Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1586
Peer-review started: September 27, 2016
First decision: October 28, 2016
Revised: November 13, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: March 7, 2017
Processing time: 160 Days and 18.9 Hours
To elucidate the role of proton pump inhibitors (PPIs) in collagenous disease, direct effect of PPI on colonocytes was examined.
Collagenous colitis is a common cause of non-bloody, watery diarrhea. Recently, there has been increasing focus on the use of proton PPIs as a risk factor for developing collagenous colitis. Mouse CT26 colonic cells were treated with PPI and/or PPI-induced alkaline media. Expression of fibrosis-associated genes was examined by RT-PCR. In human materials, collagen expression was examined by immunohistochemistry.
CT26 cells expressed a Na+-H+ exchanger gene (solute carrier family 9, member A2). Treatment with PPI and/or PPI-induced alkaline media caused growth inhibition and oxidative stress in CT26 cells. The treatment increased expression of fibrosis inducing factors, transforming growth factor β and fibroblast growth factor 2. The treatment also decreased expression of a negative regulator of collagen production, replication factor C1, resulting in increased expression of collagen types III and IV in association with lipid peroxide. In biopsy specimens from patients with collagenous colitis, type III and IV collagen were increased. Increase of type III collagen was more pronounced in PPI-associated collagenous colitis than in non-PPI-associated disease.
From these findings, the reaction of colonocytes to PPI might participate in pathogenesis of collagenous colitis.
Core tip: The main contribution of our paper is the finding of the basic mechanism of proton pump inhibitor evoking collagenous colitis with direct effects to colon epithelial cells. The collagenous colitis is a major cause of non-hemorrhagic watery diarrhea; however, the mechanism of the disease has not been fully elucidated. Our research findings show that proton pump inhibitor causes oxidative stress and collagen synthesis in colon epithelial cells, which might provide an impact to understanding pathogenesis of collagenous colitis and the side effect of proton pump inhibitor.
- Citation: Mori S, Kadochi Y, Luo Y, Fujiwara-Tani R, Nishiguchi Y, Kishi S, Fujii K, Ohmori H, Kuniyasu H. Proton pump inhibitor induced collagen expression in colonocytes is associated with collagenous colitis. World J Gastroenterol 2017; 23(9): 1586-1593
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1586
Collagenous colitis is one type of microscopic colitis and is the most common cause of non-bloody, watery diarrhea[1,2]. Originally Freeman et al[3] reported as a colitis with watery diarrhea and a lesion of colonic basement membrane. Collagenous colitis is characterized by collagenous bands at the basement membrane under microscopic examination[4,5]. This disease is treated with anti-inflammatory agents including salicylate[6]; however, untreated disease might progress into ulcerative colitis[7,8].
Recently, proton pump inhibitors (PPIs), especially lansoprazole, have been considered a potential cause of collagenous colitis[5]. Lansoprazole administration caused collagen bands in colonic mucosa in Mongolian gerbils[9]. However, the role of PPIs in the pathogenesis of collagenous colitis is not fully understood. Many pathologic mechanisms are proposed: colonic contents, mucosal immunity including autoimmunity and flora-associated immunity, eosinophilic reaction, human leukocyte antigen, biliary acids, anaerobic bacteria, fibroblast activation, and reduction of acidity in colonic contents due to suppression of acid secretion to the gastric juice[5,10]. In these candidates, the role of colonocytes' response to PPI is not given adequate attention. In the present study, we attempt to elucidate the pathologic importance of the direct effect of PPIs on colonocytes.
We reviewed the pathology and clinical data of 11 patients with collagenous colitis diagnosed in the Department of Molecular Pathology, Nara Medical University from 2012 to 2015. Two samples of non-pathological colonic mucosa were used as controls. Basic patient information is summarized in Table 1. As written informed consent was not obtained, any identifying information was removed from the samples prior to analysis, in order to ensure strict privacy protection. All procedures were performed in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government, which was approved by the Ethics Committee of Nara Medical University (Approval Number 937).
| Gene | Symbol | Accession No. | Upper primer | Lower primer |
| Collagen type IV | Col4a1 | NM_009931.2 | AAAGGGAGAAAGAGGCTTGC | CCTTTGTACCGTTGCATCCT |
| Collagen type VI | Col6a1 | NM_009933.4 | GATGAGGGTGAAGTGGGAGA | CAGCACGAAGAGGATGTCAA |
| Collagen type I | Col1a1 | NM_007742.4 | GAGCGGAGAGTACTGGATCG | GCTTCTTTTCCTTGGGGTTC |
| Collagen type III | Col3a1 | NM_009930.2 | GCACAGCAGTCCAACGTAGA | TCTCCAAATGGGATCTCTGG |
| Solute carrier family 9, member A2 | Slc9a2 | NM_001033289.2 | ACTGGGGTCACAACTTCTGG | CTTCACGGCAGTCATTGAGA |
| Transforming growth factor β1 | Tgfb1 | NM_011577.2 | TTGCTTCAGCTCCACAGAGA | TGGTTGTAGAGGGCAAGGAC |
| Fibroblast growth factor 2 | Fgf2 | NM_008006.2 | AGCGGCTCTACTGCAAGAAC | GCCGTCCATCTTCCTTCATA |
| Replication factor C1 | Rfc1 | NM_011258.2 | TGATCACAGGAGTGCTGGAG | CGAGGATTTTTGTTCCCAGA |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | NM_001289726.1 | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA |
CT26 mouse colon cancer cell line was provided by Professor I. J. Fidler (MD Anderson Cancer Center, Texas University). Cells were cultured in Dulbecco's modified Eagle's medium [DMEM; with high glucose (450 mg/dL), low glucose (100 mg/dL) or no glucose; WAKO Purechemical Ind. Ltd., Osaka, Japan), supplemented with 10% fetal bovine serum (FBS, Sigma Chemical Co., St. Louis, MO, United States) at 37 °C in 5% CO2. Alkaline (pH 8.0) medium was made from DMEM with addition of Tris-HCl (pH 9.0, Sigma). Cell growth was assessed using a tetrazolium dye assay (MTT, Sigma), as previously described[11].
Pantoprazole (Sigma), MitoGreen (Takara Bio Inc., Kusatsu, Japan), and 4-hydroxynonenal (4HNE) ELISA kit (R&D Systems Inc., Minneapolis, MN, United States) were purchased. For evaluation of MitoGreen, strength of fluorescence was measured in computer-captured images by using Photoshop Image Analyzer (Adobe Systems Inc., San Jose, CA, United States). 4HNE was measured in cell lysate according to the manufacturers' instructions. Whole-cell lysates were prepared using 0.1% nonidet 40 containing lysis buffer as previously described[12].
Consecutive 4-μm sections were immunohistochemically stained using the immunoperoxidase technique described previously[13]. Anti-type IV collagen antibody and anti-type III collagen antibody (Abcam, Cambridge, MA, United States) were used at a concentration of 0.2 μg/mL. Secondary antibodies (Medical and Biological Laboratories, Nagoya, Japan) were used at a concentration of 0.2 μg/mL. Tissue sections were color-developed with diamine benzidine hydrochloride (DAKO, Glastrup, Denmark), and counterstained with Meyer's hematoxylin (Sigma). We evaluated immunopositivity at the basement membrane and stromal fibers. Staining strength was scored from (-) to (++): (-) is no staining; (+) is staining equal to that of non-pathologic mucosa; (++) is staining more pronounced than that of non-pathologic mucosa. All samples were stained at one time to equalize staining conditions. For a negative control, unimmunized rat IgG (Santa-Cruz Biotechnology, Santa-Cruz, CA, United States) was used as primary antibody.
Reverse transcription-polymerase chain reaction (RT-PCR) of 0.5 μg total RNA extracted using an RNeasy kit (Qiagen, Germantown, MD, United States) was performed. Primer sets used in the experiment are listed in Table 1. Primers were synthesized by Sigma Genosys (Ishikari, Japan). PCR products were electrophoresed in a 2% agarose gel and stained with ethidium bromide.
Statistical significance was calculated by using two-tailed Fisher's exact, Chi-square, and unpaired Student-t tests by using InStat software (GraphPad, Los Angeles, CA, United States). Statistical significance was defined as a two-sided P-value of < 0.05.
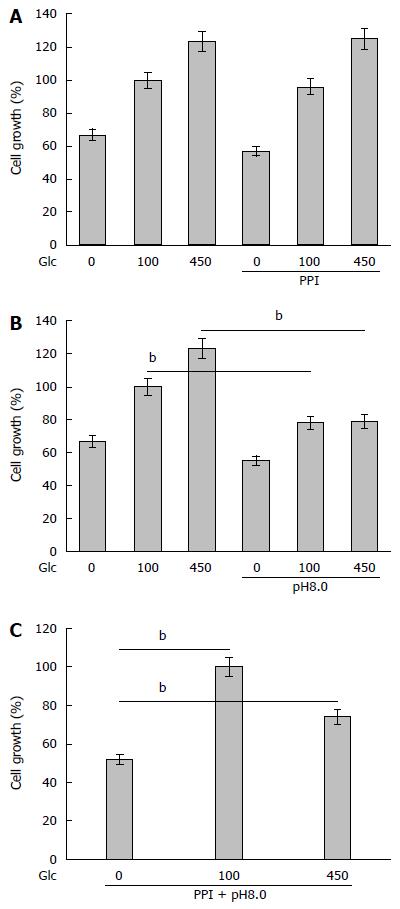
The effect of PPIs was examined by using CT26 mouse colon cancer cells treated with pantoprazole and/or alkaline media[14] (Figure 1). PPI treatment did not affect cell proliferation of CT26 cells regardless of glucose concentration (Figure 1A). In contrast, alkaline media suppressed cell proliferation at 100 and 450 mg/dL glucose concentrations (Figure 1B). When treated with PPI and alkaline media together, cell proliferation in 0 and 450 mg/dL glucose concentrations was suppressed more than in 100 mg/dL glucose concentration (Figure 1C).
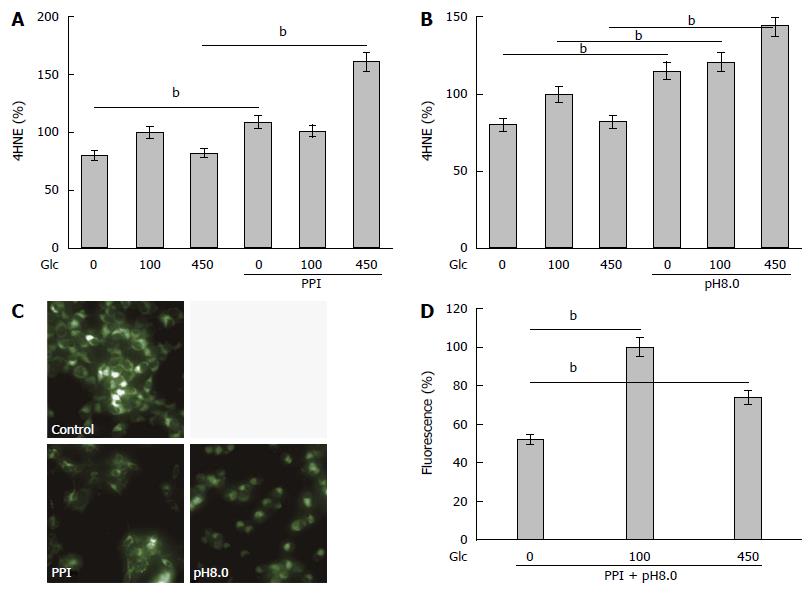
Next, oxidative stress was examined by 4HNE levels, as it is a marker for lipid peroxide (Figure 2A and B). Treatment with PPI or alkaline media increased levels of 4HNE in various glucose concentrations. Alkaline media-treated cells showed higher 4HNE levels than PPI-treated cells. Treatment with PPI or alkaline media decreased mitochondrial volume (Figure 2C), particularly in 100 and 450 mg/dL glucose concentrations compared to 0 mg/dL glucose concentration (Figure 2D).
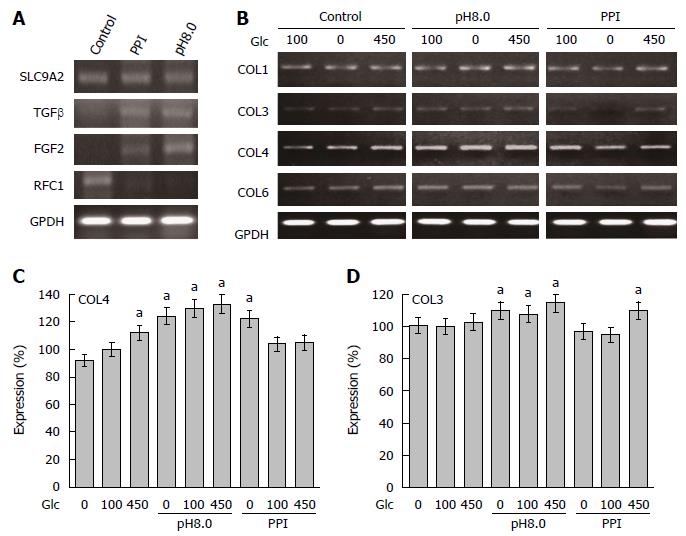
We confirmed the expression of Na+-H+ exchanger (solute carrier family 9, member A2; SLC9A2), a pharmacological target of PPIs (Figure 3A). SLC9A2 expression was not affected by treatment with PPI or alkaline media. Expression of transforming growth factor (TGF) β and fibroblast growth factor (FGF) 2 was increased by treatment with PPI or alkaline media. TGF-β and FGF2 are known to stimulate collagen-producing fibroblasts[14]. In contrast, expression of replication factor C1 (RFC1 orAlp145), a negative regulator of collagen expression[15], was downregulated by treatment with PPI or alkaline media. Compared to other collagen types, mRNA expression of type III and IV collagen was increased in CT26 cells treatment with PPI or alkaline media (Figure 3B-D).
Production of collagen type III and IV in human collagenous colitis mucosa
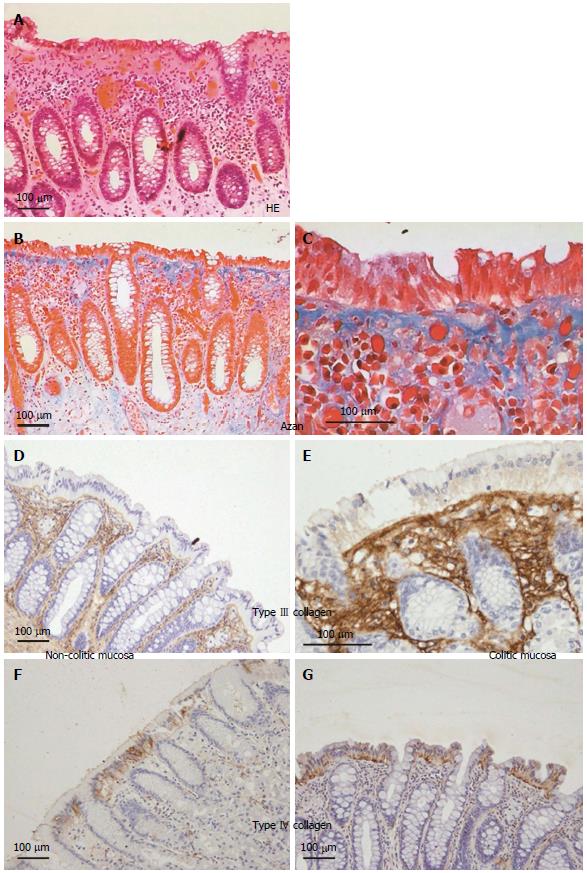
We confirmed the increase of collagen type III and IV in human colonic mucosa of patients with collagenous colitis (Figure 4). HE and Azan staining showed thickening of the basement membrane at the covering epithelium (Figure 4A-C). Immunohistochemistry of collagen type III showed that collagen fibers were increased at the basement membrane and mucosal stroma when compared with non-pathologic mucosa (Figure 4D and E). In contrast, immunohistochemistry of collagen type IV showed that collagen fibers were increased at the basement membrane alone when compared with non-pathologic mucosa.
Finally, expression of collagen type III and IV was compared in 11 collagenous colitis patients (Tables 2 and 3). The levels of collagen type III were increased in PPI-associated collagenous colitis whereas levels of collagen type IV were not different between PPI-associated and non-PPI-associated collagenous colitis.
| Case no. | Age | Sex | PPI | Expression | |
| Collagen III | Collagen IV | ||||
| 1 | 79 | F | + | ++ | ++ |
| 2 | 71 | F | + | +++ | ++ |
| 3 | 87 | F | + | ++ | ++ |
| 4 | 26 | F | + | +++ | ++ |
| 5 | 80 | M | + | +++ | + |
| 6 | 78 | M | + | +++ | ++ |
| 7 | 80 | F | - | ++ | + |
| 8 | 45 | M | - | + | ++ |
| 9 | 67 | M | - | ++ | + |
| 10 | 59 | F | - | + | ++ |
| 11 | 77 | F | - | ++ | ++ |
| PPI (+) | PPI (-) | P value | |
| n | 6 | 5 | |
| Age | 70.2 | 65.6 | NS |
| M:F | 2:4 | 2:3 | NS |
| Collagen | |||
| Type III | 2.67 ± 0.52 | 1.6 ± 0.55 | 0.0089 |
| Type IV | 1.83 ± 0.41 | 1.6 ± 0.55 | NS |
Collagenous colitis is an increasingly common microscopic colitis[1]. Recently proton pump inhibitors, especially lansoprazole, are recognized to be associated with onset of collagenous colitis[5]. Thus, the mechanism of this association is a focus of studies on collagenous colitis. In the present study, we examined the direct effect of PPIs on colonocytes, as well as the effect of increased pH of colonic contents.
We confirmed that the expression of the SLC9A2 Na+-H+ exchanger is a target of PPIs in the colonocytes. PPIs did not affect CT26 cell growth but alkaline conditions did suppress cell growth. Growth suppression of colonocytes might induce retardation of wound healing causing subsequent persistent inflammation, immune disturbances, and collagen overproduction.
PPI and alkaline condition caused increase of lipid peroxide with decrease of mitochondrial volume. Inhibition of proton efflux might induce acidic alteration of the cytoplasm, which might affect oxidative phosphorylation in mitochondria. Monitoring of mitochondrial function, ATP production and mitochondrial voltage are needed to conclude the effect of PPI on oxidative stress.
PPI and alkaline condition decreased the expression of RFC1, which is a negative regulator of collagen gene expression[16]. We then examined the alteration of expression of collagen type I, III, IV and VI. Only collagen type III and IV showed increase of mRNA levels in cells treated with PPI or alkaline conditions. In colon biopsy specimens of collagenous colitis patients, the amounts of type III and type IV collagens were increased. Type III collagen was increased in the basement membrane and mucosal stroma, whereas type IV collagen was increased only in the basement membrane. Type IV collagen is a major component of basement membrane collagens[17]. The expression level of type III collagen in PPI-associated patients was higher than in non-PPI-associated patients. These discrepancies between our data and Aigner's report might be due to differences in the pathogenesis of collagen disease or due to the influence of PPIs.
We examined pantoprazole in this study. To determine the significance of lanzoprazole in occurrence of collagenous colitis, we examine several kinds of PPIs in future. NSAIDs are also associated with collagenous colitis in cases. The mechanism is also examined to compare with that of PPIs.
In our data, PPI and alkaline condition increased the expression of TGF-β and FGF2, which are well known fibrosis-inducing factors[15]. We focused on the direct effect of PPI in colonocytes; however, stromal cells, such as fibroblasts are also involved in collagen production in collagenous colitis[5]. Our results suggest that PPIs induce fibroblast-mediated collagen production via stimulation of colonocyte secreting TGF-β and FGF2.
In the literature, alteration of gut flora is relevant for development of collagen colitis; clostridium, in particular, is associated with the disease[18-20]. Reduction of acidity in colonic contents by PPIs activates clostridium virulence[10]. The flora alteration might induce collagen production in the colonic mucosa. Anaerobic bacteria, including clostridium, induce TGF-β expression in colonocytes, which enhances collagen deposition in the colonic wall[21]. The TGF-β induction is caused by activation of the mucosal immunity[22]. Interleukin-13 increase in the inflammatory mucosa is reported to activate TGF-β expression[23]. Alkaline conditions might also affect gut flora, whose imbalance is regarded as important in causing collagenous colitis.
These findings suggest that collagen production and expression of collagen producing factors are directly stimulated by PPIs and PPI-induced alkaline change of colonic content. Colonocyte response to these factors might contribute to the pathogenesis of collagenous colitis.
The authors thank Ms. Tomomi Masutani for expert assistance with the preparation of this manuscript.
Collagenous colitis is a clinical problem recognized recently in an association with administration with proton pomp inhibitor (PPI). Since the pathogenesis is still controversy, reveal of the disease mechanism might be expected to develop a new therapy.
Many recent studies show the immunological and bacteriological effect of PPI. Study on the direct effect of PPI on colonocytes is a novel approach.
PPI and the PPI-resulted alkaline condition altered mitochondrial energy metabolism and collagen expression in colonocytes.
PPI-induced oxidative stress is not only a possible cause of collagen production in colon epithelium but also an important factor affecting tumor growth or function of mitochondria-rich tissues, such as myocardium, hepatocytes.
Interesting paper on possible mechanism for lanzoprazole associated collagenous colitis. It would be very interesting to evaluate other PPIs, such as omeprazole (a much rarer association with collagenous colitis), as well as with other agents (e.g., non-steroidal antiinflammatory drugs) to determine if this mechanism is shared with other agents.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Freeman HJ, Lakatos PL, Koulaouzidis A S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Ingle SB, Adgaonkar BD, Ingle CR. Microscopic colitis: Common cause of unexplained nonbloody diarrhea. World J Gastrointest Pathophysiol. 2014;5:48-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Bohr J, Wickbom A, Hegedus A, Nyhlin N, Hultgren Hörnquist E, Tysk C. Diagnosis and management of microscopic colitis: current perspectives. Clin Exp Gastroenterol. 2014;7:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Freeman HJ, Weinstein WM, Shnitka TK, Wensel RH, Sartor VE. Watery diarrhea syndrome associated with a lesion of the colonic basement membrane--lamina propria interface. Ann Royal Coll Phys Surg Canada. 1976;9:45. |
| 4. | Lazenby AJ. Collagenous and lymphocytic colitis. Semin Diagn Pathol. 2005;22:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Ianiro G, Cammarota G, Valerio L, Annicchiarico BE, Milani A, Siciliano M, Gasbarrini A. Microscopic colitis. World J Gastroenterol. 2012;18:6206-6215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Chande N, McDonald JW, Macdonald JK. Interventions for treating collagenous colitis. Cochrane Database Syst Rev. 2008;CD003575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Freeman HJ, Berean KW, Nimmo M. Evolution of collagenous colitis into severe and extensive ulcerative colitis. Can J Gastroenterol. 2007;21:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Giardiello FM, Jackson FW, Lazenby AJ. Metachronous occurrence of collagenous colitis and ulcerative colitis. Gut. 1991;32:447-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mizushima T, Mizoshita T, Sasaki M, Tanida S, Tsukamoto H, Shimura T, Kanematsu T, Kataoka H, Kamiya T, Tsukamoto T. Lansoprazole induces collagenous colitis in the colon of Mongolian gerbils. Asian Pac J Cancer Prev. 2011;12:2759-2762. [PubMed] |
| 10. | Hung YP, Ko WC, Chou PH, Chen YH, Lin HJ, Liu YH, Tsai HW, Lee JC, Tsai PJ. Proton-Pump Inhibitor Exposure Aggravates Clostridium difficile-Associated Colitis: Evidence From a Mouse Model. J Infect Dis. 2015;212:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H. Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol. 2005;166:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Kuniyasu H, Yasui W, Shinohara H, Yano S, Ellis LM, Wilson MR, Bucana CD, Rikita T, Tahara E, Fidler IJ. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157:1523-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Lai H, Lin K, Zhang W, Zhang Z, Jie L, Wu Y, He Q. Development of pH- and enzyme-controlled, colon-targeted, pulsed delivery system of a poorly water-soluble drug: preparation and in vitro evaluation. Drug Dev Ind Pharm. 2010;36:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Komatsu T, Kanatsu K, Kuze K, Iehara N, Takeoka H, Yamada Y, Kita T, Doi T. Demonstration of DNA replication factor C in human glomerular lesions. Clin Nephrol. 1998;49:69-73. [PubMed] |
| 17. | Randles MJ, Humphries MJ, Lennon R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Byrne MF, McVey G, Royston D, Patchett SE. Association of Clostridium difficile infection with collagenous colitis. J Clin Gastroenterol. 2003;36:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Khan MA, Brunt EM, Longo WE, Presti ME. Persistent Clostridium difficile colitis: a possible etiology for the development of collagenous colitis. Dig Dis Sci. 2000;45:998-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Gustafsson RJ, Ohlsson B, Benoni C, Jeppsson B, Olsson C. Mucosa-associated bacteria in two middle-aged women diagnosed with collagenous colitis. World J Gastroenterol. 2012;18:1628-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Mourelle M, Salas A, Guarner F, Crespo E, García-Lafuente A, Malagelada JR. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998;114:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Günaltay S, Kumawat AK, Nyhlin N, Bohr J, Tysk C, Hultgren O, Hultgren Hörnquist E. Enhanced levels of chemokines and their receptors in the colon of microscopic colitis patients indicate mixed immune cell recruitment. Mediators Inflamm. 2015;2015:132458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Fichtner-Feigl S, Strober W, Geissler EK, Schlitt HJ. Cytokines mediating the induction of chronic colitis and colitis-associated fibrosis. Mucosal Immunol. 2008;1 Suppl 1:S24-S27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |