Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1576
Peer-review started: December 9, 2016
First decision: January 10, 2017
Revised: January 24, 2017
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 7, 2017
Processing time: 87 Days and 8.2 Hours
To investigate the changes of hemodynamic and laboratory parameters during the course of acute liver failure following acetaminophen overdose.
Eight pigs underwent a midline laparotomy following jejunal catheter placement for further acetaminophen intoxication and positioning of a portal vein Doppler flow-probe. Acute liver failure was realized by intrajejunal acetaminophen administration in six animals, two animals were sham operated. All animals were invasively monitored and received standardized intensive care support throughout the study. Portal blood flow, hemodynamic and ventilation parameters were continuously recorded. Laboratory parameters were analysed every eight hours. Liver biopsies were sampled every 24 h following intoxication and upon autopsy.
Acute liver failure (ALF) occurred after 28 ± 5 h resulted in multiple organ failure and death despite maximal support after further 21 ± 1 h (study end). Portal blood flow (baseline 1100 ± 156 mL/min) increased to a maximum flow of 1873 ± 175 mL/min at manifestation of ALF, which was significantly elevated (P < 0.01). Immediately after peaking, portal flow declined rapidly to 283 ± 135 mL/min at study end. Thrombocyte values (baseline 307 × 103/µL ± 34 × 103/µL) of intoxicated animals declined slowly to values of 145 × 103/µL ± 46 × 103/µL when liver failure occurred. Subsequent appearance of severe thrombocytopenia in liver failure resulted in values of 11 × 103/µL ± 3 × 103/µL preceding fatality within few hours which was significant (P > 0.01).
Declining portal blood flow and subsequent severe thrombocytopenia after acetaminophen intoxication precede fatality in a porcine acute liver failure model.
Core tip: It still remains difficult to predict the outcome in patients with acute liver failure (ALF). Therefore we aimed to investigate the clinical course of portal blood flow (PBF) and changes in thrombocyte count in a porcine model of acetaminophen induced ALF. At manifestation of ALF, PBF increased maximally, followed by a rapidly decline until death due to multiple organ failure. In addition, thrombocytes values declined slowly at the onset of ALF. In the early ALF course, a second decline appeared 8 h after ALF escalating to a more severe thrombocytopenia after 16 h in ALF preceding fatality within few hours.
- Citation: Thiel K, Klingert W, Klingert K, Morgalla MH, Schuhmann MU, Leckie P, Sharifi Y, Davies NA, Jalan R, Peter A, Grasshoff C, Königsrainer A, Schenk M, Thiel C. Porcine model characterizing various parameters assessing the outcome after acetaminophen intoxication induced acute liver failure. World J Gastroenterol 2017; 23(9): 1576-1585
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1576.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1576
Acetaminophen (Paracetamol®) can cause severe hepatic injury if taken in large amounts either unintentionally or with suicidal intent[1]. Currently it represents the most common cause of acute liver failure (ALF) in both United States and United Kingdom with a trend to increasing incidence[2]. Various prognostic models have been established to predict the outcome of patients suffering from ALF[3-9]. The most widely accepted criteria are those defined by King's College Hospital[10], which include predominantly biochemical parameters including arterial pH, prothrombin time and serum creatinine to identify patients in need of liver transplantation. Unfortunately, a variety of biochemical parameters in ALF are deranged representing indices of altered biochemical pathways or epiphenomena of liver necrosis. Furthermore the King's criteria was questioned by Bailey et al[11] in a systematic review and meta-analysis of established prognostic scores. The authors concluded that all criteria presently available are insensitive and may miss patients requiring emergency liver transplantation. The crucial question as to whether the liver regenerates spontaneously, or that liver transplantation will be unavoidable remains the most important challenge for the attending physician. To address this problem, animal models analysing the pathophysiologic alterations in the course of ALF provide an opportunity to improve therapeutic strategies and investigate new prognostic parameters. The requirements for parameters of interest are availability, feasibility and robust assurance in diagnosis. Although acetaminophen toxicity has been extensively studied in rat[12,13] and mouse models[14,15], the precise pathogenic mechanisms of hepatocyte damage which are potentially reversible are still poorly understood. Recently, large models of acetaminophen intoxication have been established in porcine models[16,17] which are more appropriate to represent the human liver physiology and the clinical course of acetaminophen poisoning. These models are also of sufficient size to allow use of a representative human intensive care setting, which permits testing of novel therapeutic interventions.
Based on observations of a preliminary animal study of acetaminophen intoxication in which a distinctive pattern of portal blood flow (PBF) and alterations in thrombocyte count were measured[17], the following animal study was aimed to characterize the clinical course of PBF and changes in thrombocyte count during the development, onset and further course of acetaminophen induced ALF.
After approval by the institutional review board for animal experiments, all experiments were performed according to the international principles governing research on animals and under the supervision of a veterinarian, who set the guidelines for minimizing suffering.
The study was performed in eight female German landrace pigs weighing 38 ± 2 kg. Premedication, anaesthesia, intensive care medication and algorithms for standardized intensive care management have been previously reported in detail[18].
In brief, intramuscular premedication consisted of atropine 0.1% (0.05 mg/kg), ketamine (14 mg/kg), azaperone (2 mg/kg) and midazolam (0.5 mg/kg). Pigs were orally intubated and ventilated with a pressure controlled ventilation modus. Continuous intravenous anesthesia consisted of ketamine (15 mg/kg per hour), fentanyl (0.02 mg/kg per hour) and midazolam (0.9 mg/kg per hour).
The jugular and femoral veins as well as the femoral artery were instrumented to measure central venous pressure (CVP) and mean arterial pressure (MAP). A probe was inserted in the frontal brain parenchyma to record intracranial pressure. Following a median laparotomy the portal vein was separated and a 10-15 mm peri-vascular Doppler flow-probe (Medi-Stim, Oslo, Norway) was placed without restricting the portal vein diameter, and fixed to periportal tissue. PBF data were recorded electronically by a CM-2000 Doppler Flowmeter (Medi-Stim, Oslo, Norway). A jejunal catheter (Gentle-FloTM, Tyco Healthcare, Tullamore, Ireland) was inserted into the upper jejunum for further acetaminophen administration and a 14F urinary catheter (Ruesch Care, Kernen, Germany) was placed by cystostomy. The abdominal wall was closed with a running suture. Liver biopsies were sampled every 24 h following intoxication and upon autopsy. Clear ascites (500 to 1500 mL) was removed in the intoxication group during liver biopsy procedures which were performed surgically by reopening the abdomen.
Two pigs (2/8) were sham operated as a control group and received no acetaminophen intoxication. Dosage, administration and acetaminophen plasma level monitoring for a reproducible onset of acute liver failure after intrajejunal acetaminophen intoxication has been previously described in detail[17]. In brief, six pigs (6/8) received an initial enteric acetaminophen bolus of 250 mg/kg body weight via the implanted jejunal catheter. Intoxication was continued initially by an enteric maintenance dose of 2000 mg acetaminophen every hour. Acetaminophen plasma levels were recorded every four hours to adapt acetaminophen maintenance dose (1000-3000 mg) to targeted plasma levels between 300-450 mg/L.
The onset of the ALF syndrome was defined by presence of coagulopathy represented by a decline of the prothrombin time (PT) value below 30% at which point acetaminophen intoxication was stopped.
All pigs (8/8) remained under anaesthesia receiving pressure-controlled ventilation until conclusion of the study. Intensive care medication and algorithms for standardized fluid management which were used to ensure hemodynamic stability have been previously reported in detail[17,18]. Relevant vital parameters as electrocardiogram, heart rate, MAP, CVP, intracranial pressure and body temperature were recorded electronically throughout the experiment (IntelliVue MP50, Philips Medical Systems, Andover MA, United States). Arterial blood gas analysis (ABL 800, Radiometer, Copenhagen, Denmark) including haemoglobin, methaemoglobin, hematocrit, lactate, serum electrolytes, acid base balance and blood glucose levels were monitored hourly and ventilation parameters [12-30 breaths/min, tidal volume 6-12 mL/kg and oxygen concentration (FiO2) 0.3 - 1.0] were adjusted accordingly. Complete blood count, PT, aspartate aminotransferase, creatinine, albumin, bilirubin, ammonia and total plasma protein were measured before, immediately after and every eight hours following acetaminophen intoxication until study end. Norepinephrine, in combination with hydroxyethylstarch 6% (Voluven® HES 130/0.4, Fresenius, Bad Homburg, Germany) and sodium chloride solution 0.9% were used to ensure hemodynamic stability. After the onset of ALF, four fresh-frozen plasma units (300 mL/unit) were given within 24 h to avoid spontaneous bleeding complications which were observed in pilot studies. Packed erythrocyte units (300 mL/unit) were given if haemoglobin levels decreased below 6 g/dL. Blood glucose levels were maintained > 100 mg/dL with glucose 20% solution. Sodium bicarbonate 8.4% solution was used to compensate metabolic acidosis. Death was defined by a decline of MAP below 35 mmHg whilst receiving maximal vasopressor support. Sham animals were killed by a single intravenous bolus of 10 mL T 61 (Intervet, Unterscheißheim, Germany) at 48 post surgery.
At ALF, continuous venovenous hemofiltration was administered to all animals of the intoxication group (6/6). Continuous hemofiltration therapy was administered in both sham animals from 24 h (Prismaflex® system. Gambro, Hechingen, Germany) using a TF 1000 PRE SET membrane filter (Gambro Industries, Meyzieu, France). The device system was washed and primed according to the manufacturer's instruction and connected to the right femoral double-lumen catheter. Hemofiltration settings were: mean blood flow rate of 100 mL/min; filtration rate of 35 mL/kg body weight per hour; with fluid withdrawal at 60 mL/h. Unfractionated heparin (250 IU/h, B. Braun Melsungen AG, Germany) was administered to avoid clotting.
All biochemical parameters including acetaminophen plasma level were measured by the certified central laboratories of the Tuebingen University Hospital (Division of Endocrinology, Diabetology, Angiology, Nephrology, Pathobiochemistry and Clinical Chemistry, Department of Internal Medicine, Tuebingen University Hospital, Germany). Arterial albumin, lactate and creatinine (enzymatic) concentrations were determined on the ADVIA 1800 Clinical Chemistry analyzer, ammonia and acetaminophen plasma concentrations were determined on the Dimension RXL Clinical Chemistry analyzer and the ADVIA 2120 Hematology analyzer was used for blood counts (all Siemens Healthcare Diagnostics, Eschborn, Germany). Coagulation tests were performed on the ACL TOP 700 Hemostasis Testing System, (Instrumentation Laboratory, Kirchheim, Germany). Sample analysis was conducted within 1 h of collection at each time point.
Biopsy specimens of the liver were obtained every 24 h after intoxication and immediately post-mortem, and fixed in used 4% formaldehyde. Sections of the specimen were routinely stained with haematoxylin-eosin. Additionally Ki-67 immunostaining by using alkaline phosphatase staining was performed.
Mean values were compared by Wilcoxon test (JMP® 8.0, SAS Institute, Cary, NC, United States). Values of the laboratory parameters were compared to baseline values by the Wilcoxon matched pairs test. A P value < 0.05 was considered significant. Results are reported as mean ± SE. The statistical methods of this study were reviewed by Martin Schenk, University Hospital Tuebingen.
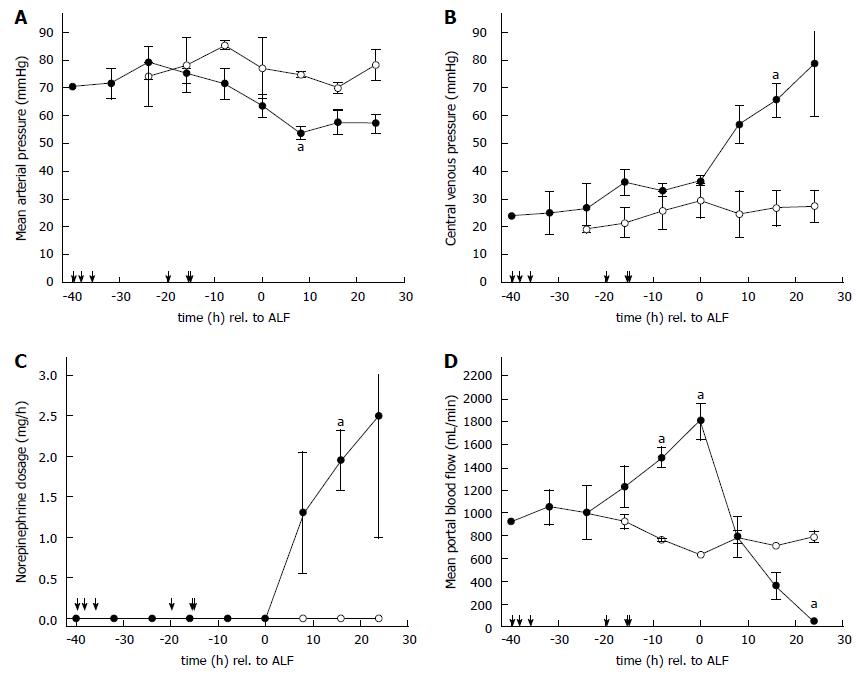
All intoxicated animals (n = 6) developed features of ALF within 28 ± 5 h, confirmed by a decrease in PT below 30%. Mortality due to ALF occurred after 21 ± 1 h due to multiple-organ failure (end of the study). The course of the hemodynamic parameters MAP, CVP, the amount of vasopressor support (norepinephrine dosage) required to maintain organ perfusion, is given in Figure 1A-C. Both sham operated animals (n = 2) survived the observation period of 48 h without substantial changes of hemodynamic and laboratory parameters. Significant hemodynamic changes in MAP occurred 8 h after the onset of ALF (p < 0.05; Figure 1A), CVP 16 h after ALF (p < 0.02; Figure 1B) and norepinephrine dosage 16 h after ALF (p < 0.04; Figure 1C). Slightly elevated intracranial pressure baseline values of 19 ± 1 mmHg (n = 8) resulted from the unphysiological supine position of the animals. An elevation due to ALF was noticed after 16 h (19 ± 2 mmHg in sham animals vs 30 ± 2 mmHg in intoxicated animals, P < 0.03) and 24 h (17 ± 1 mmHg vs 35 ± 5 mmHg) post ALF.
Portal vein flow remained hepatopetal in all animals throughout the experiment. The course of PBF during acetaminophen intoxication, manifestation of ALF and following multiple-organ failure is shown in Figure 1D. The sham operated animals started with a PBF at baseline of 1016 ± 16 mL/min. The PBF remained stable with an overall flow of 825 ± 91 mL/min throughout. At start of acetaminophen administration in the intoxication group (n = 6) baseline PBF was 1100 ± 156 mL/min. Three animals in the acetaminophen group required between 36-40 h to develop ALF, while the remainder achieved ALF within 16-20 h. Within the animals that required a prolonged acetaminophen loading phase the PBF increased only slightly for the initial period but showed a substantial rise 8 h before manifestation of ALF analogous to the course of the more susceptible animals. Maximum portal blood flow was 1873 ± 175 mL/min (n = 6) at the onset of ALF. The elevation in PBF 8 h before and at the onset of ALF was found to be significant compared to the sham animals at 24 h (p < 0.01). Immediately after ALF, PBF started to decline rapidly to a minimal value of 283 ± 135 mL/min (n = 6) at study end.
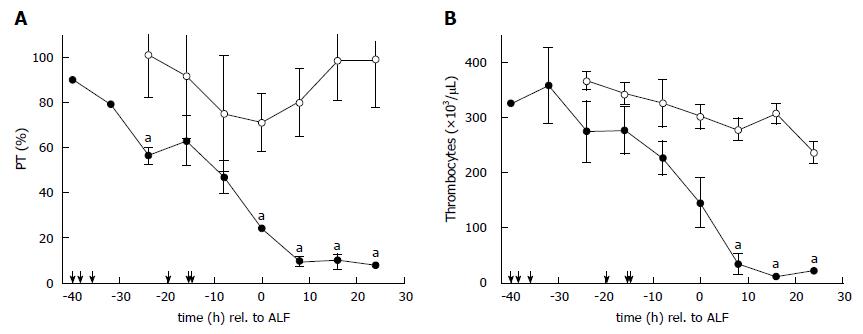
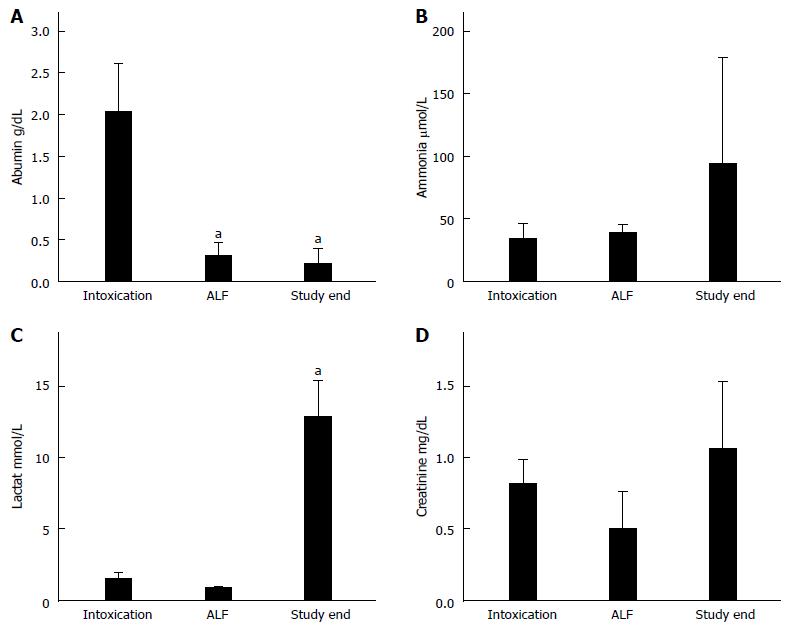
The course of PT is shown in Figure 2A. PT in all ALF animals declined constantly following acetaminophen administration. There was a transient decrease in sham animals due to the surgical trauma and volume of fluid resuscitation which later normalised. Baseline thrombocyte values for ALF animals were measured at 307 × 103/μL ± 34 × 103/μL; and 367 × 103/μL ± 18 × 103/μL in sham animals. Thrombocyte values for ALF animals declined following acetaminophen to 145 × 103/μL ± 46 × 103/μL at the onset of ALF. and were further reduced after 8 h post ALF (33 × 103/μL ± 18 × 103/μL, P < 0.01) resulting in severe thrombocytopenia with values of 11 ± 3 × 103/μL (p < 0.01) after 16 h in ALF preceding death within few hours (Figure 2B). Values of the laboratory parameters are shown in Figure 3. The decline of albumin (baseline 2.0 ± 0.2 g/dL vs ALF 0.2 ± 0.1 g/dL vs study end 0.2 ± 0.1 g/dL) was statistically significant (p < 0.05) at both time-points (Figure 3A). Plasma ammonia levels (baseline 34 ± 5 μmol/L and ALF 39 ± 3 μmol/L) increased to 95 ± 35 μmol/L at study end, though the difference was not found to be significant (Figure 3B). The increase of lactate (baseline 1.5 ± 0.5 mmol/L vs ALF 0.9 ± 0.1 mmol/L vs study end 12.9 ± 2.6 mmol/L) was significant (p < 0.05) at death compared with baseline values (Figure 3C). Creatinine values (baseline 0.8 ± 0.1 mg/dL vs ALF 0.5 ± 0.1 mg/dL vs study end 1.1 ± 0.2 mg/dL) remained near normal physiological values due to effective continuous venovenous hemofiltration therapy during the course of ALF (Figure 3D).
The increase of plasma aspartate transaminase (baseline 50 ± 9 U/L vs ALF 105 ± 28 U/L vs study end 237 ± 109 U/L) was significant raised (p < 0.02) at death compared with baseline values. Alanine aminotransferase plasma values did not change substantially during the course of ALF (baseline 33 ± 3 U/L vs ALF 23 ± 10 U/L vs study end 26 ± 7 U/L) and total bilirubin plasma levels remained in the physiological range (baseline 0.1 ± 0.0 mg/dl vs ALF 0.1 ± 0.0 mg/dl vs study end 0.3 ± 0.1 mg/dl).
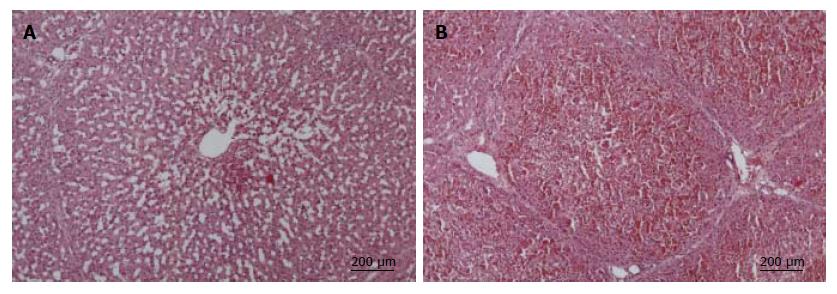
Upon autopsy, massive ascites (2000 to 3000 mL) and a dark-blue necrotic liver was found in all ALF animals. Liver biopsies taken at 24 h showed enlarged liver sinus with early centrilobular necrosis (Figure 4A). Exitus biopsy demonstrated progedient centrilobular necrosis (Figure 4B) in contrast to normal liver architecture in sham operated animals (data not shown). No evidence for increased hepatocyte proliferation (Ki-67 immunostaining) was found in any animals (data not shown). Macroscopically, kidneys were swollen with hemorrhagic infarctions. Histological examination showed acute tubular necrosis and biopsies of brain tissue revealed substantial edema (data not shown).
In this present study we sort to characterise the course of PBF and thrombocyte count in order to analyse their prognostic value for predicting a fatal outcome in a porcine model of acetaminophen intoxication. The objective of these studies is to identify the moment at which recovery will be unlikely and emergency liver transplantation remains the ultimate treatment option. The distinctive pattern of PBF and thrombocyte count in combination with worsening hemodynamics were identified as relevant parameters.
The high reproducibility of ALF resulting in 100% mortality due to multiple-organ failure confirmed the results of the our previously published porcine model of acetaminophen intoxication[17]. Within the current experimental setting, continuous venovenous hemofiltration was administrated in addition to standardized intensive care therapy in order to maximally prolong survival in ALF for patients after massive acetaminophen overdose[19]. The use of a continuous renal support device itself, applied to both sham operated animals after 24 h analogous to the onset of ALF in intoxicated animals did not influence the change of mean PBF nor the fall in thrombocytes substantially.
Unexpectedly 3/6 animals needed a longer acetaminophen loading period (36-40 h) for the development of ALF in contrast to other animals (16-20 h). Both subgroups showed different kinetics in PBF changes. The slowly intoxicating animals presented only a slight increase in PBF during the initial intoxication phase followed by a rapid increase within the last 8 h before manifestation of ALF. This phenomenon is likely associated with the animal's individual tolerance towards toxic acetaminophen metabolites. The observation that the rapid increase of PBF within 8 h before ALF in both subgroups suggests that the rapid change in PBF, even before the onset of ALF, is indicative of the extent of toxicity for the animal.
As PBF represents the main component of the liver perfusion and portal vein flow measurement is easily accessible for the attending physician in the clinical setting, we decided to focus on the changes determined in PBF. We abstained from quantifying hepatic artery flow which would increase surgical trauma and which varies considerably following the application of norepinephrine administration in ALF. Although assessment of volume blood flow by Doppler ultrasound is subject to a number of variables, errors can be minimized with careful attention to detail[20]. To evaluate a complete pattern of PBF, continuous recording was obtained by a surgically implanted Doppler flow-probe placed around the portal vein. Portal vein hemodynamics have recently been investigated in liver transplantation[21], in small for size syndrome after extended hepatectomy[22], and in living donor liver transplantation[23] in both clinical and experimental animal studies. The portal vein inflow, within its physiological range, is a stimulus for hepatic regeneration[24,25], but excessive PBF has been shown to be detrimental to the function of the affected liver[26,27]. Several studies investigating the changes of PBF in ALF have been performed in patients by serial Doppler ultrasound examinations[28] or in rats[29] by using a radioactive microsphere technique. They demonstrated a significant increase of PBF in the development and early onset of ALF followed by a steady return to baseline values during liver regeneration. The increase of PBF was clearly identified as a consequence of elevated cardiac output similar to the hemodynamic changes seen in hyperdynamic septic shock. Although the MAP during ALF could be stabilized by intensive care support, a constant decline of PBF was observed in the post ALF period. Norepinephrine which was required for hemodynamic stabilisation in ALF in our experimental setting does not influence the mesenteric venous blood flow as previously demonstrated in animal models[30,31]. This phenomenon could be related to refractivity to volume replacement and vasopressor support[32,33]. The ongoing impairment of liver perfusion additionally reduced the oxygen supply essential for survival of hepatocytes and liver regeneration.
As the current model resulted in 100% mortality, it makes the identification of any regenerative process difficult to ascertain. This could mean that the decline in PBF observed during the ALF course could be misinterpreted as a start of systemic and hepatic restoration. However, firstly the hemodynamic situation of recovering animals would certainly be stabilized and secondly the decrease of PBF, when liver restoration occurred, has already been described as a comparatively slow process in contrast to the rapid decline in the end stage of ALF[28].
It has recently been demonstrated experimentally[27,34] and clinically[35,36] that thrombocytes are able to promote liver regeneration after extended hepatectomy or liver transplantation. Their predictive value in the context of ALF remains unclear. Thrombocyte activation and increased fibrinolysis, highly suggestive of disseminated intravascular coagulation, occurs in ALF and may be related to endotoxemia or the release of thromboplastic material from the damaged liver.
The diagnosis remains difficult to substantiate as plasma concentrations of both fibrinogen and fibrin degradation products may be altered by ALF induced impairment of all serum coagulation parameters. Thrombocytopenia is a common finding in ALF from any cause[37]. Secondary to acetaminophen overdose it has been reported in a number of patients[38]. Clinical cases of acetaminophen intoxication with a plasma level of 250 mg/L ten hours after an intentional overdose of 50 g Paracetamol® tablets resulted in severe thrombocytopenia without accompanying anaemia or leucopenia approximately 48 h after ingestion[39]. A retrospective analysis of 174 patients by Fischereder and Jaffe[40] demonstrated that thrombocytopenia occurring early in the course of acetaminophen overdose was not uncommon and may identify a subset of patients with a high risk of hepatotoxicity. Our findings of a secondary sudden decline of thrombocytes following elevated acetaminophen plasma level (300-450 mg/L) support this hypothesis.
We conclude that the distinctive pattern of PBF during the intoxication process and subsequent ALF represents a reliable parameter for acetaminophen toxicity. Declining PBF in combination with ongoing worsening of hemodynamics and organ function precedes a fatal outcome after acetaminophen intoxication. An additional secondary sudden appearance of severe thrombocytopenia in ALF was found to reliably precede fatality within few hours.
The authors thank A Poven, E. Hawerkamp, J. Scheppach, J. Lauber and A. Diewold for participating in intensive care therapy of the animals. Furthermore we wish to acknowledge M. Seitzer, A. Stolz, C. Fahrner and T. O. Greiner for their excellent veterinarian and technical assistance. The authors owe great thanks to Andrea and Mark Jackson for their kind contribution to the preparation of the manuscript.
Several prognostic models have been invented to predict the outcome of patients suffering from acute liver failure (ALF). The crucial question is whether the liver regenerates spontaneously or if liver transplantation will be unavoidable. Large models of acetaminophen intoxication have been established in pigs recently.
Based on observations of a preliminary animal study of acetaminophen intoxication in which a distinctive pattern of portal blood flow (PBF) and alterations in thrombocyte count were measure, the following animal study was aimed to characterize the clinical course of PBF and changes in thrombocyte count during the development, onset and further course of acetaminophen induced ALF.
This study verifies the expected distinctive pattern of PBF during the course of acetaminophen induced ALF: a considerable increase of PBF at manifestation of ALF followed by a rapid decline of PBF until end of the study. A parallel slow decline of thrombocyte values at manifestation of ALF was observed. Subsequent severe thrombocytopenia resulted in death due to multiple organ failure within few hours.
Declining portal blood flow during the course of ALF precedes fatality. Additional sudden appearance of severe thrombocytopenia leads to death due to multiple organ failure within few hours.
This paper highlights very important issue on acetaminophen intoxication. This is very current problem for the humans. The investigations were performed on a porcine model, not mice or other small vertebrates, which make them very valuable.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Futoma-Koloch B, Kucera O S- Editor: Ma JY L- Editor: A E- Editor: Liu WX
| 1. | Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med. 2010;77:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Dabos KJ, Newsome PN, Parkinson JA, Davidson JS, Sadler IH, Plevris JN, Hayes PC. A biochemical prognostic model of outcome in paracetamol-induced acute liver injury. Transplantation. 2005;80:1712-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Mitchell I, Bihari D, Chang R, Wendon J, Williams R. Earlier identification of patients at risk from acetaminophen-induced acute liver failure. Crit Care Med. 1998;26:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transpl. 2003;9:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Pereira LM, Langley PG, Hayllar KM, Tredger JM, Williams R. Coagulation factor V and VIII/V ratio as predictors of outcome in paracetamol induced fulminant hepatic failure: relation to other prognostic indicators. Gut. 1992;33:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Schiødt FV, Bondesen S, Petersen I, Dalhoff K, Ott P, Tygstrup N. Admission levels of serum Gc-globulin: predictive value in fulminant hepatic failure. Hepatology. 1996;23:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kumar R, Shalimar H, Goyal R, Kumar A, Khanal S, Prakash S, Gupta SD, Panda SK, Acharya SK. Prospective derivation and validation of early dynamic model for predicting outcome in patients with acute liver failure. Gut. 2012;61:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1323] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 11. | Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Belardinelli MC, Pereira F, Baldo G, Vicente Tavares AM, Kieling CO, da Silveira TR, Meurer L, Soares Duarte ME, Giugliani R, Matte U. Adult derived mononuclear bone marrow cells improve survival in a model of acetaminophen-induced acute liver failure in rats. Toxicology. 2008;247:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Grypioti AD, Kostopanagiotou G, Mykoniatis M. Platelet-activating factor inactivator (rPAF-AH) enhances liver's recovery after paracetamol intoxication. Dig Dis Sci. 2007;52:2580-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Wang AY, Lian LH, Jiang YZ, Wu YL, Nan JX. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J Gastroenterol. 2010;16:384-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Yapar K, Kart A, Karapehlivan M, Atakisi O, Tunca R, Erginsoy S, Citil M. Hepatoprotective effect of L-carnitine against acute acetaminophen toxicity in mice. Exp Toxicol Pathol. 2007;59:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Newsome PN, Henderson NC, Nelson LJ, Dabos C, Filippi C, Bellamy C, Howie F, Clutton RE, King T, Lee A. Development of an invasively monitored porcine model of acetaminophen-induced acute liver failure. BMC Gastroenterol. 2010;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Thiel C, Thiel K, Etspueler A, Morgalla MH, Rubitschek S, Schmid S, Steurer W, Königsrainer A, Schenk M. A reproducible porcine model of acute liver failure induced by intrajejunal acetaminophen administration. Eur Surg Res. 2011;46:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Thiel C, Thiel K, Etspueler A, Schenk T, Morgalla MH, Koenigsrainer A, Schenk M. Standardized intensive care unit management in an anhepatic pig model: new standards for analyzing liver support systems. Crit Care. 2010;14:R138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wiegand TJ, Margaretten M, Olson KR. Massive acetaminophen ingestion with early metabolic acidosis and coma: treatment with IV NAC and continuous venovenous hemodiafiltration. Clin Toxicol (Phila). 2010;48:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Iwao T, Toyonaga A, Shigemori H, Oho K, Sumino M, Sato M, Tanikawa K. Echo-Doppler measurements of portal vein and superior mesenteric artery blood flow in humans: inter- and intra-observer short-term reproducibility. J Gastroenterol Hepatol. 1996;11:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Sainz-Barriga M, Scudeller L, Costa MG, de Hemptinne B, Troisi RI. Lack of a correlation between portal vein flow and pressure: toward a shared interpretation of hemodynamic stress governing inflow modulation in liver transplantation. Liver Transpl. 2011;17:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Chan SC, Lo CM, Ng KK, Ng IO, Yong BH, Fan ST. Portal inflow and pressure changes in right liver living donor liver transplantation including the middle hepatic vein. Liver Transpl. 2011;17:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Eguchi S, Yanaga K, Sugiyama N, Okudaira S, Furui J, Kanematsu T. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transpl. 2003;9:547-551. [PubMed] [DOI] [Full Text] |
| 25. | Maetani Y, Itoh K, Egawa H, Shibata T, Ametani F, Kubo T, Kiuchi T, Tanaka K, Konishi J. Factors influencing liver regeneration following living-donor liver transplantation of the right hepatic lobe. Transplantation. 2003;75:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Mortensen KE, Conley LN, Nygaard I, Sorenesen P, Mortensen E, Bendixen C, Revhaug A. Increased sinusoidal flow is not the primary stimulus to liver regeneration. Comp Hepatol. 2010;9:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Hisakura K, Murata S, Fukunaga K, Myronovych A, Tadano S, Kawasaki T, Kohno K, Ikeda O, Pak S, Ikeda N. Platelets prevent acute liver damage after extended hepatectomy in pigs. J Hepatobiliary Pancreat Sci. 2010;17:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Deasy NP, Wendon J, Meire HB, Sidhu PS. The value of serial Doppler ultrasound as a predictor of clinical outcome and the need for transplantation in fulminant and severe acute liver failure. Br J Radiol. 1999;72:134-143. [PubMed] [DOI] [Full Text] |
| 29. | Makin AJ, Hughes RD, Williams R. Systemic and hepatic hemodynamic changes in acute liver injury. Am J Physiol. 1997;272:G617-G625. [PubMed] |
| 30. | Woolsey CA, Coopersmith CM. Vasoactive drugs and the gut: is there anything new? Curr Opin Crit Care. 2006;12:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Di Giantomasso D, Bellomo R, May CN. The haemodynamic and metabolic effects of epinephrine in experimental hyperdynamic septic shock. Intensive Care Med. 2005;31:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bihari DJ, Gimson AE, Williams R. Cardiovascular, pulmonary and renal complications of fulminant hepatic failure. Semin Liver Dis. 1986;6:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Trewby PN, Williams R. Pathophysiology of hypotension in patients with fulminant hepatic failure. Gut. 1977;18:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Clavien PA, Graf R. Liver regeneration and platelets. Br J Surg. 2009;96:965-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Hillenbrand P, Parbhoo SP, Jedrychowski A, Sherlock S. Significance of intravascular coagulation and fibrinolysis in acute hepatic failure. Gut. 1974;15:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Gazzard BG, Clark R, Borirakchanyavat V, Williams R. A controlled trial of heparin therapy in the coagulation defect of paracetamol-induced hepatic necrosis. Gut. 1974;15:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Thornton JR, Losowsky MS. Severe thrombocytopenia after paracetamol overdose. Gut. 1990;31:1159-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Fischereder M, Jaffe JP. Thrombocytopenia following acute acetaminophen overdose. Am J Hematol. 1994;45:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |