Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.150
Peer-review started: October 31, 2017
First decision: November 8, 2017
Revised: November 18, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 7, 2018
Processing time: 68 Days and 20.2 Hours
Bone metastasis is a rare event in patients with gastric cancer, but pathologic fracture, paralysis, pain and hematological disorders associated with the bone metastasis may influence the quality of life. We report herein the case of a 53-year-old man who presented with primary remnant gastric cancer with bone metastasis. The patient requested further investigations after detection of a metastatic lesion in the 2nd lumbar vertebra during evaluation for back pain that had persisted for 3 mo. No other metastatic lesions were detected. He underwent total gastrectomy and palliative metastasectomy to aid in reduction of symptoms, and he received combination chemotherapy with tegafur (S-1) and cisplatin. The patient survived for about 60 mo after surgery. Currently, there is no treatment guideline for gastric cancer with bone metastasis, and we believe that gastrectomy plus metastasectomy may be an effective therapeutic option for improving quality of life and survival in patients with resectable primary gastric cancer and bone metastasis.
Core tip: Gastrectomy and metastasectomy may be an effective therapeutic option for improving quality of life and survival in selected gastric cancer patients with bone metastasis. Favorable factors, such as resectable solitary bone lesions, good performance status and normal serum carcinoembryonic antigen levels, should be utilized to stratify and select patients who will be good candidates for surgery.
- Citation: Choi YJ, Kim DH, Han HS, Han JH, Son SM, Kim DS, Yun HY. Long-term survival after gastrectomy and metastasectomy for gastric cancer with synchronous bone metastasis. World J Gastroenterol 2018; 24(1): 150-156
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.150
The survival rate associated with gastric cancers has improved due to improvements in diagnostic technology, surgery, adjuvant therapy and increased detection at early stages. Although the incidence of gastric cancer has gradually declined, it remains the second most common cancer in South Korea, after thyroid cancer, according to the Korea Cancer Registry statistics for the year 2010. It is also the third leading cause of cancer-related death[1].
A substantial proportion of patients are diagnosed at an advanced stage with synchronous distant metastases. Treating such patients is a therapeutic challenge for physicians, since it is generally accepted that such patients have incurable disease and that treatment is administered with a noncurative intent. Distant metastasis in gastric cancer patients is known to be one of the most important prognostic risk factors, with associated parameters like depth of invasion and lymph node metastasis[2]. Bone metastasis is more commonly observed in other cancer types, like cancers of the breast, lung and prostate, but is rather rare in gastric cancer[3].
Gastric cancer with bone metastasis is associated with poor prognosis[4,5]. Moreover, it is related to pathologic fracture, paralysis, pain and hematological disorders, which may influence quality of life and need for further treatment. Disease management in such cases is a challenge for physicians, since no guidelines exist for treatment of patients with gastric cancer and associated bone metastasis. We present here a case of long-term survival after completion of total gastrectomy and metastasectomy for primary remnant gastric cancer (RGC) with bone metastasis. We also include a review of the relevant literature.
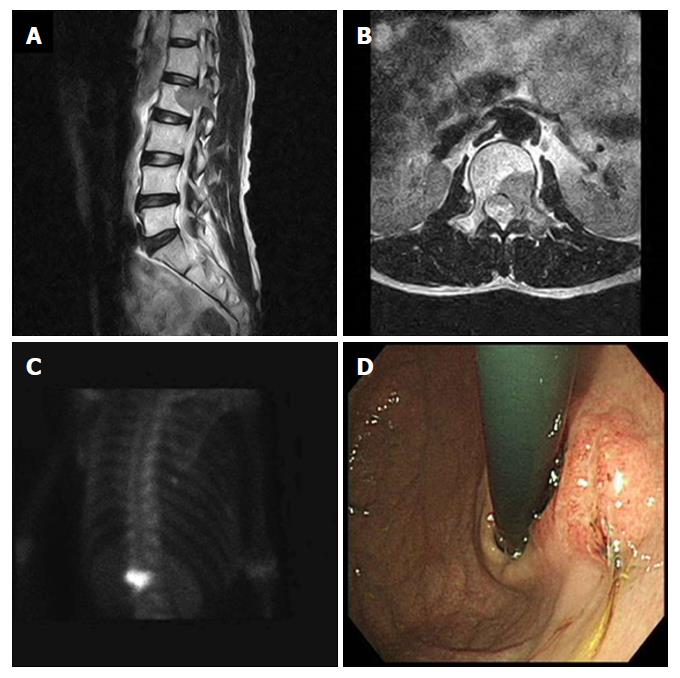
A 53-year-old male patient, who had complained of back pain, was found to have a mass in the 2nd lumbar vertebra by magnetic resonance imaging (MRI), and was transferred to our institution for further evaluation. He had no significant medical history, except for having undergone gastrectomy due to a gastric ulcer 15 years prior. At the onset, blood biochemical parameters were within normal range (alkaline phosphatase: 141 IU/L, normal range: 40-145 IU/L; calcium: 9.5 mg/dL, normal range: 8.2-10.8 mg/dL), as were the levels of carcinoembryonic antigen (CEA; 2.26 ng/mL, normal range: < 5.0 ng/mL), cancer antigen (CA) 19-9 (12.25 U/mL, normal range: < 37 U/mL) and lactate dehydrogenase (LDH; 152 IU/L, normal range: 70-178 IU/L). The MRI showed a suspicious metastatic lesion in the 2nd lumbar vertebra with enhancing signal intensity on a T2-weighted image, and bone scans showed a focal hot uptake at the 2nd lumbar vertebra (Figure 1A-C). A bone biopsy taken at the 2nd lumbar vertebra was diagnosed as metastatic adenocarcinoma. Gastroduodenoscopic findings showed a Borrmann type III lesion at the remnant stomach lesser curvature (Figure 1D), which was diagnosed as poorly differentiated adenocarcinoma by biopsy. Serum fasting gastrin level was within normal range, and Giemsa staining did not demonstrate Helicobacter pylori (H. pylori) infection in the gastric biopsy specimen. No other metastatic lesions were detected on chest computed tomography (CT) and abdominal pelvic CT scans.
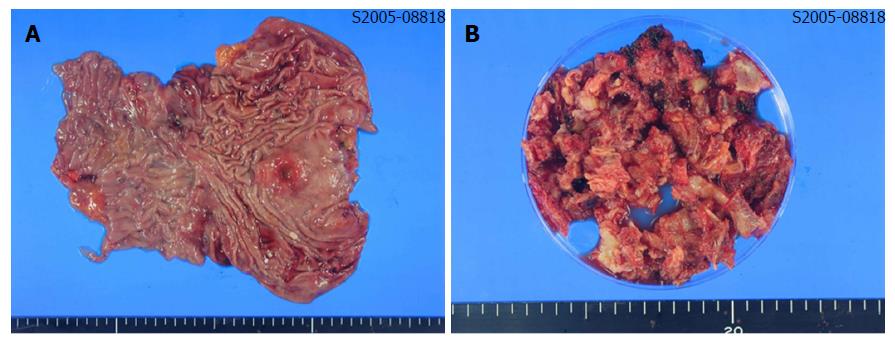
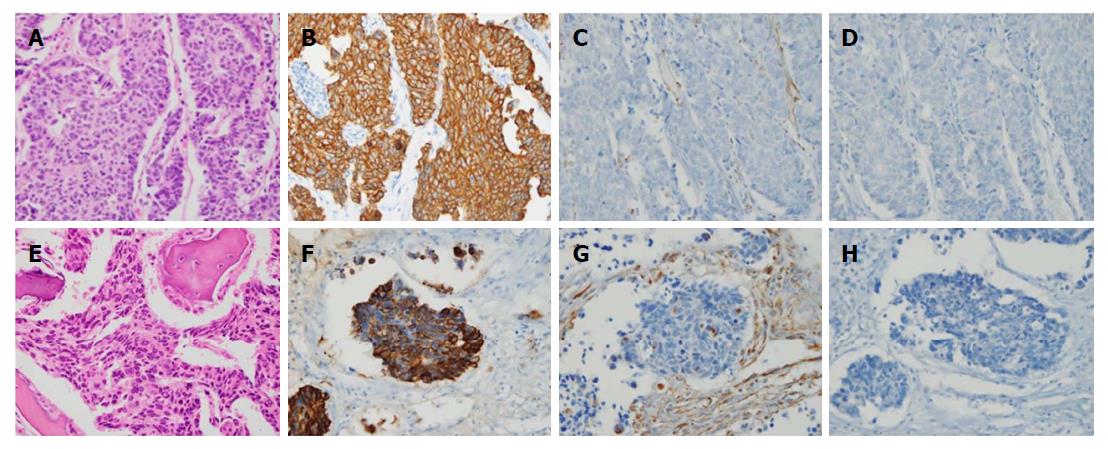
The patient underwent total gastrectomy with Roux-en-Y esophagojejunostomy and lumbar vertebrae metastasectomy (Figure 2A and B). Pathologic findings after the surgery described poorly differentiated adenocarcinoma, with invasion of serosa and massive lymphovascular invasion. Five metastatic lymph nodes were observed among the nine regional lymph nodes examined. Microscopically, the tumor consisted of solid nests of poorly differentiated tumor cells having ovoid nuclei and indistinct cytoplasm. Immunohistochemistry showed that the tumor cells exhibited diffuse immunoreactivity for cytokeratin AE1/AE3, but were negative for synaptophysin and vimentin, a test for neurofilament immunoreactivity that has been identified in most neuroendocrine tumors. These findings support the diagnosis of poorly differentiated adenocarcinoma (Figure 3A-D).
Microscopically, the tumors of lumbar vertebrae were identified as poorly differentiated, with histologic and immunohistochemical features identical to those of the carcinoma of the stomach (Figure 3E-H). Bone marrow was also obtained from the patient’s lumbar spine during surgery, and no cancer cells were detected in the bone marrow aspirate. The results of postoperative pathologic findings led to a tumor-node-metastasis classification of T4aN2M1, according to the American Joint Committee on Cancer 7th edition staging manual.
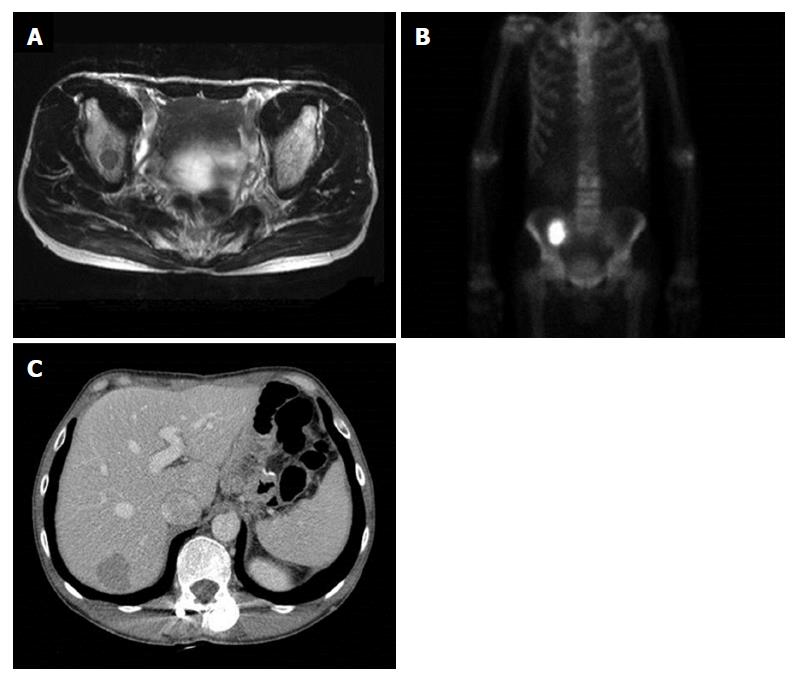
The patient received palliative first-line combination chemotherapy with tegafur (S-1) and cisplatin for a year. Duration of relapse-free survival was 25 mo, when multiple metastases were found in follow-up to have developed on the right ileum and liver (Figure 4). Right ileum radiotherapy and second-line palliative combination of folinic acid, fluorouracil and oxaliplatin (known as FOLFOX chemotherapy) was administered. Third-line combination chemotherapy of folinic acid, fluorouracil and irinotecan (known as FOLFIRI) and fourth-line docetaxel chemotherapy proceeded, and the patient died 60 mo after surgery.
In this study, we report a case of RGC with synchronous isolated bone metastasis. The average interval between initial distal gastrectomy and the second surgery for RGC is reported to be 22.0-34.6 years for benign disease and 6.8-18.8 years for gastric cancer[6]. As possible important factors for the pathogenesis for RGC, H. pylori infection, duodenogastric reflux and denervation of gastric mucosa have been considered. Also, Billroth-II (B-II) reconstruction is known to be more prevalent in developing RGC than Billroth-I (B-I) reconstruction; the possible reason is continuous bathing by duodenogastric reflux, as well as mucosal inflammation and regeneration[6,7]. In the case described herein, H. pylori infection was not identified. But, the previous B-II reconstruction and persistent duodenogastric reflux could have been the possible cause of RGC in this patient.
While the number of patients diagnosed with gastric cancer has increased with the development of endoscopy and mass screening tests, the survival rate of gastric cancer patients has also improved due to the advancement of diagnostic technology for early detection, surgery and postoperative adjuvant therapy. However, there is a substantial portion of the patient population with unresectable gastric cancer due to synchronous distant metastasis. It is now accepted that the presence of bone metastasis is an independent prognostic risk factor for advanced gastric cancer[8].
Gastric cancer with synchronous bone metastasis occurs rarely. Park et al[4] reported that synchronous bone metastases developed in about 0.9% of the total gastric cancer cases in their series. Evidence in the literature suggests that gastric cancer with synchronous bone metastasis is associated with a poor prognosis, the median survival time of which is 97 d[4]. In the majority of cases of gastric cancer with synchronous bone metastasis, the tumor is unresectable and the prognosis is very poor. There is also no established treatment guideline for these patients.
Physicians are often placed in challenging situations to manage the disease, since the difficulty is compounded by the presence of associated pathologic fractures, pain and hematologic disorders. Some studies have reported survival gain in gastric cancer patients with synchronous distant metastasis who underwent resectable gastric surgery, with or without metastasectomy[9-11]. Meta-analysis revealed that palliative gastrectomy is associated with a significant improvement in overall survival (HR = 0.62), as compared to that of patients without palliative gastrectomy[9]. Kim et al[11] studied the effect of gastrectomy and metastasectomy in patients with gastric cancer with distant metastases, who had previously received chemotherapy. They reported that the survival rate of patients who underwent gastrectomy plus metastasectomy was higher than that of patients who underwent debulking gastrectomy only, as well as that of patients who were administered chemotherapy only (median overall survival and 3-year survival rates reported as 28.0 mo, 15.5 mo and 9.0 mo and 42.8%, 8.1% and 3.5%, respectively). Meta-analysis also showed the survival improvement in metastatic gastric cancer patients who underwent visceral metastasectomy including liver, peritoneum and distant node. The mean increased difference in survival conferred by metastasectomy averaged between 9.3 mo and 15.7 mo[12].
However, the above studies were retrospective and selection bias cannot be ruled out. Evidence to support surgical intervention in these patients is not conclusive. There is a need for prospective randomized controlled studies to evaluate the value of resectable surgery on patients with gastric cancer with distant metastasis. Furthermore, in these studies, there was no case of metastasectomy in patients with synchronous bone metastasis. In addition, in the study conducted by Park et al[4] there was no evidence for curative resection of synchronous bone metastasis.
We think that for most patients with gastric cancer with synchronous bone metastases, curative resection is not an option, because the majority of bone metastases present as multiple lesions[4,13,14] and are located at unresectable anatomic locations. The most common location of bone metastasis in patients with stomach cancer is the spine, followed by the pelvis and the ribs. Hence, most patients with gastric cancer who develop bone metastases complain of back pain[15]. While there is no established treatment regimen for bone metastasis, radiation therapy is known to be effective[16,17]. In the present case, bone metastasectomy was conducted as a palliative therapy to alleviate back pain, because the solitary bone metastasis in the spine was resectable.
Clinicopathologic features associated with bone metastasis have not been fully established in the literature thus far. Sudo et al[18] reported that bone metastasis after surgery is observed mainly in cases of cancers involving the upper third or middle third of the stomach, which are associated with massive lymphatic invasion and poorly differentiated adenocarcinoma. In the present case, gastric cancer occurred in the remnant stomach of a patient who had undergone gastric ulcer surgery. Pathologic findings showed poorly differentiated adenocarcinoma with massive lymphovascular invasion. The mechanism by which bone metastasis develops in patients with gastric cancer is not known exactly, but York et al[19] observed that bone metastasis is most frequently seen in the vertebral body. Based on the location, they suggested the possibility of hematogenous spread through Batson’s vertebral plexus, bypassing portal circulation.
Known factors associated with longer median survival times for the patients with bone metastasis include isolated bone metastasis, well differentiated tumors, palliative chemotherapy and zoledronic acid treatment[20]. On the other hand, high-level LDH, CEA or CA19-9, serum hypercalcemia, poor performance status, and involvement of multiple bones are associated with shorter survival times[4,5,20]. Usually, an increase of serum alkaline phosphatase levels is observed in 45.3%-66.0% of patients with bone metastasis[14,15]. In the present case, CEA, serum alkaline phosphatase and serum calcium levels were within normal range.
In the majority of cases, gastric cancer with synchronous bone metastasis is unresectable, and prognosis is usually very poor. In the present case, however, performance status was good, and the metastasis was a solitary, resectable bone lesion. These factors and aggressive palliative chemotherapy probably supported long-term survival of the patient after surgery. Therefore, we hypothesize that gastrectomy plus metastasectomy may be an effective therapeutic option for improving quality of life and survival in selected patients with bone metastasis. Favorable factors, such as resectable solitary bone lesions, good performance status and normal serum CEA levels, should be utilized to stratify and select patients who will be good candidates for surgery.
In conclusion, aggressive local therapy, including gastrectomy with metastasectomy and palliative chemotherapy, may be an effective therapeutic option for improving survival in patients with resectable primary gastric cancer and bone metastasis.
A 53-year-old man, referred after detection of a tumorous bony lesion in the 2nd lumbar vertebra during evaluation for back pain.
The patient had no significant medical history, except for having undergone gastrectomy due to gastric ulcer 15 years prior.
Primary bone neoplasm, metastatic bone tumor.
Serum alkaline phosphatase, calcium, carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9 and lactate dehydrogenase (LDH) levels were within normal range.
Magnetic resonance imaging of the spine showed a suspicious metastatic lesion in the 2nd lumbar vertebra, with enhancing signal intensity on a T2-weighted image. Gastroduodenoscopy showed a Borrmann type III lesion at the remnant stomach lesser curvature.
A bone biopsy taken at the 2nd lumbar vertebra led to diagnosis of metastatic adenocarcinoma. Gastric lesion was diagnosed as poorly differentiated adenocarcinoma by biopsy.
The patient underwent total gastrectomy and lumbar vertebrae metastasectomy, followed by aggressive palliative chemotherapy.
Gastric cancer with bone metastasis is relatively rare and associated with poor prognosis. Known factors associated with longer median survival times for patients with bone metastasis include isolated bone metastasis, well differentiated tumors, palliative chemotherapy and zoledronic acid treatment. On the other hand, high-level LDH, CEA and CA19-9, serum hypercalcemia, poor performance status, and involvement of multiple bones are associated with shorter survival times. Small studies have reported survival gain in gastric cancer patients with synchronous distant metastasis who underwent resectable gastric surgery plus metastasectomy.
Gastrectomy is a partial or total surgical removal of the stomach. Metastasectomy is the surgical removal of metastases, which are secondary cancerous growths that have spread from cancer originating in another organ in the body.
Aggressive local therapy, including gastrectomy with metastasectomy and palliative chemotherapy, may be an effective therapeutic option for improving survival in patients with resectable primary gastric cancer and bone metastasis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aoyagi K, Guner A, Koch TR S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1-14. [PubMed] [DOI] [Full Text] |
| 2. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [PubMed] [DOI] [Full Text] |
| 3. | Nishidoi H, Koga S. [Clinicopathological study of gastric cancer with bone metastasis]. Gan To Kagaku Ryoho. 1987;14:1717-1722. [PubMed] |
| 4. | Park HS, Rha SY, Kim HS, Hyung WJ, Park JS, Chung HC, Noh SH, Jeung HC. A prognostic model to predict clinical outcome in gastric cancer patients with bone metastasis. Oncology. 2011;80:142-150. [PubMed] [DOI] [Full Text] |
| 5. | Kim YJ, Kim SH, Kim JW, Lee JO, Kim JH, Bang SM, Lee JS, Lee KW. Gastric cancer with initial bone metastasis: a distinct group of diseases with poor prognosis. Eur J Cancer. 2014;50:2810-2821. [PubMed] [DOI] [Full Text] |
| 6. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [PubMed] [DOI] [Full Text] |
| 7. | Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 2014;20:13734-13740. [PubMed] [DOI] [Full Text] |
| 8. | Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886-891. [PubMed] [DOI] [Full Text] |
| 9. | Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, Zhou B, Xu H. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577. [PubMed] [DOI] [Full Text] |
| 10. | Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ; Dutch Gastric Cancer Group. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438-1443. [PubMed] [DOI] [Full Text] |
| 11. | Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ, Kim JH, Kim HH, Lee JS. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer. 2011;14:130-138. [PubMed] [DOI] [Full Text] |
| 12. | Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D, Yakoub D. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. J Surg Oncol. 2015;112:38-45. [PubMed] [DOI] [Full Text] |
| 13. | Nakanishi H, Araki N, Kuratsu S, Narahara H, Ishikawa O, Yoshikawa H. Skeletal metastasis in patients with gastric cancer. Clin Orthop Relat Res. 2004;208-212. [PubMed] [DOI] [Full Text] |
| 14. | Choi CW, Lee DS, Chung JK, Lee MC, Kim NK, Choi KW, Koh CS. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin Nucl Med. 1995;20:310-314. [PubMed] [DOI] [Full Text] |
| 15. | Ahn JB, Ha TK, Kwon SJ. Bone metastasis in gastric cancer patients. J Gastric Cancer. 2011;11:38-45. [PubMed] [DOI] [Full Text] |
| 16. | Murai N, Koga K, Nagamachi S, Nishikawa K, Matsuki K, Kusumoto S, Watanabe K. [Radiotherapy in bone metastases--with special reference to its effect on relieving pain]. Gan No Rinsho. 1989;35:1149-1152. [PubMed] |
| 17. | McQuay HJ, Collins SL, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database Syst Rev. 2000;CD001793. [PubMed] [DOI] [Full Text] |
| 18. | Sudo H, Takagi Y, Katayanagi S, Hoshino S, Suda T, Hibi Y, Ito K, Tsutida A, Aoki T. [Bone metastasis of gastric cancer]. Gan To Kagaku Ryoho. 2006;33:1058-1060. [PubMed] |
| 19. | York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL. Gastric cancer and metastasis to the brain. Ann Surg Oncol. 1999;6:771-776. [PubMed] [DOI] [Full Text] |
| 20. | Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, Yalcin S. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164-172. [PubMed] [DOI] [Full Text] |