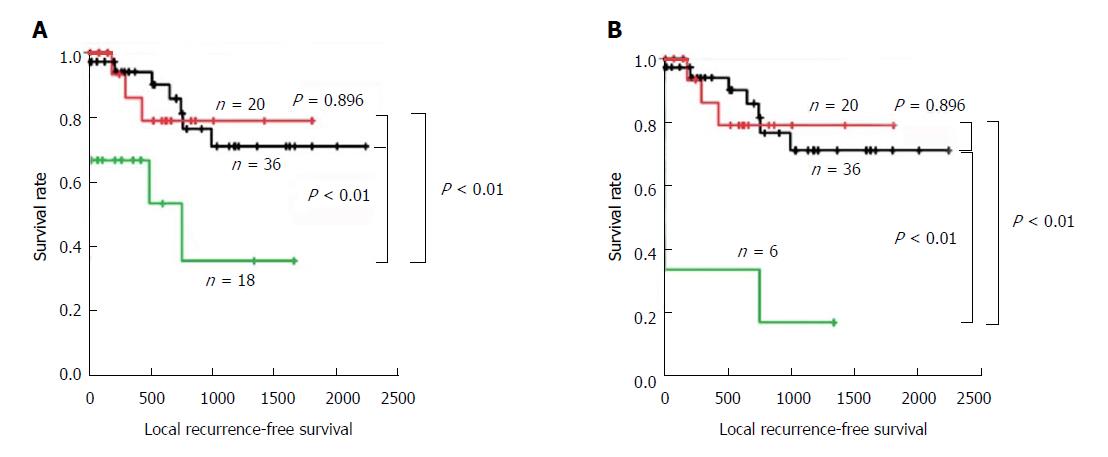
Local RFS analysis according to the epithelial and total status assessed by PSD demonstrated similar results (Figure 4 and Table 8). Patients with positive margins in the total and epithelial layers demonstrated significantly worse survival compared with those with negative or borderline margins (all P < 0.01). In contrast, patients with negative and borderline margins in the total and epithelial layers showed similar prognoses (both P = 0.896).
| Duration (yr) | Negative | Borderline | Positive | |
| Total layer | ||||
| Number of cases | 36 | 20 | 18 | |
| 1 | 0.940 | 0.862 | 0.667 | |
| 3 | 0.710 | 0.790 | 0.356 | |
| 5 | 0.710 | ND | ND | |
| 1Epithelial layer | ||||
| Number of cases | 36 | 20 | 6 | |
| 1 | 0.940 | 0.862 | 0.333 | |
| 3 | 0.710 | 0.790 | 0.167 | |
| 5 | 0.710 | ND | ND |
Based on the results of survival analysis, borderline margins in the epithelial layer were regarded substantially as negative. Histopathological diagnosis in the epithelial layer was reclassified into positive or negative, and the concordance rate between FSD and PSD was revised (Table 3). The concordance rates in the total, epithelial, and subepithelial layers were 95.0%, 93.0%, and 98.0%, respectively. These results suggest that FSD is a reliable method to evaluate margin status of the bile duct intraoperatively.
The status of the bile duct margin has been assessed by intraoperative FSD for complete resection of eCCA. However, the usefulness of FSD is controversial owing to the frequent discordance between FSD and PSD. Okazaki et al[5] reported that concordance rate between FSD and PSD was only 56.5%, and concluded that FSD should not be carried out for patients with a high risk of hepatic failure. Yamaguchi et al[7] reported that diagnosis of resected bile duct margin was altered from FSD to PSD in five of 20 patients with gall bladder or bile duct cancer who underwent surgical resection. Endo et al[9] reported that discrepancies between FSD and PSD were observed in 10 of 101 patients with pCCA who underwent surgery. In the present study, we experienced diagnostic discordance in 28 of 100 duct margins between FSD and PSD. This high discordance rate was probably due to the grouping method of histopathological results. The margin status was classified into either positive or negative by Yamaguchi et al[7] and either positive/suspicious or negative in the study by Endo et al[9], in which only invasive carcinoma was diagnosed as positive. In contrast, the margin status in the present study was grouped into three categories of positive, borderline, or negative. Diagnostic discordance in this study was only 2% if only invasive cancer was classified as positive.
Discordance between FSD and PSD was observed in 28 bile duct margins; 31 in the epithelial layer and two in the subepithelial layer. The most frequent alteration was borderline to negative in the epithelial layer. During frozen sample preparation, epithelium easily detaches from basement membrane, and becomes twisted, folded, and overlapped. The nuclei are often swollen because of rapid freezing, which makes it difficult to discriminate the epithelium from dysplasia and/or carcinoma in situ. We assume that the low quality of the frozen section sample may have been the greatest cause of the discordance (Figure 2A and B). The low quality of the frozen section sample was also demonstrated by the low remaining rate of the epithelium for histopathological evaluation. In this study, more than half of the epithelium was lost during sample preparation, and this might have resulted in underdiagnosis in the epithelial layer. In light of the similar extent of the remaining epithelium between FSD and PSD, the epithelial layer might be lost during resection for intraoperative diagnosis by surgeons. We experienced two cases of alteration from negative to positive. These were caused by sampling different sections within the bile duct margin (Figure 2C and D). The cut-surface of the permanent histology was different from that of frozen section histology. We did not overlook cancer cells within frozen section samples. Marked stromal cell infiltration into the tumor is an inherent characteristic of CCA, which may fundamentally underlie inaccurate FSD. Mucosal inflammation caused by the catheter for preoperative biliary drainage may also mislead the frozen diagnosis[5,7]. It may cause regenerative atypia of normal mucosa with thick and multilayered atypical epithelial cells and immature mesenchymal cells, which may be misdiagnosed as malignant epithelium showing sarcomatous changes. In this study, the rate of borderline was significantly higher in the epithelial layer than in the subepithelial layer on FSD, and borderline epithelial margins significantly decreased but borderline subepithelial margins did not change on PSD. This may be partly explained by the fact that almost all patients underwent biliary drainage tube insertion preoperatively (Table 1).
In the present study, patients with negative margins by FSD demonstrated a significantly favorable local RFS compared with patients with positive margins, suggesting that FSD is useful for complete resectability and predicting good prognosis. Positive margins in this study included the presence of cancer cells in the epithelial and/or subepithelial layers, of the bile duct submitted for diagnosis. However, clinical significance of positive surgical margins of the bile duct is controversial. Some authors reported no correlation between positive margins and postoperative local recurrence of pCCA[11]. In contrast, a strong correlation has been reported by other authors[9,12-14]. For example, pCCA patients with positive bile duct margins by paraffin section histology demonstrated significantly worse disease-specific survival compared with those with negative bile duct margins[9]. Bile duct margin was evaluated as positive only when invasive cancer was confirmed histologically[9]. In addition, local recurrence of gall bladder and bile duct cancer was slightly associated with the margin status by paraffin section histology, that is, 4/7 positive patients versus 9/37 negative patients (P = 0.081)[7]. In the patients with positive margins, local recurrence occurred only when cancer cells were observed in the subepithelial layer[7]. In the study of middle and distal bile duct cancer, PSD of the hepatic-side duct margin predicted local recurrence with marginal significance, that is, 2 of 6 (33%) positive patients versus 4 of 45 (9%) negative patients (P = 0.08)[4]. Localization of cancer cells in the surgical margin was not described in that study[4].
It has been reported that the presence of epithelial dysplasia at the bile duct margin confirmed postoperatively is not associated with survival of patients who undergo R0 resection[9,12,13]. Yamaguchi et al[7] also reported that local recurrence occurred in neither of the two patients with carcinoma in situ of the bile duct margin by permanent histopathology. In the present study, local recurrence was observed in all six patients with positive margins in the epithelial layer by FSD, suggesting the need for accurate intraoperative diagnosis of BilIN-3/severe dysplasia/in situ carcinoma. However, FSD of the bile duct is often difficult even for experienced pathologists. In addition, there is some interobserver variation in the evaluation of the grade of biliary dysplasia. In contrast, diagnosis of invasive carcinoma in the subepithelial layer is easier, especially when there is perineural invasion. Analysis of a greater number of cases is awaited to clarify the significance of BilIN-3/in situ carcinoma in the bile duct margin.
One of the main purposes of this study was to determine the relevance of borderline lesions, consisting of BilIN-1 and 2 and indefinite for neoplasia, diagnosed intraoperatively. Approximately 40% of the epithelial layer was diagnosed as borderline, while only 1% of the subepithelial layer was diagnosed as borderline by FSD. We thought that the difference was due to the following reasons: (1) intrinsic borderline lesion, such as BilIN-1 and 2, is defined as a diagnostic category in the epithelial lesion; (2) epithelium is more vulnerable to artifacts than subepithelial stromal tissue is; and (3) impact of preoperative biliary drainage tube insertion. Because our patients had pCCA and dCCA, which are locoregionally different tumors, we investigated only local recurrence rate and not overall survival rate. By survival analysis, patients with borderline margins in the epithelial layer demonstrated a comparable local RFS compared with patients with negative margins. These data suggested that epithelial borderline lesions might be interpreted substantially as negative margins and that additional ductal resection might not be necessary in such institutions as having well-experienced pathologists.
On the other hand, it is quite likely that some borderline margins may ultimately turn out to be positive in a larger series with more diverse pathologist. Hence, if the first margin is borderline and additional margin can be safely obtained, additional ductal resection will be desirable to achieve negative margin as the local recurrence is very high in positives.
In the present study, four of 26 patients with negative frozen margins had local recurrence. PSD was also negative in all these patients. This may have been because CCA sometimes shows discontinuous longitudinal spread or tumorigenesis from separate foci along the bile duct[14,15].
In conclusion, FSD of the bile duct margin was reliable enough to provide useful information for deciding the extent of resection of eCCA regardless of technical limitations in sample preparation. Positive margins in the epithelial layer was significantly associated with local recurrence, while the borderline margins demonstrated a similar local recurrence rate to that of negative margins. Although it is desirable to achieve negative margin if the first margin is borderline, epithelial borderline lesions could be regarded substantially as negative margins in such institutions as with well-experienced pathologists.
Cholangiocarcinoma (CCA) is a rare malignancy with poor prognosis. Complete surgical resection is the only treatment with the potential for cure, and the status of the final ductal margin is strongly associated with prognosis. Intraoperative frozen section diagnosis (FSD) of the bile duct margins has traditionally been used to guide the extent of operative resection, but its usefulness has been controversial until now.
Because of the rarity and locoregional anatomical complexity of CCA, few centers have substantial clinical experience of managing this disease, and few pathologists have expertise in characterizing resected specimens accurately. In addition, quality of FSD samples is very low. Hence, discordance between FSD and permanent section diagnosis (PSD) that reuses frozen samples often occurs.
The primary purpose of this study was to examine reliability of intraoperative FSD to evaluate the margin status. The secondary purpose was to clarify clinical relevance of borderline lesions that could not be definitely determined whether malignant or benign. Borderline in the present study included such lesions as low-grade and intermediate-grade dysplasia [biliary intraepithelial neoplasia (BilIN)-1 and BilIN-2] and lesions indefinite for neoplasia.
We retrospectively analyzed 74 consecutive patients who underwent surgery for extrahepatic CCA (eCCA) from 2012 to 2017, during which FSD of bile duct margins was performed. They consisted of 40 distant CCAs (dCCAs) and 34 perihilar CCAs (pCCAs) (45 and 55 bile duct margins, respectively). The diagnosis was classified into three categories: negative, borderline, or positive. FSD in the epithelial layer, subepithelial layer, and total layer was compared with corresponding PSD postoperatively. Then, association between FSD and local recurrence was analyzed. The concordance rate between FSD and PSD was investigated at the margin and patient levels, but survival analysis was performed solely at the patient level.
Analysis of 100 duct margins revealed that original concordance rate between FSD and PSD was 68.0% in the total layer, 69.0% in the epithelial layer, and 98.0% in the subepithelial layer. The extent of remaining biliary epithelium was comparable between FSD and PSD, and more than half of the margins lost > 50% of the entire epithelium, suggesting low quality of the samples. In FSD, the rate of negative margins decreased and that of borderline and positive margins increased according to the extent of the remaining epithelium, suggesting proportional sensitivity to the remaining rate and intrinsic difficulty in the assessment of the epithelial layer. Diagnostic discordance between FSD and PSD was observed in 31 epithelial layers and two subepithelial layers. Although the discordance rate in the epithelial layer was somewhat higher in pCCA than dCCA, there was no significant difference between them in the epithelial layer and subepithelial layer. Alteration from borderline to negative was the most frequent (20 of the 31 epithelial layers). Less frequently, alterations from negative to borderline (4 margins) and positive to borderline (4 margins) were observed in the epithelial layer. Although some authors reported no correlation between positive margins and postoperative local recurrence, in the present study patients with positive margin in the total and epithelial layers by FSD demonstrated a significantly worse local recurrence-free survival (RFS) compared with patients with borderline and negative margins. On the other hand, patients with borderline and negative margins in the total and epithelial layers by FSD revealed comparable local RFS. Patients with borderline and negative margins in the epithelial layer by PSD also revealed comparable local RFS. These results suggested that epithelial borderline might be regarded substantially as negative in such institutions as having well-experienced pathologists. However, if the first margin is borderline and additional margin can be safely obtained, additional ductal resection will be desirable to achieve negative margin, because it is quite likely that some borderline margins may ultimately turn out to be positive in a larger series with more diverse pathologist and the local recurrence is very high in positive margins. When classifying the status of the epithelial layer either as negative or positive, concordance rates between FSD and PSD in the total, epithelial, and subepithelial layers were 95.0%, 93.0%, and 98.0%, respectively. These results suggest that FSD is a reliable method to evaluate margin status of the bile duct intraoperatively.
FSD of the bile duct margin was reliable enough to provide useful information for deciding the extent of resection of eCCA regardless of technical limitations in sample preparation. In contrast to the previous reports, positive margins in the epithelial layer was significantly associated with local recurrence, while the borderline margins demonstrated a similar local recurrence rate to that of negative margins. Although negative margin is desirable, epithelial borderline lesions could be regarded substantially as negative in such institutions as with well-experienced pathologists. These findings would aid surgeons to determine the resection range of the bile duct and better manage the patients with eCCA.
Intraoperative FSD of the bile duct margins has traditionally been used to guide the extent of operative resection, but the usefulness of FSD has been controversial until now. In the present study, we clearly demonstrated that FSD was reliable enough for pathological diagnosis by comparing FSD and PSD and based on the results of survival analysis. In addition, in contrast to some previous reports, we demonstrated that positive FSD in the epithelial layer was significantly associated with local recurrence and that borderline FSD in the epithelial layer could be substantially regarded as negative. Our results may be partly due to a relatively large number of eCCA cases. This study also highlighted the need for precise and detailed histopathological diagnosis. In this respect, the future challenge is more objective differential diagnosis of BilIN-1, 2, and 3 by FSD. Development of morphometric analysis, special staining procedure, immunohistochemistry, and molecular diagnostics which can be available over a short time of intraoperative FSD are awaited. It will be also necessary to develop the training program of pathologists who can make a correct diagnosis of bile duct margin by intraoperative FSD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bramhall S, Kapoor S, Lin J S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2053] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 2. | About Bile Duct Cancer. American Cancer Society. Downloaded on October 30. 2017; Available from: https://www.cancer.org/content/dam/CRC/PDF/Public/8552.00.pdf. |
| 3. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 966] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 4. | Sakamoto Y, Kosuge T, Shimada K, Sano T, Ojima H, Yamamoto J, Yamasaki S, Takayama T, Makuuchi M. Prognostic factors of surgical resection in middle and distal bile duct cancer: an analysis of 55 patients concerning the significance of ductal and radial margins. Surgery. 2005;137:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Okazaki Y, Horimi T, Kotaka M, Morita S, Takasaki M. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology. 2002;49:625-627. [PubMed] |
| 6. | Hirohashi K, Uenishi T, Kubo S, Yamamoto T, Tanaka H, Shuto T, Yamasaki O, Horii K, Kinoshita H. Histologic bile duct invasion by a mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2002;9:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi K, Shirahane K, Nakamura M, Su D, Konomi H, Motoyama K, Sugitani A, Mizumoto K, Tanaka M. Frozen section and permanent diagnoses of the bile duct margin in gallbladder and bile duct cancer. HPB (Oxford). 2005;7:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lechago J. Frozen section examination of liver, gallbladder, and pancreas. Arch Pathol Lab Med. 2005;129:1610-1618. [PubMed] |
| 9. | Endo I, House MG, Klimstra DS, Gönen M, D’Angelica M, Dematteo RP, Fong Y, Blumgart LH, Jarnagin WR. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:2104-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Bosman FT, Carneiro F, Hruban RH, and Theise ND, eds ; Albores-Saavedra J, Adsay NV, Crawford JM et al. Carcinoma of the gall bladder and extrahepatic bile ducts. WHO classification of tumours of the digestive system, 4th ed. Lyon: IARC Press 2010; 266-273. |
| 11. | Bhuiya MR, Nimura Y, Kamiya J, Kondo S, Nagino M, Hayakawa N. Clinicopathologic factors influencing survival of patients with bile duct carcinoma: multivariate statistical analysis. World J Surg. 1993;17:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Sasaki R, Takeda Y, Funato O, Nitta H, Kawamura H, Uesugi N, Sugai T, Wakabayashi G, Ohkohchi N. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007;31:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |