Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1228
Peer-review started: November 6, 2017
First decision: November 27, 2017
Revised: December 24, 2017
Accepted: January 16, 2018
Article in press: January 16, 2018
Published online: March 21, 2018
Processing time: 130 Days and 19.8 Hours
To assess the levels of different immune modulators in patients with hepatocellular carcinoma (HCC), in relation to other hepatic diseases.
Eighty-eight patients were included in the current study and represented patients with HCC (20), liver cirrhosis (28) and chronic hepatitis (CH; 25), and normal controls (NC; 15). Peripheral blood was isolated for immunophenotyping of active myeloid dendritic cells (mDCs; CD1c and CD40), mature inactive myeloid cells (CD1c and HLA), active plasmacytoid cells (pDCs; CD303 and CD40), mature inactive pDCs (CD30 and HLA), active natural killer (NK) cells (CD56 and CD161), active NK cells (CD56 and CD314) and inactive NK cells (CD56 and CD158) was done by flow cytometry. Serum levels of interleukin (IL)-2, IL-10, IL-12, IL-1β, interferon (IFN)-α, IFN-γ and tumor necrosis factor (TNF)-αR2 were assessed by ELISA.
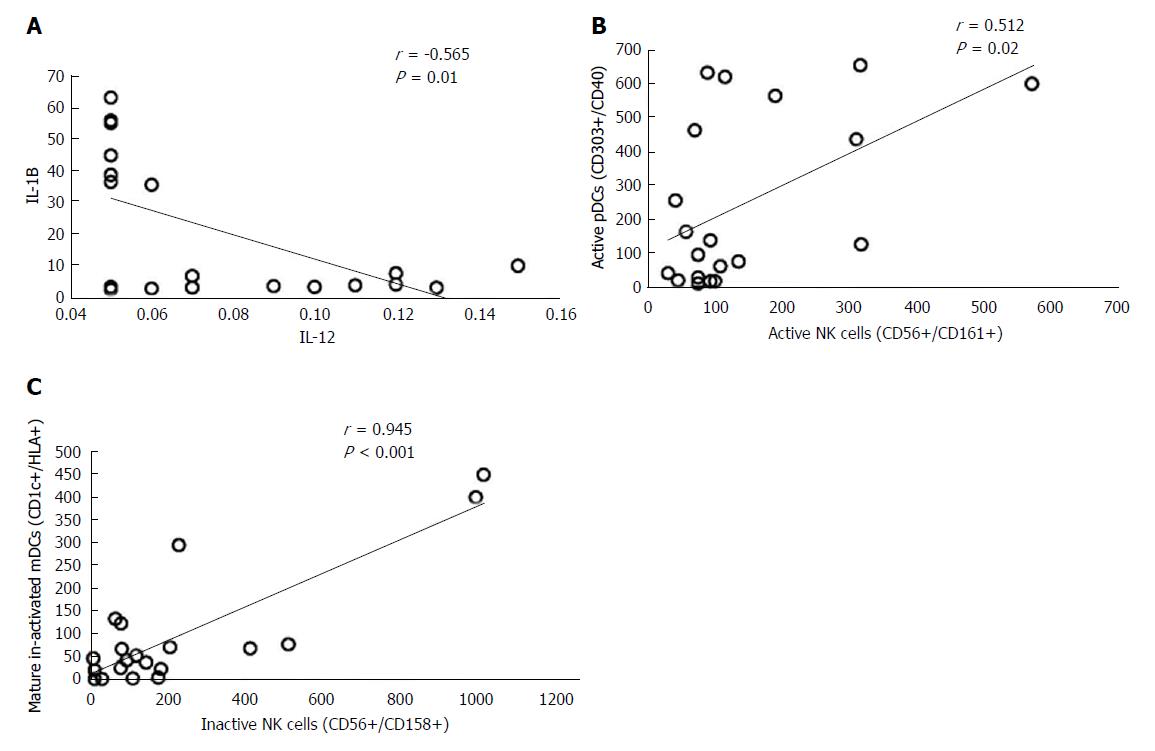
Active mDCs (CD1C+/CD40+) and inactive mDCs (CD1c+/HLA+) were significantly decreased in HCC patients in relation to NC (P < 0.001). CD40+ expression on active pDCs was decreased in HCC patients (P < 0.001), and its level was not significantly changed among other groups. Inactive pDCs (CD303+/HLA+), inactive NKs (CD56+/CD158+) and active NKs (CD56+/CD161+) were not statistically changed among the four groups studied; however, the latter was increased in CH (P < 0.05). NKG2D was statistically decreased in HCC, CH and cirrhosis (P < 0.001), and it was not expressed in 63% (12/20) of HCC patients. There was significant decrease of IL-2, IFN-α and IFN-γ (P < 0.001), and a significant increase in IL-10, IL-1β, and TNF-αR2 (P <0.01, P < 0.001 and P < 0.001; respectively) in HCC patients. There was inverted correlation between IL-12 and IL-1β in HCC (r = -0.565, P < 0.01), with a strong correlation between pDCs (CD303+/CD40+) and NKs (CD56+/CD161+; r = 0.512, P < 0.05) as well as inactive mDCs (CD1c+/HLA+) and inactive NK cells (CD56+/CD158+; r = 0.945, P < 0.001).
NKG2D, CD40, IL-2 and IL-10 are important modulators in the development and progression of HCC.
Core tip: We assessed the levels of different immune modulatory cytokines and innate immune cells as natural killer (NK) cells and dendritic cells (DCs) in patients with disease progression of hepatocarcinogenesis. Our results showed significant down-regulation in active mDCs and pDCs expressing CD40 as well as NK cells expressing NKG2D. The expression of NKG2D on NKs was not expressed in 63% of hepatocellular carcinoma (HCC) patients. Also, there was significant decrease of interleukin (IL)-2, interferon-α and interferon-γ, and a significant increase in IL-10, IL-1β, and TNF-αR2 in HCC patients. These factors could be implicated in the pathogenesis of HCC, and represent attractive targets for therapy in chronic hepatitis C virus hepatitis and HCC.
- Citation: Zekri ARN, El Deeb S, Bahnassy AA, Badr AM, Abdellateif MS, Esmat G, Salama H, Mohanad M, El-dien AE, Rabah S, Abd Elkader A. Role of relevant immune-modulators and cytokines in hepatocellular carcinoma and premalignant hepatic lesions. World J Gastroenterol 2018; 24(11): 1228-1238
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1228
Hepatocellular carcinoma (HCC) is a major health problem worldwide. It is the sixth most common cancer and the second leading cause of cancer-related death in the world[1]. In Egypt, HCC ranks the first among cancers in males (33.6%), and the 2nd in females after breast cancer (13.5%)[2]. This high incidence is attributed to the high prevalence of hepatitis C virus (HCV) infection, especially of genotype IV, in Egypt[3]. Despite advances in treatment modalities, sorafenib is still the only treatment approved by the Food and Drug Administration (FDA) for HCC; however, it extends the overall survival by 3 mo only. Hence, it is crucial to understand the underlying biological and immunological changes in HCC and to develop new treatment modalities based on these data[4,5].
The body’s immune defense mechanism(s) plays an important role in the inhibition or progression of cancer. However, tumors can escape immune surveillance by producing a local and/or systemic immune-suppressive environment[6]. Although the underlying molecular mechanisms are not yet fully clear, recent studies show that the immune response in cancer patients is usually down-regulated by immunosuppressive cells, mainly T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which suppress the immune system and promote immunologic tolerance[7]. These inhibitory cells accumulate during advanced cancer stages. They are involved in chronic inflammation and tumor progression and they can inhibit many immune cells (e.g., CD4+, CD8+, natural killer (NK) cells)[8]. They also secrete many immune-suppressive cytokines, such as interleukin (IL)-6, IL-10, and transforming growth factor (TGF)-β, creating a tolerogenic and suppressive environment[9].
The NK cells represent 25%-30% of the human liver lymphocytes, compared to 10%-20% of the total peripheral blood lymphocytes[10]. They are the main effector cells of innate immunity, which play an important role in tumor surveillance. The NK cells achieve their functions through the release of cytolytic mediators, such as perforin and granzymes, or the induction of apoptosis via the expression of tumor necrosis factor (TNF) ligands and interferon (IFN)-γ[11]. Activation of NK cells is strictly regulated by a balance between activating and inhibitory signals, whereas the inhibitory signals are mainly induced by receptors for MHC class I molecules (KIRs, CD94/NKG2A). The activating signals are mainly achieved through NKG2D, a C-type lectin-like receptor which is expressed on NK cells, γδ T cells and CD8+ T cells[12,13]. NKG2D is responsible for detection and elimination of transformed cells via binding to MICA (MHC class I–related chain), MICB and the UL16-binding proteins[14]. Activation of NKG2D provides unique costimulation to antigen-specific CD8 T cells that is non-redundant to CD28 costimulation[15].
Dendritic cells (DCs) are the most professional antigen-presenting cells which respond rapidly to microenvironment signals. Upon maturation by tumor antigen, cross-priming to T and B lymphocytes occurs to produce antitumor adaptive immune responses[16]. There are two subsets of DCs: the myeloid dendritic cells (mDCs), which are characterized by their ability to produce IL-12; and, the plasmacytoid cells (pDCs), which are responsible for the production of over 95% of type-I IFNs in response to viral infection[17]. On activation, the CD8+ cytotoxic T cells (CTLs) eliminate tumor cells by the production of IFN-γ, whereas CD4+ T helper cells stimulate B cells to support the cytotoxic and humoral immune response[18].
IL-1β is a regulatory cytokine produced by tumor and immune cells, such as MDSCs. It also induces COX-2 expression, which prevents maturation and activation of antigen presenting cells at the tumor site[19].
In the current study, we assessed the role(s) of different immune regulatory cells and cytokines in the development and progression of chronic liver disease (chronic active hepatitis, cirrhosis and HCC) compared to the normal healthy volunteers. We believe that this could allow for better understanding of different pathways implicated in the development and progression of hepatocarcinogenesis, and could also help in designing new immune-therapeutic drugs.
The current study included 88 patients who attended the medical oncology clinics of the National Cancer Institute (NCI), Cairo University during the period from 2014 to 2016. Patients were divided into four groups: HCC (20 patients - G1), liver cirrhosis (28 patients - G2), chronic hepatitis (CH; 25 patients - G3) and normal healthy volunteers as a control group (NC; 15 persons, matched for age and sex - G4). The Ethical Committee of the NCI, Egypt approved the study protocol and an informed consent was obtained from all participants before enrollment in the study.
Peripheral blood samples were isolated from patients and healthy subjects for immunophenotyping of active mDCs (CD1c and CD40), mature inactive myeloid cells (CD1c and HLA), active pDCs (CD303 and CD40), mature inactive pDCs (CD303 and HLA), active NK cells (CD56 and CD161), active NK cells (CD56F CD314) and inactive NK cells (CD56 and CD158) using the specific monoclonal antibodies according to manufacturer’s protocols (all monoclonal antibodies were purchased from Invitrogen, eBioscience, San Diego, CA, United States). Samples were then analyzed by flow cytometry (FACSCaliber; Becton and Dickenson, Franklin Lakes, NJ, United States).
Serum levels of IL-2, IL-10, IL-12, IL-1β, IFN-α, IFN-γ and TNF-αR2 were measured by the ELISA technique, according to manufacturer’s instructions (Invitrogen, eBioscience), using ELISA reader (Sunrise; Tecan, Mannedorf, Switzerland).
Data were expressed as mean ± SE of mean for continuous variables. Comparison between cytokine levels and immune cells were performed using one-way analysis of variance followed by Tukeys’ post hoc test. Pearson’s correlation was used to assess the strength of correlation between different variables and a two-tailed P-value was determined. The P-value was considered significant at < 0.05. All statistical analyses were performed using SPSS, version 22 (IBM SPSS, Armonk, NY, United States).
The mean age of the patients included in the current study was 57.1 ± 4.76 for HCC, patients, 51.29 ± 9.03 for liver cirrhosis patients, 49.64 ± 7.2 for CH patients and 42.9 ± 10.2 for NC (Table 1).
| Normal | Chronic hepatitis | Cirrhosis | HCC | |
| Sex | ||||
| Male | 11 (73.3 ) | 9 (36.0) | 19 (67.9) | 19 (95.0) |
| Female | 4 (26.7) | 16 (64.0) | 9 (32.1) | 1 (5.0) |
| Age, mean ± SD | 42.9 ± 10.2 | 49.64 ± 7.2 | 51.29 ± 9.03 | 57.1 ± 4.76 |
| HCV | +ve | +ve | +ve | +ve |
| Total | 15 | 25 | 28 | 20 |
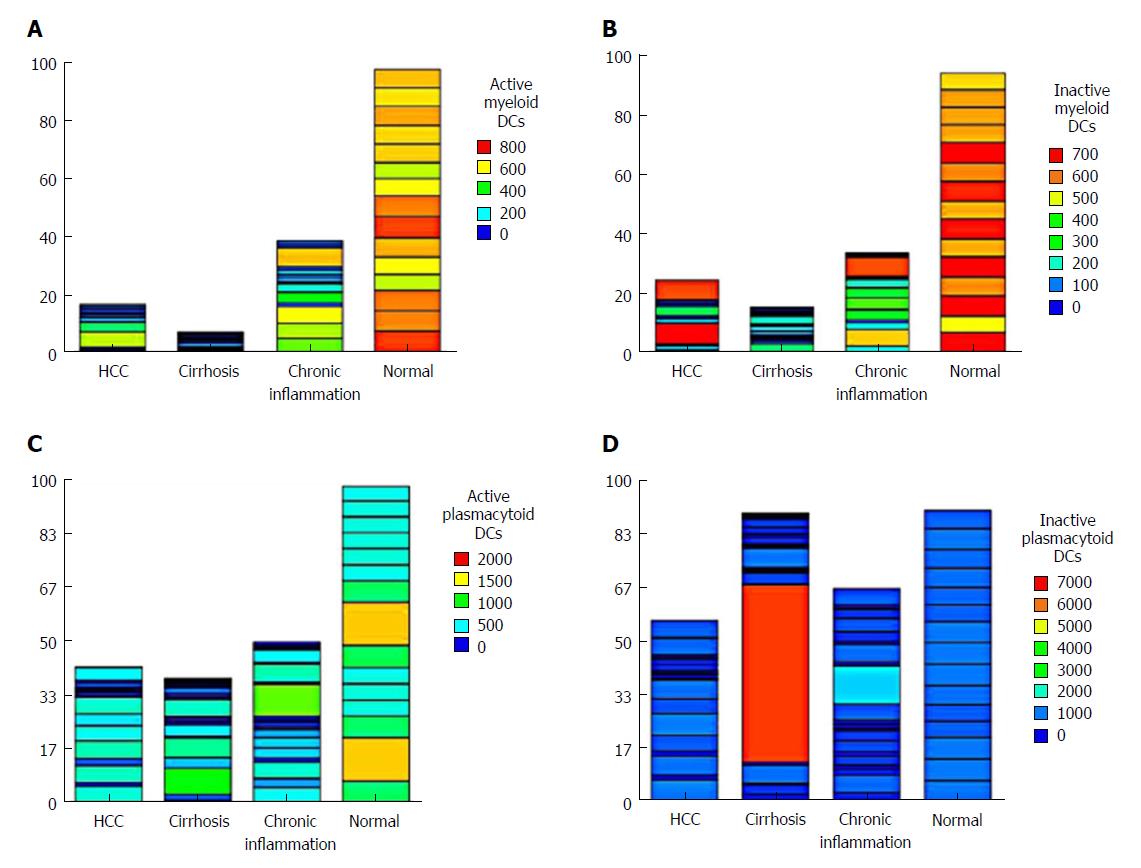
There was a significant decrease in active mDCs (CD1C+/CD40+) and inactive mDCs (CD1c+/HLA+) in HCC patients compared to the NC group (P < 0.001), as well as in active mDCs (CD1C+/CD40+) in cirrhotic patients compared to CH patients (P = 0.003; Table 2). Also, the expression level of CD40+ on active pDCs (CD303+) was significantly decreased in HCC compared to the NC group (P < 0.001). However, there was no significant difference from the other groups (cirrhosis and CH). Meanwhile, the level of inactive pDCs (CD303+/HLA+) did not differ significantly between the four groups studied (Table 2, Figure 1).
| Normal | Chronic hepatitis | Cirrhosis | HCC | P value | |
| Active mDCs (CD1C+/CD40+) | 652.4 ± 15.9A1 | 154.1 ± 40.9B | 25.5 ± 4.3C | 82.8 ± 28.7BC | < 0.001 |
| Inactive mature mDCs (CD1c+/HLA+) | 627.1 ± 14.8A | 134.4 ± 37.1B | 54.7 ± 12.3B | 121.8 ± 44.8B | < 0.001 |
| Active pDCs (CD303+/CD40+) | 782.2 ± 89.6A | 237.8 ± 56.2B | 164.1 ± 45.9B | 251 ± 55.9B | < 0.001 |
| Inactive mature pDCs (CD303+/HLA+) | 727.5 ± 18.7A | 318.5 ± 61.4A | 385.7 ± 233.3A | 339.2 ± 67.5A | 0.339 |
| Active NK cells (CD56+/CD161+) | 535 ± 83.4AB | 1166.3 ± 426.3B | 399.8 ± 139.8AB | 145.5 ± 30.2A | < 0.05 |
| Inactive NK cells (CD56+/CD158+) | 710.3 ± 127.9A | 656.3 ± 235.7A | 462.6 ± 105.3A | 456.7 ± 218.1A | 0.708 |
| Active NK cells (CD56+/CD314+) | 585.5 ± 35.9A | 188.5 ± 69.4B | 123.5 ± 47.6B | 196 ± 110.8B | < 0.001 |
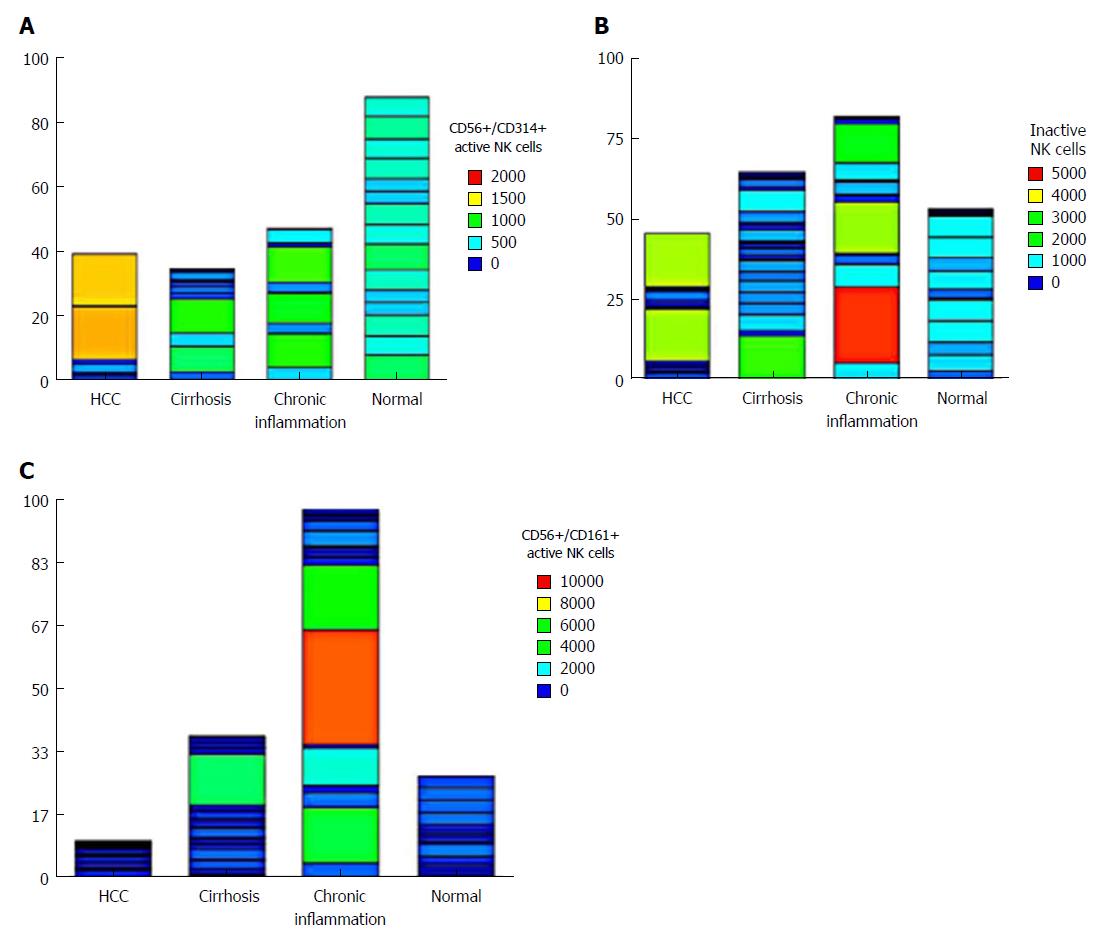
The level of the nonactive NK cells (CD56+/CD158+) did not differ significantly among groups. However, the active NK cells (CD56+/CD161+) showed a significant increase in the CH group (P < 0.05), whereas the level of active NK cells (CD56+/CD314+) was statistically decreased in HCC, CH and cirrhotic patients compared to the NC group (P < 0.001), indicating an important role of these cells in the pathogenesis of HCC (Table 2). The expression of all cell types in each patient showed that active NK cells (CD56+/CD314+) were not expressed in 63% (12/20) of HCC patients. However, 2 patients showed unexplainable high variability (Figure 2).
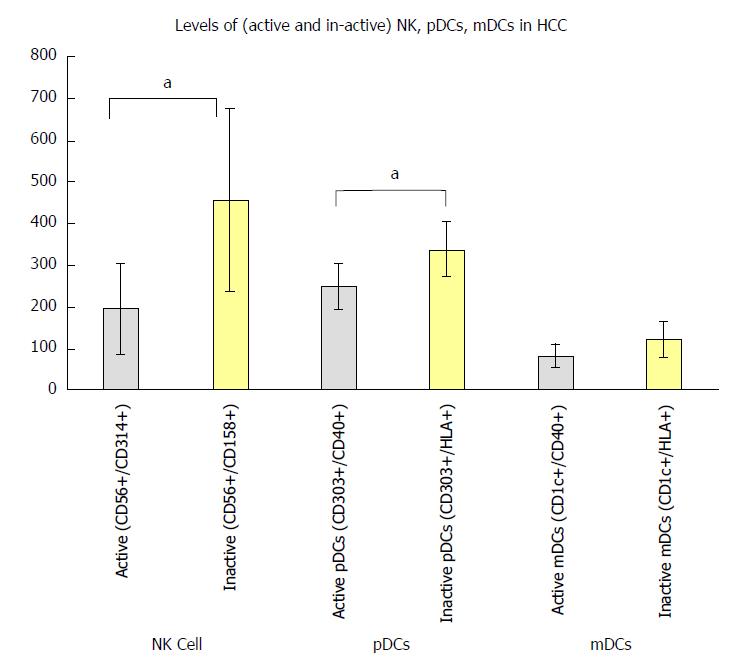
We also found a significant decrease in the active NK cells (CD56+/CD314+; P < 0.05) and active pDCs (CD303+, CD40+; P < 0.05) compared to the inactive NK cells (CD56+/CD158+) and inactive pDCs (CD303+/HLA+) respectively in HCC. To the contrary, the NK cells (CD56+/CD161+) and the mDCs did not differ significantly between groups (Figure 3).
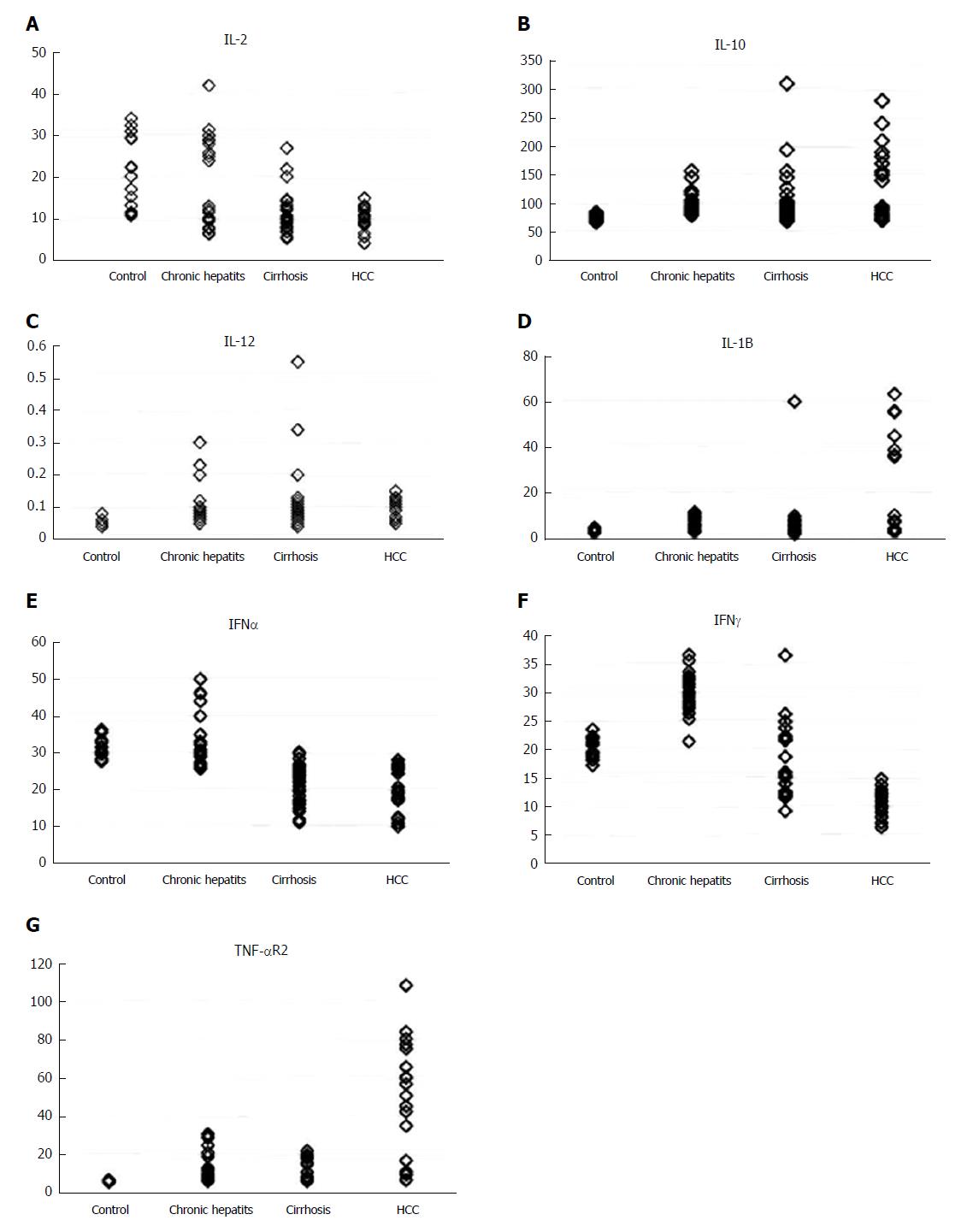
There was a significant decrease in serum levels of IL-2, IFN-α and IFN-γ in HCC patients compared to the NC group (P < 0.001), and a significant increase in serum levels of IL-10, IL-1β and TNF-αR2 in HCC compared to the NC group (P < 0.01, P < 0.001 and P < 0.001 respectively). However, there was no statistically significant change in the serum level of IL-12 (P = 0.393) among the four groups studied (Table 3, Figure 4).
| Normal | Chronic hepatitis | Cirrhosis | HCC | P value | |
| IL-2 | 22.13 ± 2.21A1 | 16.28 ± 2.12B | 11.36 ± 0.95C | 10.24 ± 0.64C | < 0.001 |
| IL-10 | 74.8 1 ± 1.34A | 98.46 ± 4.01AB | 104.11 ± 9.35B | 132.1 ± 14.26C | < 0.01 |
| IL-12 | 0.06 ± 0.01A | 0.09 ± 0.01A | 0.15 ± 0.06A | 0.08 ± 0.01A | 0.393 |
| IL-1β | 3.46 ± 0.15A | 6.48 ± 0.58A | 6.71 ± 2.39A | 21.38 ± 4.88B | < 0.001 |
| IFN-α | 31.20 ± 0.67A | 32.28 ± 1.44A | 21.31 ± 1.01B | 18.49 ± 1.37B | < 0.001 |
| IFN-γ | 20.47 ± 0.49A | 29.75 ± 0.68B | 17.51 ± 1.18C | 10.46 ± 0.56D | < 0.001 |
| TNF-αR2 | 6.03 ± 0.07A | 13.25 ± 1.73A | 10.09 ± 0.97A | 47.42 ± 6.74B | < 0.001 |
Significant correlation was also reported in HCC patients between (1) serum level of IL-12 and IL-1β (r = -0.565, P < 0.01), (2) expression of CD40 on pDCs (CD303+, CD40+) and active NK cells (CD56+/CD161+; r = 0.512, P < 0.05), and (3) inactive mDCs (CD1c+/HLA+) and inactive NK cells (CD56+/CD158+; r = 0.945, P < 0.001) (Figure 5A-C).
Patients with chronic active hepatitis showed strong significant correlation between active NK cells (CD56+/CD161+) and both IL-2 and IL-12 (r = 0.549, P = 0.004 and r = 0.660, P < 0.001 respectively). Strong correlations were also reported between the expression of CD314 on NK cells and IL-2 (r = 0.548, P < 0.01) and active mDCs CD1c+/CD40+ (r = 0.577, P = 0.003). The level of IL-10 was strongly correlated with (1) increased inactive NK cells (CD56+/CD158+; r = 0.604, P = 0.001) and (2) inactive mDCs CD1c+/HLA+ (r = 0.588, P = 0.002; Table 4).
Identification of inflammatory mediators which promote or prevent the progression of cancer depends on the tumor microenvironment. Thus, this study was designed to assess the levels of different immune modulators and cytokines in patients with HCC, nonmalignant hepatic diseases (e.g., CH and liver cirrhosis) compared to a normal group. This would enable us to study different pathways that help in identifying immune cells or biomarkers that may be implicated in hepatocellular carcinogenesis. This may also explain why liver disease progresses more rapidly in some patients than in others and provides additional therapeutic modalities for viral infections and HCC (immunotherapy).
One of the most powerful antigen presenting cells is the DC, which plays an important role in antitumor immune response. Our data revealed that active and inactive mDCs were significantly decreased in HCC patients compared to the NC group (P < 0.001); however, the level of active mDCs (CD1C+/CD40+) was significantly decreased in cirrhosis compared to CH patients (P = 0.003). These data are consistent with Obermajer et al[20], who reported that the inhibition of DC maturation in HCC may prove to be a critical feature of tumor escape, as well as with another two recent studies which reported numerical and functional defects in the peripheral DCs in hepatitis B- and C-associated HCC patients[21,22].
The pDC (CD303+, CD40+) represents the major cell responsible for antiviral immunity, and the main source of IFN-α in the body. Our data revealed that the expression level of CD40+ on active pDCs was significantly decreased in HCC patients compared to NC (P < 0.001). Meanwhile, inactive pDCs (CD303+/HLA+) did not change significantly among the four groups studied. This was also confirmed by the significant decrease of serum IFN-α, and the increase of IL-10 in HCC patients compared to the NC group (P < 0.001 and P < 0.01 respectively). This is in concordance with previous reports showing that IL-10 inhibits IFN-α production and promotes apoptosis of human pDCs[23,24]. Moreover, Gonzalez-Carmona et al[25] demonstrated that cancer patients had low CD40 expression on DCs or CD40L on T cells, which is associated with an impaired immune response, and Shurin et al[26] reported that the tumor-derived IL-10 inhibits CD40 expression on DCs and DC precursors and suppresses their maturation and function.
NK cells play an important role in controlling viral hepatitis, liver fibrosis and carcinogenesis. Their functions are modulated by different classes of receptors present on its surface[27]. Thus, it is essential to identify the roles of these cells in different stages of HCC development. Among these, we investigated the role of the killer cell immunoglobulin-like receptors (KIR; CD158), NKR-P1A (CD161) and NKG2D (CD314) in HCV CH patients, and in liver cirrhosis and its progression to HCC.
Our results revealed that the expression of the inhibitory KIR (CD158+) receptors and the activating NKR-P1A (CD161) on NK cells was not statistically changed between HCC and NC (P = 0.827 and P = 0.788 respectively). Thus, these two pathways may not be involved in the pathogenesis of HCC. However, the latter were statistically increased in CH (P < 0.05). This result was supported by our finding of a strong statistical correlation between the expression of CD161 on NK cells and IL-12 (r = 0.77, P < 0.001) in patients with HCV chronic active hepatitis. This illustrates the role of NK cells (CD56+/CD161+) in viral hepatitis.
On the other hand, the level of active NK cells expressing NKG2D were statistically decreased in HCC, CH and cirrhosis in relation to the NC group (P < 0.001), which could indicate the implication of these cells in the progression of HCC. This was clarified by representing its expression in each patient, it was not expressed in 63% (12/20) of HCC patients, emphasizing its important role in HCC development. These data were consistent with Lanier et al[28]. Yoon et al[29] reported that direct interaction between human NK cells and HCV-infected hepatoma cells down-regulates NK cell NKG2D expression and effector function with diminished IFN-γ production. Another study carried out by Kamiya et al[30] demonstrated that NKG2D was an important mediator of antiHCC activity. On the contrary, Oliviero et al[31] found a decreased percentage of NK cells expressing the inhibitory receptor KIR3DL1 and a concomitant increase in the proportion of NKG2D+ NK cells in Italian patients with chronic HCV. This could be explained by racial and environmental differences with different viral genotypes, and also according to the clinical stage.
Our results regarding DCs and NK cells were supported by assessment of the cytokine serum levels in the four groups studied. We found that there was a significant increase in IL-1β (P < 0.001) in HCC patients compared to the NC group. However, there was no statistically significant change in the serum level of IL-12 (P = 0.393) among the four groups studied. Furthermore, we had a significant increase in the serum level of IL-10 in the cirrhosis and HCC groups compared to the NC group. This is consistent with Accapezzato et al[32]. IL-10 is important for allowing liver NK cells to maintain immune-tolerant states[33]. Its significant increase in HCC patients is responsible for the abnormal tolerant NK cells that cannot eliminate infected or transformed cells, which leads to immune evasion.
In the current study, there was a significant decrease of the serum levels of IFN-γ in chronic HCV hepatitis, cirrhosis and HCC patients in comparison to NC (P < 0.001). This is in agreement with Li et al[34], who found that NK cells cultured with cancer-associated fibroblasts from HCC (H-CAFs) down-regulate NKG2D and NKp46 and decrease expression of granzyme B, perforin and INF-γ. Later, another study showed that TGF-β and IL-10 in hepatic carcinoma patients induce the expression of microRNA (miR)-146a, which causes reduced IFN-γ production and cytotoxicity, resulting in a poorer prognosis[35]. Meanwhile, Zhang et al[36] found that MDSCs selectively suppressed the IFN-γ production deriving from NKT cells through membrane-bound TGF-β.
IL-2 plays a critical role in activation of the immune system, which could be used to eradicate cancer. It has been demonstrated as a monotherapy, and was approved for metastatic renal cell carcinoma and metastatic melanoma by the FDA[37]. Our results showed a significant increase in TNF-αR2 (P < 0.001) and significant decrease in IL-2 (P < 0.001) in HCC patients in relation to other groups, consistent with our previous results[38,39] that concluded the possible use of serum TNFR-II, IL-2Ra and IL-8 as combined biomarkers in HCV-infected patients at high risk of developing HCC.
Another important finding in the current study was that HCC patients had a significant decrease of active NK cells (CD56+/CD314+; P < 0.05) and active pDCs (CD303+, CD40+; P < 0.05) in comparison to inactive NK cells (CD56+/CD158+) and inactive pDCs (CD303+/HLA+) respectively. NK cells expressing CD56+/CD161+ and mDCs were not statistically affected. This revealed the importance of these two subsets of cells [active NK cells (CD56+/CD314+), and active pDCs (CD303+, CD40+)] in the pathogenesis of HCC, and allows for further research around the state of this imbalance that emerges in HCC patients.
We conclude that there are immunological changes occurring in HCC patients which could be possible candidate(s) for future immunotherapy for these patients. Among these are the NK cells expressing NKG2D and pDCs expressing CD40, IL-2 and IL-10. These factors could be implicated in the pathogenesis of HCC, and provide an attractive target for therapeutics in chronic HCV hepatitis and liver cancer.
Hepatocellular carcinoma (HCC) is a major health problem worldwide and mainly in Egypt due to the high prevalence of hepatitis C virus (HCV) infection, especially of genotype IV. It ranks the first among cancers in males (33.6%), and the 2nd in females after breast cancer. Sorafenib is still the only treatment approved by the Food and Drug Administration for HCC; however, it extends the overall survival by 3 mo only. Hence, it is crucial to understand the underlying biological and immunological changes in HCC Egyptian patients, and to develop new treatment modalities based on these data.
The immune system plays an important role in suppression of cancer. However, tumors can escape immune surveillance by producing an immune-suppressive environment, for which the underlying mechanisms are not fully clear. Recent studies show that the immune response in HCC patients is usually down-regulated by immunosuppressive cells (myeloid-derived suppressor cells and T regulatory cells) that are involved in chronic inflammation and tumor progression. Also, these inhibitory cells secrete many immune-suppressive cytokines, such as interleukin (IL)-6, IL-10 and transforming growth factor-β, creating a tolerogenic and suppressive environment[9].
The objective of this study was to assess the levels of different immune modulators and cytokines that may play a role in the pathogenesis of HCC and other hepatic diseases (e.g., chronic hepatitis and liver cirrhosis) compared to a normal group. This would help in identifying additional therapeutic modalities for viral infections and HCC in the context of immunotherapy.
This retrospective cohort study included 88 patients who attended the medical oncology clinics of the National Cancer Institute, Cairo University during the period from 2014 to 2016. Patients were divided into the HCC group (20 patients-G1), liver cirrhosis group (28 patients-G2), chronic hepatitis group (CH; 25 patients-G3) and normal healthy volunteers as a control group (NC; 15 persons). The immune system of the patients was assessed through immunophenotyping of CD1c and CD40, CD1c and HLA, CD303 and CD40, CD303 and HLA, CD56 and CD161, CD56 and CD314, and CD56 and CD158 by flow cytometry. On the other hand, serum levels of IL-2, IL-10, IL-12, IL-1β, interferon (IFN)-α, IFN-γ and tumor necrosis factor (TNF)-αR2 were measured by the ELISA technique, according to the manufacturer’s instructions. Data were expressed as mean ± SE of mean, and statistical comparison between cytokine levels and immune cells were performed.
There was a significant decrease in active and inactive mDCs in HCC patients compared to the NC group, as well as active mDCs (CD1C+/CD40+) in cirrhotic patients compared to CH patients. The expression level of CD40+ on active pDCs (CD303+) was significantly decreased in HCC compared to the NC. However, there was no significant difference with the other groups (cirrhosis and CH). Meanwhile, the level of inactive pDCs (CD303+/HLA+) and inactive NK cells (CD56+/CD158+) did not differ significantly between the four groups studied.
The active NK cells (CD56+/CD161+) showed a significant increase in the CH group, whereas the level of active NK cells (CD56+/CD314+) was statistically decreased in HCC, CH and cirrhotic patients compared to the NC group, indicating an important role of these cells in the pathogenesis of HCC. The individual expression of each cell type in patients showed that active NK cells (CD56+/CD314+) were not expressed in 63% (12/20) of HCC patients.
There was a significant decrease in serum levels of IL-2, IFN-α and IFN-γ in HCC patients compared to the NC group, and a significant increase in serum levels of IL-10, IL-1β and TNF-αR2 in HCC compared to the NC group. However, there was no statistically significant change in the serum level of IL-12 among the four groups studied.
We conclude that there are immunological changes occurring in HCC patients in relation to other liver diseases. The related immunological factors are NKG2D expressed on NK cells, and pDCs expressing CD40, IL-2 and IL-10. These factors could be implicated in the pathogenesis of HCC, and represent attractive targets for therapeutics in chronic HCV hepatitis and liver cancer.
NKG2D, CD40, IL-2 and IL-10 could be a possible candidate for future immunotherapy for HCC patients. However, further studies are recommended regarding correlation of these factors and clincopathological features as well as the overall survival of the patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu XL S- Editor: Wang JL L- Editor: Filipodia E- Editor: Huang Y
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 2. | Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 3. | Zekri AR, Hafez MM, Bahnassy AA, Hassan ZK, Mansour T, Kamal MM, Khaled HM. Genetic profile of Egyptian hepatocellular-carcinoma associated with hepatitis C virus Genotype 4 by 15 K cDNA microarray: preliminary study. BMC Res Notes. 2008;1:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Knudsen ES, Gopal P, Singal AG. The changing landscape of hepatocellular carcinoma: etiology, genetics, and therapy. Am J Pathol. 2014;184:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Ostrand-Rosenberg S. Tolerance and immune suppression in the tumor microenvironment. Cell Immunol. 2016;299:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2369] [Cited by in RCA: 2867] [Article Influence: 220.5] [Reference Citation Analysis (0)] |
| 8. | Shrihari TG. Dual role of inflammatory mediators in cancer. Ecancermedicalscience. 2017;11:721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Zeromski J, Mozer-Lisewska I, Kaczmarek M, Kowala-Piaskowska A, Sikora J. NK cells prevalence, subsets and function in viral hepatitis C. Arch Immunol Ther Exp (Warsz). 2011;59:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell. 2016;166:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 487] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 12. | Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2096] [Cited by in RCA: 2177] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 13. | Dai Z, Turtle CJ, Booth GC, Riddell SR, Gooley TA, Stevens AM, Spies T, Groh V. Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus. J Exp Med. 2009;206:793-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 633] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 15. | Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Tel J, van der Leun AM, Figdor CG, Torensma R, de Vries IJ. Harnessing human plasmacytoid dendritic cells as professional APCs. Cancer Immunol Immunother. 2012;61:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 19. | Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498-5505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 21. | Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 258] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, Yildiz Y, Sievers E, Schmidt-Wolf IG, Caselmann WH. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology. 2008;48:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 29. | Yoon JC, Lim JB, Park JH, Lee JM. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85:12557-12569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Kamiya T, Chang YH, Campana D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol Res. 2016;4:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151-1160, 1160.e1-1160.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 32. | Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 33. | Wu Y, Tian Z, Wei H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front Immunol. 2017;8:930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 34. | Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012;318:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 35. | Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol. 2017;14:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Zhang H, Li Z, Wang L, Tian G, Tian J, Yang Z, Cao G, Zhou H, Zhao L, Wu Z. Critical Role of Myeloid-Derived Suppressor Cells in Tumor-Induced Liver Immune Suppression through Inhibition of NKT Cell Function. Front Immunol. 2017;8:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 38. | Zekri AR, Youssef AS, Bakr YM, Gabr RM, El-Rouby MN, Hammad I, Ahmed EA, Marzouk HA, Nabil MM, Hamed HA. Serum biomarkers for early detection of hepatocellular carcinoma associated with HCV infection in egyptian patients. Asian Pac J Cancer Prev. 2015;16:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Zekri AR, Alam El-Din HM, Bahnassy AA, Zayed NA, Mohamed WS, El-Masry SH, Gouda SK, Esmat G. Serum levels of soluble Fas, soluble tumor necrosis factor-receptor II, interleukin-2 receptor and interleukin-8 as early predictors of hepatocellular carcinoma in Egyptian patients with hepatitis C virus genotype-4. Comp Hepatol. 2010;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |