Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.139
Peer-review started: October 9, 2017
First decision: October 25, 2017
Revised: November 13, 2017
Accepted: November 21, 2017
Article in press: November 21, 2017
Published online: January 7, 2018
Processing time: 91 Days and 18.1 Hours
To define probiotic monotherapy effect on Helicobacter pylori (H. pylori) status by performing a systematic review.
Methods of analysis and inclusion criteria were based on PRISMA recommendations. Relevant publications were identified by searching PubMed, MEDLINE, Science Direct, and EMBASE. The end-point was to estimate eradication rate and urea breath test delta value before and after probiotic monotherapy across all studies and, overall, with a pooled data analysis. Adverse events of probiotic therapy were evaluated. The data were expressed as proportions/percentages, and 95%CIs were calculated. For continuous variables, we evaluated the weighted mean difference. Odd ratios (ORs) were calculated according to the Peto method for the comparison of eradication rates between probiotics and placebo.
Eleven studies were selected. Probiotics eradicated H. pylori in 50 out of 403 cases. The mean weighted eradication rate was 14% (95%CI: 2%-25%, P = 0.02). Lactobacilli eradicated the bacterium in 30 out of 235 patients, with a mean weighted rate of 16% (95%CI: 1%-31%). Saccharomyces boulardii achieved eradication in 6 out of 63 patients, with a pooled eradication rate of 12% (95%CI: 0%-29%). Multistrain combinations were effective in 14 out of 105 patients, with a pooled eradication rate of 14% (95%CI: 0%-43%). In the comparison of probiotics vs placebo, we found an OR of 7.91 in favor of probiotics (95%CI: 2.97-21.05, P < 0.001). Probiotics induced a mean reduction in delta values higher than placebo (8.61% with a 95%CI: 5.88-11.34, vs 0.19% for placebo, P < 0.001). Finally, no significant difference in adverse events was found between probiotics and placebo (OR = 1, 95%CI: 0.06-18.08).
Probiotics alone show a minimal effect on H. pylori clearance, thus suggesting a likely direct role.
Core tip: Despite several lines of evidence in the literature having demonstrated a pivotal role of probiotics as adjunctive treatment for Helicobacter pylori (H. pylori) eradication, national and international guidelines do not have a uniform consensus about their clinical application. Many meta-analyses have confirmed that co-administration of probiotics may have a beneficial effect on the prevention of side effects and eradication rates. Herein, we found that probiotic monotherapy may eradicate H. pylori in 14% of cases. Lactobacilli, Saccharomyces boulardii and multistrain combinations eradicated the bacterium with a rate of 16%, 12% and 14%, respectively. Probiotics were significantly more effective than placebo (OR = 7.91).
- Citation: Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, Di Leo A. Probiotic monotherapy and Helicobacter pylori eradication: A systematic review with pooled-data analysis. World J Gastroenterol 2018; 24(1): 139-149
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.139
Helicobacter pylori (H. pylori) is a ubiquitous Gram-negative, flagellated organism, residing in the human stomach, where it may cause both malignant and nonmalignant diseases[1-3]. The treatment of H. pylori relies mainly on a combination of antibiotics. However, despite several therapeutic schemes having been proposed, the way towards ideal therapeutic management remains an unsolved issue[4].
Until a few years ago, triple therapy (based on a proton pump inhibitor, amoxicillin and clarithromycin) was considered as the standard first-line regimen. However, failure rates have increased recently, due to the spreading of antibiotic resistances, which are due to point mutations of the H. pylori genome[5]. For this reason, alternative first-line regimens have been proposed (sequential, concomitant, quadruple with and without bismuth, and hybrid). In this context, the geographic pattern of antibiotic resistances must also be studied as a relevant matter[6-9]. To now, the “ideal therapy” does not exist and this is the real limit for worldwide effective therapeutic guidelines[6].
A relevant problem related to H. pylori therapy failure is linked to patient compliance, which is often affected by antibiotic-associated adverse events, including diarrhea, nausea, vomiting and abdominal pain. Therefore, the development of a new strategy which could improve the eradication rate as well as reduce the frequency of adverse effects is advisable.
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”[10]. The intestinal microbiota is the community of microorganisms which colonizes the gut. It is an essential component of the luminal intestinal environment. Antibiotic-induced alteration of the microbiota may lead to diarrhea and other side effects[11]. Consequently, probiotic supplementation could restore microbial balance, thus preventing antibiotic-associated adverse events[12,13]. In particular, this benefit may be useful in H. pylori management for the need to administer a combination of antibiotics at high dose.
Furthermore, it is supposed that probiotics could interfere with potential pathogens which may colonize the stomach[14]. Indeed, probiotics may compete with H. pylori for host surface receptors and, thereby, inhibit its adhesion to epithelial cells[15]. Furthermore, it has been demonstrated that, L. acidophilus may hamper H. pylori urease activity in vitro[16]. Finally, lactobacilli produce lactic acid, which is able to counteract H. pylori-induced hypochlorhydria and has bactericidal effect itself[17]. For these reasons, it is possible to hypothesize that probiotics may exert a direct inhibitory effect on H. pylori growth.
Several meta-analyses have demonstrated that probiotics, when given in combination with the standard therapy, induce an improvement in both eradication rates and reduction of adverse events. In this regard, Zhang et al[18] demonstrated that probiotic administration along with triple therapy achieved a success rate of 82.31% (against the 72.08% of the control group), with a risk ratio of 1.11 in favor of probiotics. Another study[19] showed that probiotics have a positive effect on preventing diarrhea [odds ratio (OR) = 0.21] and increase the eradication rate, with an OR of 1.68.
Until now, meta-analyses have investigated probiotic effects on H. pylori only in association with antibiotics. To the best of our knowledge, there are no meta-analyses concerning probiotic monotherapy effects on H. pylori infection. Therefore, our aim was to perform a systematic review with pooled data analysis regarding this uninvestigated topic.
Methods of analysis and inclusion criteria were based on “Preferred Reporting Items for Systematic reviews and Meta-Analyses” (PRISMA) recommendations[20], and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist has been enclosed as supplementary material. We excluded review articles, experimental in vitro studies and single case reports.
A literature search was performed in May 2017. Relevant publications were identified by a search of PubMed, MEDLINE, Science Direct and Scopus. The search terms were Helicobacter pylori, probiotics, lactobacilli, bifidobacteria, saccharomyces, treatment, eradication, breath test. We used the following string, with Boolean operators AND/OR: ([Helicobacter pylori OR H. pylori] AND [probiotic* OR lactobacil* OR bifidobacteria OR saccharomyces OR bacillus OR treatment OR eradication OR breath test]). We excluded studies that used probiotics in combination with antibiotics, while co-administration of other molecules, such as proton pump inhibitors, was not considered as an exclusion criterion. We excluded, as well, studies in which patients with major gastrointestinal surgery interventions were enrolled.
Titles and abstracts of papers were screened by two reviewers (Losurdo G and Ierardi E). Studies were independently prescreened in blinded fashion for relevance by the two reviewers using full reports. Discussion put an end to any disagreements. Successively, data were extracted from the relevant studies by one reviewer and checked by a second reviewer, and thus inserted into dedicated tables. A third reviewer (Leandro G) came to a decision on any disagreements.
Reviewers independently extracted the following data from each paper: (1) year of publication; (2) country where the study was performed; (3) single- or multicenter study; (4) study design; (5) number of patients included; (6) mean age and sex of enrolled patients; (7) test used to diagnose H. pylori infection; (8) type of probiotic and modality of administration; (9) success rate; (10) delta values of urea breath test (UBT); and (11) adverse events. We did not include studies reporting only the results of UBT delta value without detailing eradication rate.
The end-point was to estimate the mean eradication rate and variations of delta value at UBT across all studies and, overall, with a pooled data analysis. The data were expressed as proportions/percentages, and 95%CIs were calculated using the generic inverse variance method, as described in the Cochrane Handbook, Chapter 9.4.3.2[21], and as we already performed in a previous meta-analysis[22]. The inverse variance methods allow a “weighting” of the eradication rate according to the sample size. For continuous variables (delta value of UBT), we entered mean, standard deviations and sample size in order to calculate the weighted mean difference. OR and 95%CI were calculated, where available, based on the Peto method, for the comparison of two groups (probiotics vs placebo).
Data were entered into the RevMan 5.3 software (The Nordic Cochrane Centre, Copenhagen, Denmark) (Cochrane library) in order to draw forest plots. A P value < 0.05 was considered statistically significant. Heterogeneity was assessed by using the χ2 and I2 statistics. In particular, heterogeneity was considered to be present if the χ2 test delivered a P < 0.05 and, therefore, the I2 statistic was used to quantify the proportion of heterogeneity between the studies. In the presence of heterogeneity, a revision of included studies was carried out to assess the main reasons explaining the phenomenon and, therefore, a subgroup analysis was performed. Only if this attempt failed, a random effects model was employed, in order to minimize the impact of heterogeneity. We preferred a fixed effects model if less than 4 studies per outcome were included in the analysis[23].
The degrees of freedom (df) were reported for each analysis. We evaluated the quality of enrolled studies by the Jadad scale[24] for randomized clinical trials (RCTs) or by the Quality Assessment Tool for Case Series Studies (QATCSS) of the National Institutes of Health[25] for nonrandomized, open label pilot studies. Finally, when comparison between two groups (probiotics vs placebo) was performed, we drew funnel plots and applied Egger’s regression method to estimate the asymmetry of the funnel plots, considering non-statistically significant results as absence of publication bias[26].
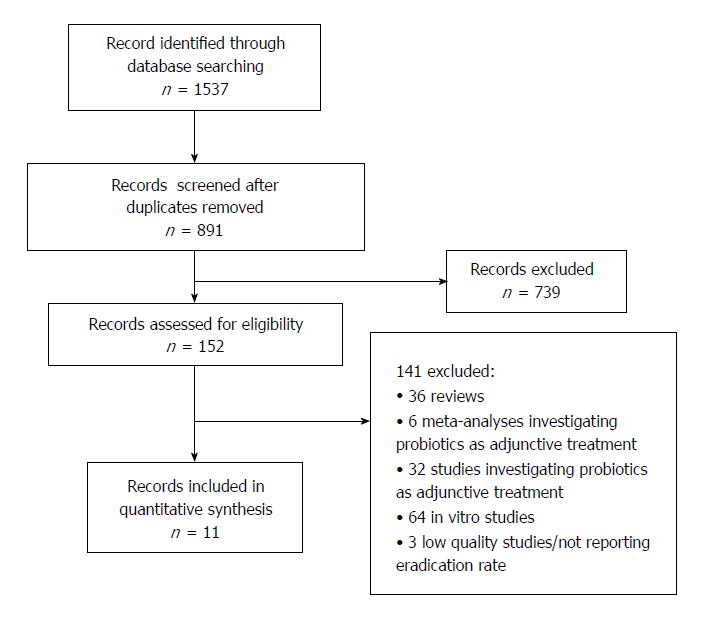
The literature search found 1537 articles overall. After study selection, reported in detail in Figure 1, 11 studies were eligible for the analysis[27-37]. Only 7 of them were RCTs[27,29,30,32,33,36,37]. A total of 517 H. pylori-infected patients were recruited. Of these, 114 received a placebo treatment and served as a control group, and the remaining 403 had probiotic supplementation. In all studies except 2, the diagnosis was achieved by UBT[27,37], but in most cases the initial diagnosis was established by the combination of more than one test, including UBT, upper endoscopy with histology or rapid urease test, serology or stool antigen test (SAT). The verification of eradication of treatment was performed by UBT in all but 2[27,37], which used SAT both for diagnosis and eradication control. Details of the cut-offs used for diagnosis and timing of UBT are reported in Table 1.
| Ref. | Nation | Age and sex | Probiotic strain and dose | Diagnosis | Control of eradication | Eradication rate % (n/N) |
| Boonyaritichaikij et al[27], 2009 | Japan | 62 ± 14 yr | Cheese with L. gasseri OLL2716 5 × 108 CFU/g for 12 mo | SAT | SAT after 12 mo | Probiotic: 29.3% (24/82) |
| Male sex: 54.5% | Placebo: 0% (0/6) | |||||
| Dore et al[28], 2014 | Italy | Mean age: 51 yr (range, 21-68) | L. reuteri 108 CFU/tablet bid + Pantoprazole 20 mg bid for 60 d | UBT | UBT after 30-40 d | 14.3% (3/21) |
| Male sex: 13.6% | ||||||
| Francavilla et al[29], 2008 | Italy | 53.3 ± 13.3 yr (probiotics) | L. reuteri ATCC55730 108 CFU/tablet bid for 28 d | UBT (cut-off 3.5%), SAT, RUT, histology | UBT after 4 wk | Probiotic: 0% (0/20) |
| 52.4 ± 13.1 yr (placebo) | Placebo: 0% (0/20) | |||||
| Male sex: 57.5% | ||||||
| Gotteland et al[30], 2005 | Chile | 8.5 ± 1.7 | L. acidophilus LB 109/tablet bid or S. boulardii 250 mg + inulin 5 g bid for 8 wk | UBT (cut-off 5‰) | UBT after 1 d | 9.3% (9/97) |
| Male sex: 49.6% | L. acidophilus 6.5% (3/46) | |||||
| S. boulardii 11.8% (6/51) | ||||||
| Myllyluoma et al[31], 2007 | Finland | Mean age: 51 yr (range, 40-69) | Multi-strain (L. rhamnosus GG, L. rhamnosus LC705, P. freudenreichii JS, B. lactis Bb12) 2.5 × 109 CFU/day for 8 wk | UBT (cut-off 2.2%), RUT, histology | UBT after 8 wk | 0% (0/6) |
| Pantoflickova et al[32], 2003 | Switzerland | 25 ± 5 yr | L. johnsonii bid for 3 wk, then once daily for 13 wk | UBT (cutoff 5%), histology, culture, RUT, serology | UBT, culture at the end of treatment | Probiotic: 0% (0/25) |
| Male sex: 50% | Placebo: 0% (0/25) | |||||
| Rosania et al[33], 2012 | Italy | 52.4 ± 21.7 yr (probiotics) | Multi-strain (S. termophilus, L. acidophilus, B. longum, L. plantarum, B. brevis, L. paracasei, B. infantis, L. delbrueckii) 1800 × 109/d for 10 d | UBT (cut-off 4%) | UBT after 4 wk | Probiotic: 32.5% (13/40) |
| 48.7 ± 25.3 yr (placebo) | Placebo: 0% (0/40) | |||||
| Male sex: 42.5% | ||||||
| Sakamoto et al[34], 2001 | Japan | 50.1 ± 7.4 yr | Yoghurt + L. gasseri OLL2716 1-1.4 × 107 CFU/g bid for 8 wk | UBT (cut-off 5%) | UBT after 9 wk | 0% (0/29) |
| Male sex: 93.1% | ||||||
| Shimizu et al[35], 2002 | Japan | Mean age: 12.1 yr (range, 7.4-15.8) Male sex: 41.7% | Yoghurt + L. gasseri OLL2716 1-1.4 × 107 CFU/g bid for 8 wk | SAT, UBT | SAT, UBT after 4 and 10 wk | 0% (0/12) |
| Wang et al[36], 2004 | China | Not available | Multi-strain yoghurt (L. acidophilus La5, B. lactis Bb12, L. bulgaricus, S. termophilus) > 107 bacteria/mL for 6 wk | UBT (cut-off 3.5%), histology | UBT after 8 wk | Probiotic: 1.7% (1/59) |
| Placebo: 0% (0/11) | ||||||
| Namkin et al[37], 2016 | Iran | Age range of 9-12 yr | S. boulardii 250 mg/d for 1 mo | SAT | SAT after 8 wk | Probiotic: 0% (0/12) |
| Male sex: 20.8% | Placebo: 0% (0/12) |
Only 3 studies were conducted on the pediatric population[30,35,37]. In most cases (7), a lactobacilli-based formulation was employed, while only 2 studies administered Saccharomyces boulardii[28,35] and 3 investigated probiotic multistrain formulations[31,33,36]. Of note, only in 1 study[28] was probiotics given in combination with proton pump inhibitor. The duration of probiotic supplementation varied across the studies, from 10 d to 1 year. Quality assessment is reported in Table 2.
| Ref. | Type of study | Jadad score1 | QATCSS score2 |
| Boonyaritichaikij et al[27], 2009 | Randomized, single blind placebo-controlled, pilot | 3 | NA |
| Dore et al[28], 2014 | Prospective, single center, open label pilot study | NA | 8 |
| Francavilla et al[29], 2008 | Randomized, double blind placebo-controlled | 4 | NA |
| Gotteland et al[30], 2005 | Randomized, open study | 3 | NA |
| Myllyluoma et al[31], 2007 | Prospective, single center, open label pilot study | NA | 7 |
| Pantoflickova et al[32], 2003 | Randomized, double blind placebo-controlled | 4 | NA |
| Rosania et al[33], 2012 | Randomized, double blind placebo-controlled | 4 | NA |
| Sakamoto et al[34], 2001 | Single center, open label pilot study | NA | 6 |
| Shimizu et al[35], 2002 | Single center, open label pilot study | NA | 6 |
| Wang et al[36], 2004 | Randomized, double blind placebo-controlled | 2 | NA |
| Namkin et al[37], 2016 | Randomized, double blind placebo-controlled | 5 | NA |
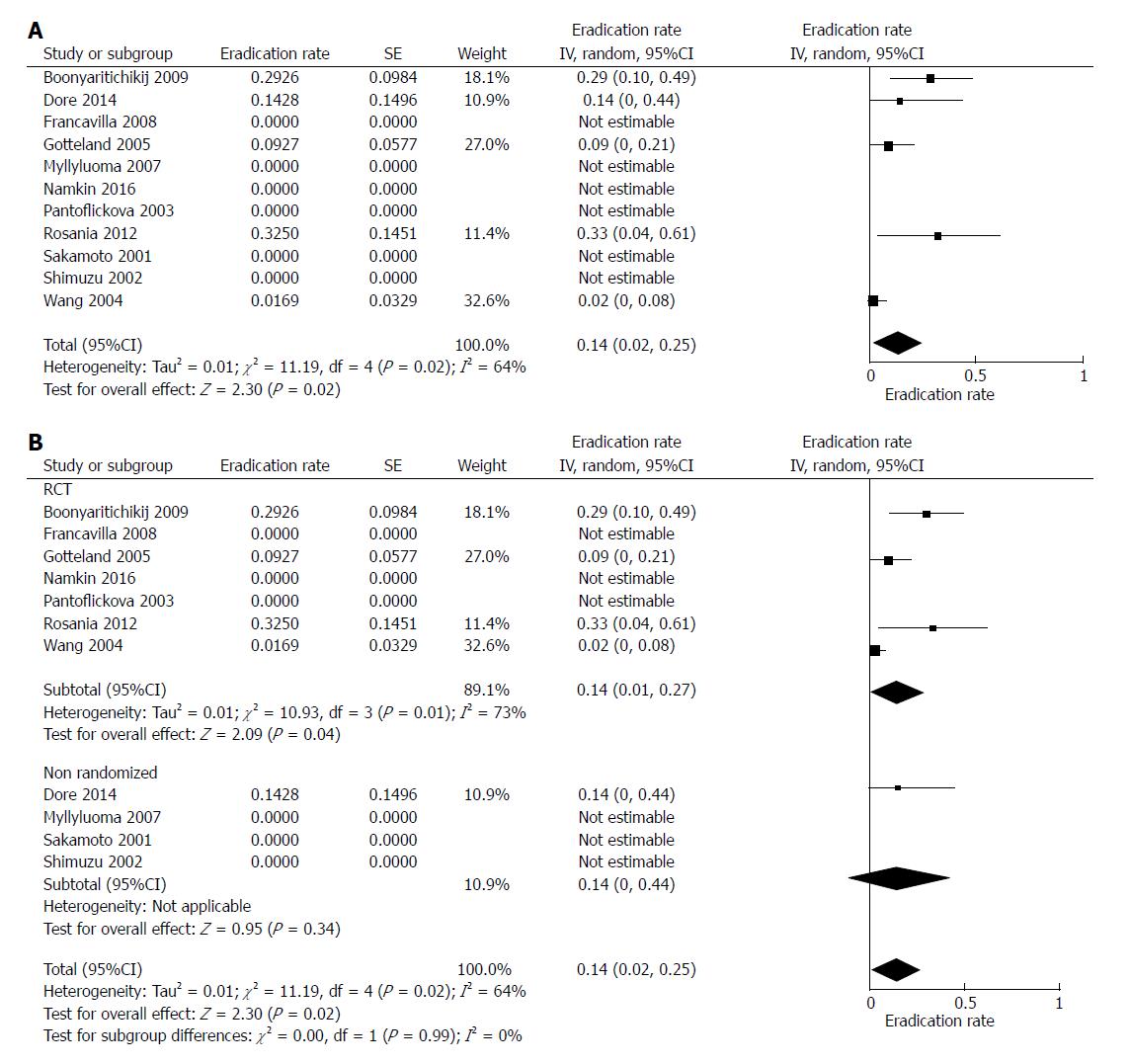
In the 11 selected studies, probiotics eradicated H. pylori in 50 out of 403 cases. The mean weighted eradication rate was 14%, with a 95%CI of 2%-25% (df = 4, P = 0.02). In 6 studies, probiotic treatment was unsuccessful[29,31,32,34,35,37], while the highest percentage of eradication (32.5%) was achieved in an Italian study[33]. The forest plot of such analysis is displayed in Figure 2A.
Further, we performed a sub-analysis comparing the success rate in RCT vs non-randomized studies (Figure 2B). The pooled rate was 14% for RCT (95%CI: 1%-27%, df = 3, P = 0.04) and 14% for non-randomized trials (95%CI: 0%-44%, P = 0.34). No difference was found between these two groups (P = 0.99).
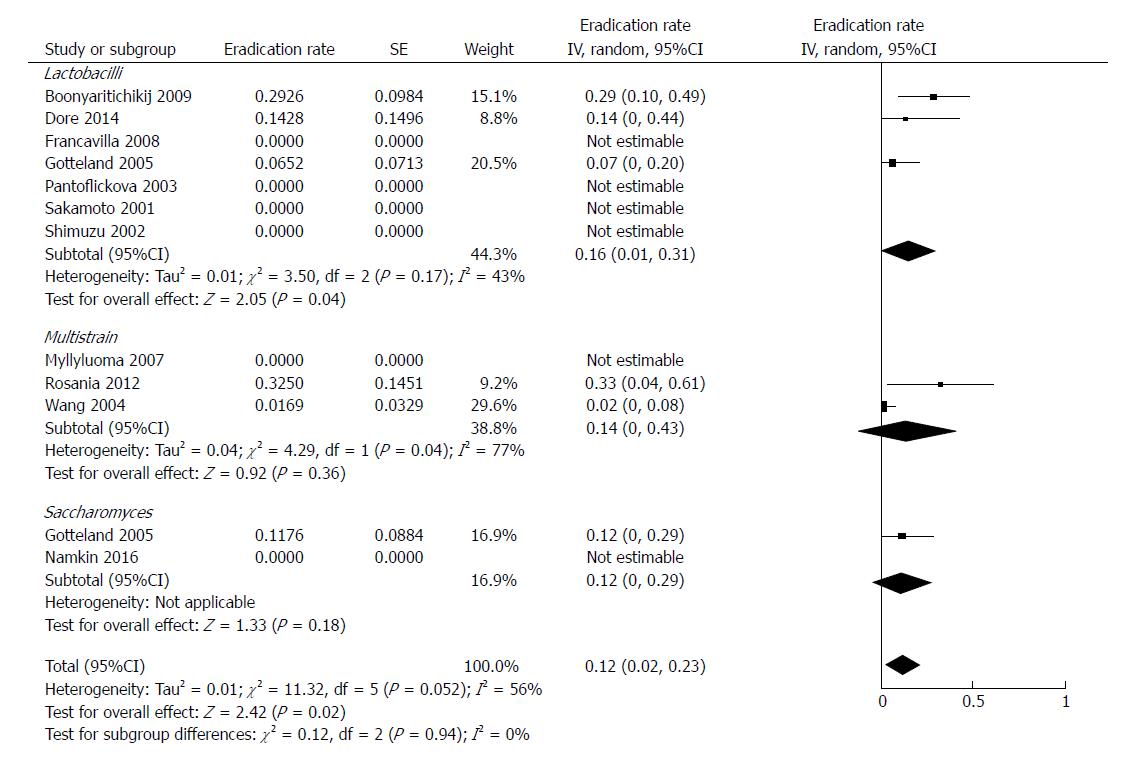
Most of studies investigated a probiotic formulation based on a single lactobacilli strain (further details of species are listed in Table 1)[27-30,32,34,35]. Lactobacilli eradicated the bacterium in 30 out of 235 patients, with a mean weighted rate of 16% (95%CI: 1%-31%, df = 2). Multistrain combinations[31,33,36] were effective in 14 out of 105 patients, with a pooled eradication rate of 14% (95%CI: 0%-43%, df = 1). In the two studies evaluating Saccharomyces boulardii[30,37], the treatment was successful in 6 out of 63 subjects (pooled rate of 12%, 95%CI: 0%-29%). We did not find any statistically significant difference among these three formulations (P = 0.94). The forest plot of this analysis is reported in Figure 3.
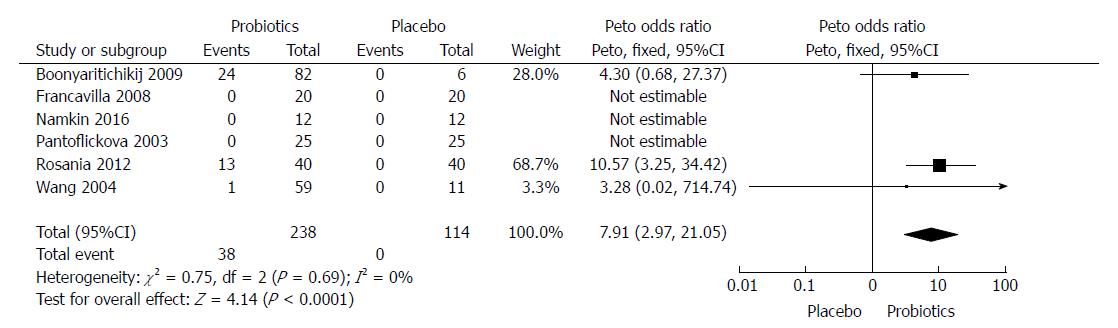
Six RCTs[27,29,32,33,36,37] compared probiotics to a placebo (see Figure 4). In total, probiotics eradicated the bacterium in 38 out of 238 patients (15.9%), while placebo alone did not achieve any success (0 out of 114, 0%). The analysis, reported in Figure 4, provided an OR of 7.91 in favor of probiotics, with a 95%CI of 2.97-21.05. In this case, we used a fixed effects model since heterogeneity was absent (χ2 = 0.75, df = 2, P = 0.69). A funnel plot, reported in Figure 5, showed that a possible bias could be detected, as confirmed by Egger’s test (P = 0.02). However, the low number of included studies and the presence of 0% eradication rates (which are void for the test) imply that the test has a low statistical power, and therefore the possibility of bias is questionable anyway.
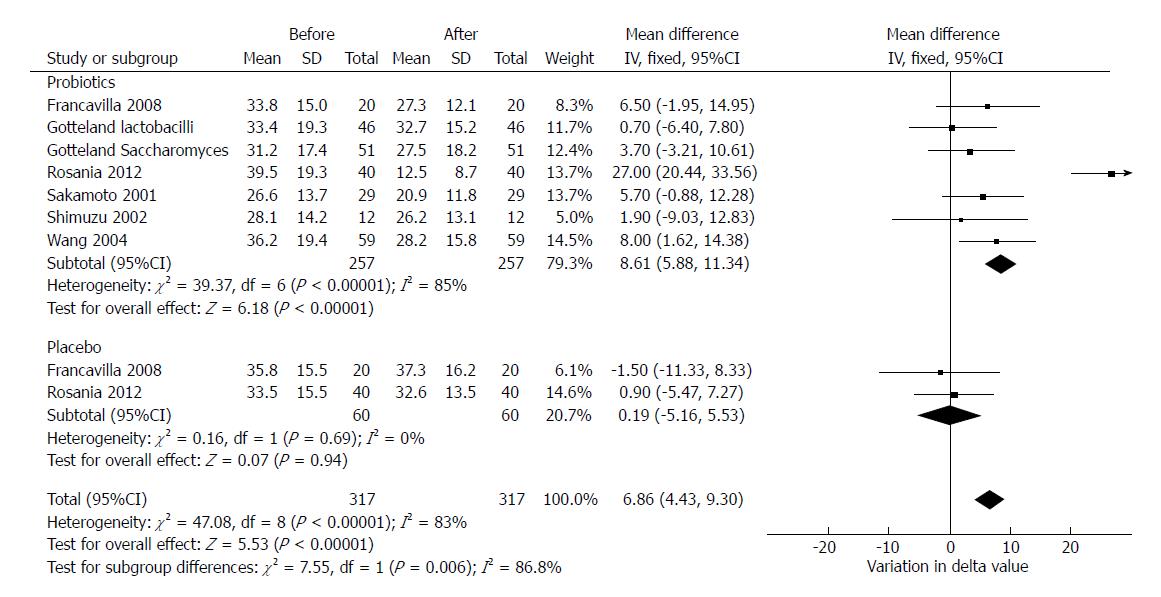
We aimed to evaluate whether probiotics’ administration alone could reduce the expired 14C-marked CO2 during the UBT. Six studies provided sufficient data (delta values expressed as ‰) to perform such analysis[29,30,33-36]. In two studies, delta values for placebo were reported[29,33].
Overall, probiotics induced a statistically significant mean reduction in delta values of 8.61‰ (95%CI: 5.88-11.34, df = 6) which was statistically significant. On the other hand, placebo implied a reduction of 0.19‰, which was not statistically significant (95%CI: -5.16-5.53, P = 0.94, df = 1). The test for subgroup differences demonstrated that probiotics significantly reduced delta compared to placebo (P = 0.006). In this analysis, despite a high heterogeneity (χ2 = 47.08, df = 8, P < 0.001, I2 = 83%) we used a fixed effects model since the number of included studies was low and the heterogeneity could be explained by the different type of probiotics and the different study design of enclosed trials. The forest plot of this analysis is reported in Figure 6.
Only 3 studies described adverse events during probiotic administration[28,30,37], and only 1 case of side effect was reported in 39 treated patients, with a pooled prevalence of 8% (95%CI: 0%-39%, P = 0.59). In only 1 study[37], side effect rate was reported both for placebo and probiotic groups. In this case, the meta-analysis did not show any difference between the two groups (OR = 1, 95%CI: 0.06-18.08, P = 1).
Despite several lines of evidence in the literature having demonstrated a consistent role of probiotics as adjunctive treatment for H. pylori eradication[38], national and international guidelines do not address a uniform consensus about their clinical application. The last Maastricht guidelines state that certain probiotics may have a beneficial impact on the eradication[39]. Similarly, Italian guidelines advise their use since they may reduce antibiotics-related side effects[40]. On the other hand, Toronto guidelines discourage routine probiotic administration in order to reduce side effects and improve the efficacy, since clinical trials and meta-analyses are characterized by low quality[41].
The most important issue that sets a limit to draw conclusions about the effects of probiotics in the treatment of H. pylori is that they have been considered only as an adjunctive treatment to antibiotics. In this context, probiotics demonstrated effectiveness mainly in reducing adverse events (especially diarrhea). However, these studies did not provide adequate evidence regarding a direct role in the eradication. Few studies have focused probiotic alone activity on bacteriotherapy in this field and, to date, this is the first systematic review on this topic.
In our analysis, the exclusive inclusion of studies using probiotics alone allowed us to draw more solid conclusions about the role of probiotics, since we removed the interference of factors and bias related to antibiotics such as inhomogeneous resistance pattern, variations in doses and administration modalities, patient compliance and adverse events. On the other hand, our analysis implied other limitations, such as the low number of enrolled patients, the differences of administered probiotic strains and the lack of randomization and/or a placebo arm as control group. For this reason, we attempted to limit these sources of heterogeneity by adding subgroup analyses and by choosing a random effect model heterogeneity that was high, a strategy that can minimize this phenomenon[23]. Finally and unfortunately, none of the included studies reported any data about smoking habits nor on alcohol assumption. Therefore, we were unable to perform a sub-analysis. This is another drawback, since it is known that such factors could influence the eradication. However, most of studies were conducted in pediatric populations, so that we may assume that such cases patients did not consume alcohol nor cigarettes.
The first relevant finding of this review is that probiotics alone may eradicate H. pylori, in 14%. From a clinical point of view, this is an unsatisfactory rate; however, taking into account that this percentage is considerably higher than placebo (0%, with a Peto OR = 7.91; Figure 4), we could assume that probiotic direct antibacterial action against H. pylori is consistent. Our analysis failed to ascertain whether some formulations may be more effective than others, but this limitation is due to the low number of included studies. Indeed, better outcome (32.5% of successful eradication) was achieved in the study which employed a multistrain combination with the highest bacterial charge[33]. On the other hand, in 4 out of 7 studies using a single lactobacillus strain, no eradication was recorded. These observations may suggest that an association of more bacterial species could be more effective[42]. One study explored the effect of Saccharomyces boulardii, a yeast species, demonstrating a success rate of 11.8% and, thus, indicating a reliable performance in H. pylori gastritis[43,44].
The second important result concerns the variations in delta values for UBT. Indeed, as shown in Figure 6, in all studies, a reduction of delta values was observed in the probiotic arm, while delta values remained stable in subjects assuming placebo. This result is in agreement with evidence from the literature[45,46] and may suggest that probiotics could reduce the bacterial load in any case, despite a complete eradication not being obtained[47,48]. Indeed, labeled CO2 in the expirate is considered as an indirect indicator of the density of gastric H. pylori colonization[49,50]. A probiotic-induced intragastric bacterial load reduction has been confirmed by histological semiquantitative analysis in some included studies[31] and even by a study, which used an original assessment of bacterial stool antigen[29,51].
In conclusion, preliminary data show that a primary therapeutic effect of probiotics may be hypothesized for H. pylori, but the low number of studies, their inhomogeneity in the design and the low number of enrolled patients are a critical limit to drawing evidence-based conclusions. However, the modulation of gastric microbiota could represent an intriguing aspect, since it does not imply the drawback of antibiotics (induction of dysbiosis, side effects) and is safe and probably more acceptable for patients[11,52].
Probiotics have been largely used as adjunctive treatment for Helicobacter pylori (H. pylori) eradication, showing good results.
Until now, meta-analyses have investigated probiotic effects on H. pylori only in association with antibiotics. Therefore, our aim was to perform a systematic review with pooled data analysis regarding this uninvestigated topic.
The objective was to perform a meta-analysis aiming to calculate a pooled eradication rate for probiotic monotherapy, overall and according to the strain.
Article search and selection was conducted according to the PRISMA criteria. We performed a pooled-data analysis using to the inverse variance method to calculate the mean weighted eradication rate. Peto odd ratio (OR) was calculated for the comparison “probiotics vs placebo”. For continuous variables (delta value of urea breath test), we entered mean, standard deviations and sample size in order to calculate the weighted mean difference.
We found that probiotic monotherapy may eradicate H. pylori in 14% of cases. Lactobacilli, Saccharomyces boulardii and multistrain combinations eradicated the bacterium with a rate of 16%, 12% and 14%, respectively. Probiotics were significantly more effective than placebo (OR = 7.91). Moreover, probiotics were able to reduce delta values in the expirate of urea breath test.
The eradication rate of probiotics’ monotherapy is disappointing; however, our meta-analysis showed that, in some cases, they are able to defeat the bacterium. They compete with H. pylori for host surface receptors and, thereby, inhibit its adhesion to epithelial cells. Furthermore, it has been demonstrated that probiotics could hamper H. pylori urease activity. On these bases, since probiotics administration does not carry the risk of antibiotic resistance, it could represent an optimal strategy in selected cases.
Further studies on large sample size are necessary to draw more solid conclusions about a direct inhibitory effect of probiotics on H. pylori.
The authors are thankful to Vito Bellomo for graphical assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Islek A, Nagahara H, Slomiany BL, Zhu YL S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Ierardi E, Goni E, Losurdo G, Di Mario F. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2014;19 Suppl 1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Franceschi F, Gasbarrini A, Polyzos SA, Kountouras J. Extragastric Diseases and Helicobacter pylori. Helicobacter. 2015;20 Suppl 1:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Helicobacter pylori and Gastrointestinal Malignancies. Helicobacter. 2015;20 Suppl 1:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Georgopoulos SD, Papastergiou V, Karatapanis S. Current options for the treatment of Helicobacter pylori. Expert Opin Pharmacother. 2013;14:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ierardi E, Giorgio F, Iannone A, Losurdo G, Principi M, Barone M, Pisani A, Di Leo A. Noninvasive molecular analysis of Helicobacter pylori: Is it time for tailored first-line therapy? World J Gastroenterol. 2017;23:2453-2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J Gastroenterol. 2013;19:8168-8180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53:1374-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 679] [Article Influence: 32.3] [Reference Citation Analysis (2)] |
| 8. | Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Losurdo G, Leandro G, Principi M, Giorgio F, Montenegro L, Sorrentino C, Ierardi E, Di Leo A. Sequential vs. prolonged 14-day triple therapy for Helicobacter pylori eradication: the meta-analysis may be influenced by ‘geographical weighting’. Int J Clin Pract. 2015;69:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria (PDF), in Food and Agriculture Organization of the United Nations. World Health Organization. . |
| 11. | Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 12. | Ianiro G, Bibbò S, Gasbarrini A, Cammarota G. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets. 2014;15:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Lionetti E, Miniello VL, Castellaneta SP, Magistá AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Ianiro G, Molina-Infante J, Gasbarrini A. Gastric Microbiota. Helicobacter. 2015;20 Suppl 1:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:G941-G950. [PubMed] |
| 16. | Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573-4580. [PubMed] |
| 17. | De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett. 2006;259:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Zhang MM, Qian W, Qin YY, He J, Zhou YH. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:4345-4357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 19. | Zhu R, Chen K, Zheng YY, Zhang HW, Wang JS, Xia YJ, Dai WQ, Wang F, Shen M, Cheng P. Meta-analysis of the efficacy of probiotics in Helicobacter pylori eradication therapy. World J Gastroenterol. 2014;20:18013-18021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 10990] [Article Influence: 686.9] [Reference Citation Analysis (0)] |
| 21. | Cochrane Handbook for Systematic Reviews of Interventions. Accessed August 31 2017. Available from: http://training.cochrane.org/handbook. |
| 22. | Losurdo G, Marra A, Shahini E, Girardi B, Giorgio F, Amoruso A, Pisani A, Piscitelli D, Barone M, Principi M. Small intestinal bacterial overgrowth and celiac disease: A systematic review with pooled-data analysis. Neurogastroenterol Motil. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Leandro G. Meta-analysis in Medical Research. The handbook for the understanding and practice of meta-analysis. Malden,Massachusetts, USA: Blackwell Publishing, Inc 2005; . |
| 24. | Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16:62-73. [PubMed] |
| 25. | National Heart, Lung, and Blood Institute. Quality Assessment Tool for Case Series Studies. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series. |
| 26. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 27. | Boonyaritichaikij S, Kuwabara K, Nagano J, Kobayashi K, Koga Y. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter. 2009;14:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Dore MP, Cuccu M, Pes GM, Manca A, Graham DY. Lactobacillus reuteri in the treatment of Helicobacter pylori infection. Intern Emerg Med. 2014;9:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008;13:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Gotteland M, Poliak L, Cruchet S, Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:1747-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Myllyluoma E, Kajander K, Mikkola H, Kyrönpalo S, Rasmussen M, Kankuri E, Sipponen P, Vapaatalo H, Korpela R. Probiotic intervention decreases serum gastrin-17 in Helicobacter pylori infection. Dig Liver Dis. 2007;39:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Pantoflickova D, Corthésy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F, Enslen M, Blum AL. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Rosania R, Minenna MF, Giorgio F, Facciorusso A, De Francesco V, Hassan C, Panella C, Ierardi E. Probiotic multistrain treatment may eradicate Helicobacter pylori from the stomach of dyspeptics: a placebo-controlled pilot study. Inflamm Allergy Drug Targets. 2012;11:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Shimizu T, Haruna H, Hisada K, Yamashiro Y. Effects of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in children. J Antimicrob Chemother. 2002;50:617-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, Jan CM, Lai CH, Wang TN, Wang WM. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr. 2004;80:737-741. [PubMed] |
| 37. | Namkin K, Zardast M, Basirinejad F. Saccharomyces Boulardii in Helicobacter Pylori Eradication in Children: A Randomized Trial From Iran. Iran J Pediatr. 2016;26:e3768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Lü M, Yu S, Deng J, Yan Q, Yang C, Xia G, Zhou X. Efficacy of Probiotic Supplementation Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11:e0163743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1969] [Article Influence: 246.1] [Reference Citation Analysis (1)] |
| 40. | Zagari RM, Romano M, Ojetti V, Stockbrugger R, Gullini S, Annibale B, Farinati F, Ierardi E, Maconi G, Rugge M. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig Liver Dis. 2015;47:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 628] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 42. | McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4:546-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Ianiro G, Bruno G, Lopetuso L, Beghella FB, Laterza L, D’Aversa F, Gigante G, Cammarota G, Gasbarrini A. Role of yeasts in healthy and impaired gut microbiota: the gut mycome. Curr Pharm Des. 2014;20:4565-4569. [PubMed] |
| 44. | Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2015;41:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2004;9:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Hsieh PS, Tsai YC, Chen YC, Teh SF, Ou CM, King VA. Eradication of Helicobacter pylori infection by the probiotic strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter. 2012;17:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Holz C, Busjahn A, Mehling H, Arya S, Boettner M, Habibi H, Lang C. Significant Reduction in Helicobacter pylori Load in Humans with Non-viable Lactobacillus reuteri DSM17648: A Pilot Study. Probiotics Antimicrob Proteins. 2015;7:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Zagari RM, Pozzato P, Martuzzi C, Fuccio L, Martinelli G, Roda E, Bazzoli F. 13C-urea breath test to assess Helicobacter pylori bacterial load. Helicobacter. 2005;10:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | De Francesco V, Zullo A, Perna F, Giorgio F, Hassan C, Vannella L, Cristofari F, Panella C, Vaira D, Ierardi E. Helicobacter pylori antibiotic resistance and [13C]urea breath test values. J Med Microbiol. 2010;59:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Ierardi E, Margiotta M, Monno R, De Francesco V, Minenna MF, Burattini O, Faleo D, Panella C, Francavilla A, Cuomo R. A new semiquantitative method of quantifying Helicobacter pylori in antigen stools. J Clin Gastroenterol. 2002;35:375-378. [PubMed] |
| 52. | Cammarota G, Ianiro G, Bibbò S, Gasbarrini A. Gut microbiota modulation: probiotics, antibiotics or fecal microbiota transplantation? Intern Emerg Med. 2014;9:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |