Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1513
Peer-review started: November 27, 2016
First decision: December 19, 2016
Revised: January 6, 2017
Accepted: February 16, 2017
Article in press: February 17, 2017
Published online: March 7, 2017
Processing time: 101 Days and 17.6 Hours
The gastrointestinal barrier is constantly exposed to numerous environmental substrates that are foreign and potentially harmful. These xenobiotics can cause shifts in the intestinal microbiota composition, affect mucosal immune responses, disturb tissue integrity and impair regeneration. The multidrug transporter ABCB1/MDR1 p-glycoprotein (p-gp) plays a key role at the front line of host defence by efficiently protecting the gastrointestinal barrier from xenobiotic accumulation. This Editorial discusses how altered expression and function of ABCB1/MDR1 p-gp may contribute to the development and persistence of chronic intestinal inflammation in inflammatory bowel diseases (IBD). Recent evidence implies multiple interactions between intestinal microbiota, innate immunity and xenobiotic metabolism via p-gp. While decreased efflux activity may promote disease susceptibility and drug toxicity, increased efflux activity may confer resistance to therapeutic drugs in IBD. Mice deficient in MDR1A develop spontaneously chronic colitis, providing a highly valuable murine IBD model for the study of intestinal epithelial barrier function, immunoregulation, infectious co-triggers and novel therapeutic approaches. Possible associations of human ABCB1 gene polymorphisms with IBD susceptibility have been evaluated, but results are inconsistent. Future studies must focus on further elucidation of the pathophysiological relevance and immunological functions of p-gp and how its ambiguous effects could be therapeutically targeted in IBD.
Core tip: Altered levels of p-glycoprotein (p-gp) expression as well as genetic variants of ABCB1/MDR1 have been associated with inflammatory bowel diseases (IBD). Decreased efflux activity of p-gp may promote disease susceptibility, while increased efflux activity may impair drug responses in IBD. In this Editorial, I highlight what we need to know about this transporter and xenobiotic signaling pathways in order to better understand its potential pathophysiology in IBD and develop targeted therapies.
- Citation: Cario E. P-glycoprotein multidrug transporter in inflammatory bowel diseases: More questions than answers. World J Gastroenterol 2017; 23(9): 1513-1520
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1513
The gastrointestinal (GI) barrier is constantly exposed to numerous environmental substrates that are foreign and potentially harmful, so-called xenobiotics. Toxic compounds can cause shifts in the intestinal microbiota composition, affect host innate and adaptive immune responses, disturb tissue integrity and impair regeneration. Several dysfunctions in xenobiotic recognition and metabolism have previously been implicated in the pathogenesis of inflammatory bowel diseases (IBD)[1-3]. To maintain mucosal homeostasis and prevent immunotoxic effects of xenobiotics, the GI barrier is equipped with a variety of detoxification mechanisms, including efflux transporters.
This Editorial focuses on recent insights into the ABCB1/MDR1 (multi-drug resistance) - encoded p-glycoprotein (p-gp), which represents the most investigated ATP-dependent efflux transporter pump of xenobiotics (including metabolic products, toxins and drugs) in the intestine, and its impact on IBD pathophysiology. Growing evidence implies that altered expression and function of ABCB1/MDR1 p-gp may contribute to the development and persistence of chronic intestinal inflammation in IBD. While decreased efflux function may mediate disease susceptibility and trigger drug toxicity, increased efflux activity may confer resistance to drug therapy in IBD.
P-gp, cloned in 1985[4], was initially described as a control mechanism of drug permeation and release at the membrane surface of colchicine-resistant Chinese hamster ovary cells[5]. In humans, the drug transporter p-gp is encoded by the ABCB1/MDR1 gene (located on chromosome 7q21), while in rodents, p-gp is encoded by two genes, Abcb1a/Mdr1a and Abcb1b/Mdr1b. The N-terminal glycosylated protein consists of 1280 amino acids with a molecular mass of approximately 170 kDa. Murine p-gp shares 87% amino acid sequence identity with the human homologue[6], which makes knockout (KO) mouse models useful to study.
The secondary structure of ABCB1/MDR1 p-gp contains two symmetrical halves of an ATP-binding domain (also known as "nucleotide binding domain") in the cytoplasm and a transmembrane domain with six hydrophobic α-helices, which are separated by a highly charged "linker region"[7,8]. Its transport activity depends on energetic metabolism and ATP hydrolysis. Once a substrate gets captured within the internal cavity of p-gp, ATP binds to its domains which causes a large conformational change presenting the substrate und drug-binding site to the extracellular space[6]. Thus, p-gp efficiently detoxifies cells by exporting hundreds of chemically and pharmacologically unrelated substances, including many important IBD drugs, such as steroid hormones (glucocorticosteroids), immunosuppressive agents (cyclosporine, tacrolimus), antimetabolites (methotrexate) or antibiotics (levofloxacin), and metabolic products. In addition, p-gp may also be involved in the transmembrane transport of pro-inflammatory cytokines, such as interleukin (IL)-2 and interferon-gamma (IFN-γ)[9], however, it remains to be shown how cytokine release could be directly regulated by p-gp signaling.
The basal expression pattern of p-gp shows high inter- and also intraindividual variability along the GI tract, with a general increase from proximal to distal parts[10]. While ABCB1/MDR1 p-gp is constitutively expressed at the frontline of the mucosal barrier, i.e. at the apical pole of intestinal epithelial cells, it is also inducibly expressed by many other cell types (e.g. macrophages[11] and T cell subsets[12]) in the lamina propria.
P-gp expression and function can be modulated by numerous exogenous and endogenous factors - based on the activation state of the individual cell and influences of its surrounding environment. Innate and adaptive immune responses, oxidative or inflammatory stress, dietary antigens, gut microbiota and other environmental triggers may differentially influence host metabolic signaling and xenobiotic transport via p-gp in the intestinal mucosa. The human ABCB1/MDR1 promoter region contains multiple transcription factor-binding sequences, including specificity protein 1 (Sp-1), activator protein 1 (AP-1), nuclear factor interleukin-6 (NF-IL-6), forkhead transcription factor (FKHR) or T-cell factor/lymphoid enhancer factor (TCF/LEF), which points to complex regulation[13]. Upstream, the nuclear pregnane X receptor (PXR) may control convergence between xenobiotic detoxification and innate immunity by modulating transcription of p-gp as well as activation of (NACHT-, LRR- and PYD-containing Protein 3 (NLRP3)[14] and Toll-like receptor 4 (TLR4) signaling[15].
Downregulation of p-gp expression has been associated with acute intestinal inflammation, such as in the experimental mouse model of dextran sulphate sodium (DSS)-induced colitis[16] or in some patients with active ulcerative colitis (UC)[17]. Increased mucosal levels of tumor necrosis factor alpha (TNFα) in active IBD suppress gene transcription of ABCB1/MDR1 in intestinal epithelial cells, thus impairing xenobiotic efflux via p-gp[18]. Other major cytokines in IBD, such as IL-1ß or IL-6[19], may also interfere with p-gp expression and function. Interestingly, rifaximin, a non-absorbable antibiotic potentially beneficial for inducing remission in Crohn's disease (CD)[20], may antagonize TNFα-induced inhibition of p-gp via PXR[21].
Varying levels of p-gp in the intestinal mucosa may also be attributed to circadian rhythms caused by clock gene products which - at least in part - control ABCB1/MDR1 gene expression[22]. Circadian expression of p-gp in the intestine may functionally affect the pharmacokinetics of its substrates, leading to temporal changes in intestinal absorption and excretion[23]. Of note, changes in the expression of several circadian genes have also been observed in active IBD[24]. Future research is needed to clarify the potential role of IBD-related circadian alterations in disturbing xenobiotic metabolism via p-gp.
Mice deficient in MDR1A, first described by Dr. Alfred Schinkel in 1994, have initially been shown to be highly sensitive to the pesticide ivermectin and the chemotherapy drug vinblastine due to a blood-brain barrier defect[25]. Few years later, Dr. Jo Viney's group observed that MDR1A KO mice develop spontaneously chronic colitis that resembles human UC in several histopathological features[26,27]. Since then, numerous reports have proven that MDR1A KO colitis provides a highly valuable murine IBD model for the study of intestinal epithelial barrier function[28,29], immunoregulation[30-32], infectious co-triggers[33-35], and novel therapeutic approaches[36,37].
Typically, MDR1A KO pancolitis involves massive inflammatory thickening of the mucosa, increased crypt length with occasional abscesses, and goblet cell loss[26,27]. MDR1A KO colitis is driven by aberrant Th1 cytokine responses, associated with increased numbers of infiltrating CD4+ and TCRαβ+ T cells to the lamina propria[26] and intraepithelial lymphocyte alterations[30]. Dr. Robin Lorenz' group has recently shown that MDR1A KO mice display decreased numbers of CD4+Foxp3+ regulatory T cells in intestinal lymphoid tissues prior to the onset of disease, implying a primary defect in mucosal immunoregulation in the context of MDR1A deficiency[32].
Based on the MDR1A KO colitis model, it has been proposed that mechanisms involving mucosal upregulation of p-gp expression and/or function could have therapeutic potential in ameliorating acute IBD. Examples of potential p-gp inducers are listed in[38]. In addition, administration of Keratinocyte Growth Factor 2 or probiotics leads to increased mucosal p-gp expression[39,40], which is associated with attenuation of acute intestinal inflammation[41,42]. Future research must provide functional proof that upregulation of p-gp directly contributes to anti-inflammatory effects in the intestine.
The gut microbiome is involved in the pathogenesis of IBD. Tolerance to bacterial antigens is broken in active IBD and alterations in gut microbiota diversity contribute to inflammation and effector immune responses[43]. Several lines of evidence link gut microbiota and xenobiotic metabolism[44] via p-gp in the intestine.
Intestinal inflammation in MDR1A KO mice is commensal microbiota-dependent. MDR1A KO mice housed under germ-free conditions do not develop colitis[28] and oral antibiotic treatment significantly ameliorates disease[26,36]. Although commensal-mediated spontaneous colitis of MDR1A KO is not transmissible to wild-type animals[26], disease is exacerbated by infection with various pathogens, including bacteria (e.g., Helicobacter bilis[33]), viruses (e.g., murine norovirus[35]) or parasites (e.g., Trichuris muris[45]). Animal feed, often contaminated by bacterial antigens[46], may also aggravate intestinal inflammation in MDR1A KO mice[47].
Intestinal p-gp limits bacterial invasion and dissemination. For instance, overexpression of p-gp in intestinal epithelial cells leads to increased resistance to Listeria monocytogenes or Salmonella typhimurium infection[48,49], while mice deficient in MDR1A exhibit enhanced burden of Listeria monocytogenes as compared to wildtype after infection[48]. But it remains unclear whether p-gp is capable of directly expelling virulence factors and toxins of bacterial pathogens from host cells. Signaling via p-gp might also fight infection by activating distinct immune processes, such as inducing production of type I interferon in response to Listeria monocytogenes[50].
Dysbiosis precedes the onset of overt colonic inflammation in MDR1A KO mice[51], allowing certain, yet unknown, microbial species to colonize and expand. Lack of p-gp causes intestinal epithelial cell and barrier defects[28,29,52], leading to increased permeability and bacterial translocation which may induce excessive innate immune activation in the underlying lamina propria. Enhanced lipopolysaccharide signaling via MD-2/TLR4 in the intestinal mucosa seems to be required for perpetuation of colitis in MDR1A KO mice[36]. It remains to be tested whether genetic deficiency of MDR1A primarily determines changes in the microbial composition, or rather secondarily subverts the host innate immune response for creating an aberrant mucosal microenvironment that favours microbial misrecognition and shifts.
One may also speculate that impaired efflux pump activity in MDR1A deficiency could lower the threshold for dysbiosis and pro-inflammatory conditions by accumulation of harmful xenobiotic compounds and metabolites in the intestinal mucosa. Xenobiotics and their metabolites may shape the complex dynamics of the gut microbiome[53] by providing substrates for selective growth of certain bacterial species, modulating gene expression[54], breaking microbial tolerance and triggering immune hypersensitivity to otherwise harmless commensals in the intestinal mucosa. Future studies must identify unremoved xenobiotic metabolites in intestinal MDR1A deficiency, examine their environmental effects on the microbiome and the mucosal immune system and analyse how they may contribute to colitis development.
Conversely, gut microbiota may directly affect host xenobiotic metabolism and detoxification by modifying p-gp signaling in the intestinal mucosa[53]. For instance, pathogenic Salmonella typhimurium dampens p-gp expression in intestinal epithelial cells[49]. So far, it is unclear which virulence factors or components of Salmonella typhimurium may be involved in impairing host p-gp function[55]. On the other hand, commensal Lactobacilli strains are capable of stimulating p-gp expression via the involvement of c-Fos/c-Jun in intestinal epithelial cells[40,56]. It is likely that this effect is mediated by TLR2 activation, as TLR2 signaling, which is induced by Lactobacillus[57,58], modulates ABCB1/MDR1-encoded p-gp synthesis and efflux function in intestinal cells[59]. Interestingly, deletion of TLR2 causes fulminant exacerbation of pancolitis in the context of MDR1A deficiency[36]. TLR2/MDR1A double KO intestinal myeloid cells hyperrespond to non-pathogenic Escherichia coli with excessive cellular stress, including increased reactive oxygen species generation, associated lysosomal damage and caspase-1-dependent IL-1ß production, leading to pyroptosis - a form of microbial-induced pro-inflammatory cell death[36]. Blockade of IL-1β activity by treatment with IL-1R antagonist (anakinra) inhibits colitis acceleration in TLR2/MDR1A double deficiency[36]. These data uncover an unexpected combinatory function between host innate immunity (TLR2) and xenobiotic metabolism (MDR1A) in controlling antimicrobial host defence in the lamina propria.
Taken together, xenobiotic metabolism via p-gp is tightly intertwined in a multi-dimensional network with the gut microbiota and the host innate immune system. But several questions arise from these results which remain to be answered, e.g., What commensal community (if any) is responsible for driving murine colitis in the context of MDR1A deficiency? Does MDR1A deficiency sensitize otherwise tolerogenic mucosal immune cells to specific microbial ligands and/or xenobiotics? How do bioactive microbial metabolites modulate xenobiotic signaling via p-gp? Do xenobiotic compounds directly activate TLR (and other innate immune) signaling pathways to control p-gp activity? How is p-gp-mediated transport involved in microbial efflux from host cells?
Several studies have evaluated the potential association of human ABCB1 gene polymorphisms with IBD susceptibility. The ABCB1/MDR1 single nucleotide polymorphism C3435T, which has been correlated with lower expression of p-gp in the intestine[60], was found in patients with extensive UC in some populations[61-64], but not all[65-68]. Two meta-analyses[69,70] did not help to resolve this apparent contradiction, as they produced conflicting results as well. Using a robust gene-wide "block-free" haplotype tagging approach, Dr. Jack Satsangi's group previously identified six SNPs in the ABCB1/MDR1 gene which were significantly associated with UC, but not CD, in their cohort[62]. In addition, a significant association of Ala893Ser/Thr (G2677) with IBD was shown in a large, multicentre North American study[71]. However, none of these ABCB1/MDR1 gene variants were captured as major hits by the recent genome-wide screens in UC (personal communication, Dr. Judy Cho). These contrasting results may reflect differences in the populations studied. It is possible that certain ABCB1/MDR1 gene associations may only be clearly detectable in refined case cohorts with distinct IBD sub-phenotypes.
So far, detailed studies examining the effects of these putative causal variants on gene function are missing in IBD. Based on the findings from the MDR1A KO colitis model, future studies will need to determine whether these variations in the ABCB1/MDR1 gene may indeed alter xenobiotic metabolism, innate immune responses and host-commensal interactions in the human intestine. It must be clarified mechanistically how human ABCB1/MDR1 gene defects may influence detoxification, antimicrobial defences and commensal composition.
Enhanced multidrug resistance via p-gp may limit the individual drug response. Several drugs central to IBD therapy represent p-gp substrates, including glucocorticoids[72] or cyclosporine[73]. Elevated p-gp expression levels have been shown in peripheral blood lymphocytes of those IBD patients who fail therapy with glucocorticoids[74]. High-dose administration of glucocorticoids may result in increased expression of ABCB1/MDR1 mRNA in patients with UC[75]. Recently a human pathogenic Th17-cell subset which stably expresses p-gp was identified in patients with CD. These MDR1+-Th17 cells were refractory to different glucocorticosteroids[12], thus likely contributing to steroid-resistant chronic inflammation in IBD. Reversely, inhibition of p-gp significantly increases intracellular cortisol and cyclosporine levels in vitro, implying a potential target approach for overcoming the poor response to immunosuppressant therapy in refractory IBD[76]. However, in vivo proof remains so far lacking. Three generations of inhibitors of p-gp have largely failed to demonstrate any improvement in therapeutic efficacy in other clinical settings[77]. Future research will need to show whether the design of novel p-gp inhibitors, e.g., based on recent advances in phytochemistry[78], would help overcome drug resistance in IBD.
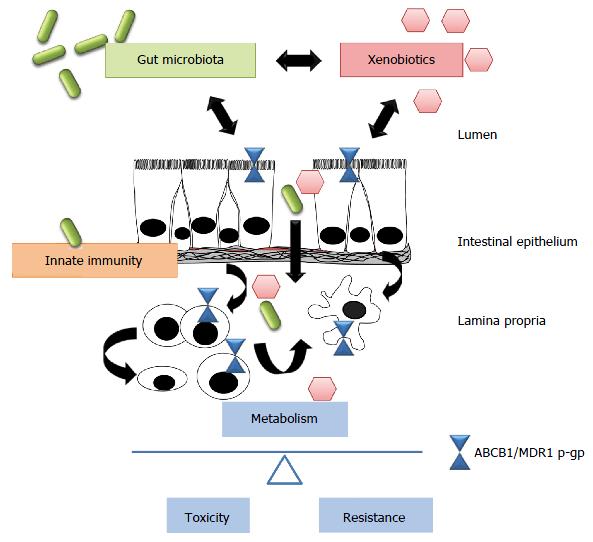
It has become evident that gut microbiota and host innate immunity interact in a multi-dimensional network that controls xenobiotic metabolism to maintain normal mucosal homeostasis in the intestine. Imbalanced host-bacterial interactions may alter xenobiotic metabolism via ABCB1/MDR1 p-gp, contributing to intestinal inflammatory processes, drug toxicity and resistance development in IBD (Figure 1).
Novel, large-scale approaches, including in-silico and complex computational tools[79], are now needed to provide in-depth elucidation of the possible pathways through which the gut microbiota may modify xenobiotics and vice versa as well as their combined metabolic effects via ABCB1/MDR1 p-gp (and other transporters) on host immunity and functions in IBD pathogenesis.
The potential therapeutic value of ABCB1/MDR1 p-gp as a molecular target requires further clarification in IBD. But it is clear that any p-gp targeting will become a delicate balancing act. Its ambivalent effects will make treatment development difficult. Future research will need to look at different therapeutic approaches, either to activate "underactive" p-gp in order to attenuate acute inflammation or to inactivate "overactive" p-gp in order to overcome therapy resistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Gangl A, Wu ZQ S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26-40. [PubMed] |
| 2. | Englund G, Jacobson A, Rorsman F, Artursson P, Kindmark A, Rönnblom A. Efflux transporters in ulcerative colitis: decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm Bowel Dis. 2007;13:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Deuring JJ, de Haar C, Koelewijn CL, Kuipers EJ, Peppelenbosch MP, van der Woude CJ. Absence of ABCG2-mediated mucosal detoxification in patients with active inflammatory bowel disease is due to impeded protein folding. Biochem J. 2012;441:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Riordan JR, Deuchars K, Kartner N, Alon N, Trent J, Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature. 1985;316:817-819. [PubMed] |
| 5. | Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162. [PubMed] |
| 6. | Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1662] [Cited by in RCA: 1514] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 7. | Higgins CF, Callaghan R, Linton KJ, Rosenberg MF, Ford RC. Structure of the multidrug resistance P-glycoprotein. Semin Cancer Biol. 1997;8:135-142. [PubMed] [DOI] [Full Text] |
| 8. | Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics. 2011;21:152-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Drach J, Gsur A, Hamilton G, Zhao S, Angerler J, Fiegl M, Zojer N, Raderer M, Haberl I, Andreeff M. Involvement of P-glycoprotein in the transmembrane transport of interleukin-2 (IL-2), IL-4, and interferon-gamma in normal human T lymphocytes. Blood. 1996;88:1747-1754. [PubMed] |
| 10. | MacLean C, Moenning U, Reichel A, Fricker G. Closing the gaps: a full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metab Dispos. 2008;36:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Cory TJ, He H, Winchester LC, Kumar S, Fletcher CV. Alterations in P-Glycoprotein Expression and Function Between Macrophage Subsets. Pharm Res. 2016;33:2713-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 13. | Katayama K, Noguchi K, Sugimoto Y. Regulations of P-Glycoprotein/ABCB1/MDR1 in Human Cancer Cells. New J Sci. 2014;476974. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Wang S, Lei T, Zhang K, Zhao W, Fang L, Lai B, Han J, Xiao L, Wang N. Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells. J Biol Chem. 2014;289:30075-30081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 727] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 16. | Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, Nakamura K, Kobayashi S, Nakashima E. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci. 2003;92:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Gutmann H, Hruz P, Zimmermann C, Straumann A, Terracciano L, Hammann F, Lehmann F, Beglinger C, Drewe J. Breast cancer resistance protein and P-glycoprotein expression in patients with newly diagnosed and therapy-refractory ulcerative colitis compared with healthy controls. Digestion. 2008;78:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Belliard AM, Lacour B, Farinotti R, Leroy C. Effect of tumor necrosis factor-alpha and interferon-gamma on intestinal P-glycoprotein expression, activity, and localization in Caco-2 cells. J Pharm Sci. 2004;93:1524-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Fernandez C, Buyse M, German-Fattal M, Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci. 2004;7:359-371. [PubMed] |
| 20. | Sartor RB. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;43 Suppl 1:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, Renga B, Fiorucci S. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80:1700-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Murakami Y, Higashi Y, Matsunaga N, Koyanagi S, Ohdo S. Circadian clock-controlled intestinal expression of the multidrug-resistance gene mdr1a in mice. Gastroenterology. 2008;135:1636-1644.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Iwasaki M, Koyanagi S, Suzuki N, Katamune C, Matsunaga N, Watanabe N, Takahashi M, Izumi T, Ohdo S. Circadian modulation in the intestinal absorption of P-glycoprotein substrates in monkeys. Mol Pharmacol. 2015;88:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Palmieri O, Mazzoccoli G, Bossa F, Maglietta R, Palumbo O, Ancona N, Corritore G, Latiano T, Martino G, Rubino R. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. 2015;32:903-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491-502. [PubMed] |
| 26. | Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733-5744. [PubMed] |
| 27. | Wilk JN, Bilsborough J, Viney JL. The mdr1a-/- mouse model of spontaneous colitis: a relevant and appropriate animal model to study inflammatory bowel disease. Immunol Res. 2005;31:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153-G162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Ey B, Eyking A, Gerken G, Podolsky DK, Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284:22332-22343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Eisenbraun MD, Mosley RL, Teitelbaum DH, Miller RA. Altered development of intestinal intraepithelial lymphocytes in P-glycoprotein-deficient mice. Dev Comp Immunol. 2000;24:783-795. [PubMed] |
| 31. | Staley EM, Dimmitt RA, Schoeb TR, Tanner SM, Lorenz RG. Critical role for P-glycoprotein expression in hematopoietic cells in the FVB.Mdr1a(-/-) model of colitis. J Pediatr Gastroenterol Nutr. 2011;53:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Tanner SM, Staley EM, Lorenz RG. Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis. Mucosal Immunol. 2013;6:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a-/-) mice. Am J Pathol. 2002;160:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a-/- mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease. Comp Med. 2008;58:522-533. [PubMed] |
| 36. | Ey B, Eyking A, Klepak M, Salzman NH, Göthert JR, Rünzi M, Schmid KW, Gerken G, Podolsky DK, Cario E. Loss of TLR2 worsens spontaneous colitis in MDR1A deficiency through commensally induced pyroptosis. J Immunol. 2013;190:5676-5688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, Stevens E, Bigler J, Davis JA, Rottman JB. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 38. | Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remião F. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 39. | Saksena S, Priyamvada S, Kumar A, Akhtar M, Soni V, Anbazhagan AN, Alakkam A, Alrefai WA, Dudeja PK, Gill RK. Keratinocyte growth factor-2 stimulates P-glycoprotein expression and function in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G615-G622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Saksena S, Goyal S, Raheja G, Singh V, Akhtar M, Nazir TM, Alrefai WA, Gill RK, Dudeja PK. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1115-G1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Miceli R, Hubert M, Santiago G, Yao DL, Coleman TA, Huddleston KA, Connolly K. Efficacy of keratinocyte growth factor-2 in dextran sulfate sodium-induced murine colitis. J Pharmacol Exp Ther. 1999;290:464-471. [PubMed] |
| 42. | Nanda Kumar NS, Balamurugan R, Jayakanthan K, Pulimood A, Pugazhendhi S, Ramakrishna BS. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J Gastroenterol Hepatol. 2008;23:1834-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Sartor RB, Wu GD. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology. 2017;152:327-339.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 610] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 44. | Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016;14:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 45. | Bhardwaj EK, Else KJ, Rogan MT, Warhurst G. Increased susceptibility to Trichuris muris infection and exacerbation of colitis in Mdr1a-/- mice. World J Gastroenterol. 2014;20:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Crump JA, Griffin PM, Angulo FJ. Bacterial contamination of animal feed and its relationship to human foodborne illness. Clin Infect Dis. 2002;35:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Neudeck BL, Loeb JM, Faith NG, Czuprynski CJ. Intestinal P glycoprotein acts as a natural defense mechanism against Listeria monocytogenes. Infect Immun. 2004;72:3849-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Siccardi D, Mumy KL, Wall DM, Bien JD, McCormick BA. Salmonella enterica serovar Typhimurium modulates P-glycoprotein in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1392-G1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Sigal N, Kaplan Zeevi M, Weinstein S, Peer D, Herskovits AA. The human P-glycoprotein transporter enhances the type I interferon response to Listeria monocytogenes infection. Infect Immun. 2015;83:2358-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Nones K, Knoch B, Dommels YE, Paturi G, Butts C, McNabb WC, Roy NC. Multidrug resistance gene deficient (mdr1a-/-) mice have an altered caecal microbiota that precedes the onset of intestinal inflammation. J Appl Microbiol. 2009;107:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Collett A, Higgs NB, Gironella M, Zeef LA, Hayes A, Salmo E, Haboubi N, Iovanna JL, Carlson GL, Warhurst G. Early molecular and functional changes in colonic epithelium that precede increased gut permeability during colitis development in mdr1a(-/-) mice. Inflamm Bowel Dis. 2008;14:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Lu K, Mahbub R, Fox JG. Xenobiotics: Interaction with the Intestinal Microflora. ILAR J. 2015;56:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 594] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 55. | Mercado-Lubo R, McCormick BA. The interaction of gut microbes with host ABC transporters. Gut Microbes. 2010;1:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Priyamvada S, Anbazhagan AN, Kumar A, Soni V, Alrefai WA, Gill RK, Dudeja PK, Saksena S. Lactobacillus acidophilus stimulates intestinal P-glycoprotein expression via a c-Fos/c-Jun-dependent mechanism in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2016;310:G599-G608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Galdeano CM, Perdigón G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 58. | Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Walker MR, Marinshaw JM, Stappenbeck TS, Stenson WF. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 59. | Frank M, Hennenberg EM, Eyking A, Rünzi M, Gerken G, Scott P, Parkhill J, Walker AW, Cario E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol. 2015;194:1983-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 60. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 893] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 61. | Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, Stange E, Herfarth H, Schoelmerich J, Gregor M. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 62. | Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, Arnott ID, Satsangi J. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128:288-296. [PubMed] |
| 63. | Farnood A, Naderi N, Moghaddam SJ, Noorinayer B, Firouzi F, Aghazadeh R, daryani NE, Zali MR. The frequency of C3435T MDR1 gene polymorphism in Iranian patients with ulcerative colitis. Int J Colorectal Dis. 2007;22:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Juyal G, Midha V, Amre D, Sood A, Seidman E, Thelma BK. Associations between common variants in the MDR1 (ABCB1) gene and ulcerative colitis among North Indians. Pharmacogenet Genomics. 2009;19:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Croucher PJ, Mascheretti S, Foelsch UR, Hampe J, Schreiber S. Lack of association between the C3435T MDR1 gene polymorphism and inflammatory bowel disease in two independent Northern European populations. Gastroenterology. 2003;125:1919-1920; author reply 1919-1920. [PubMed] |
| 66. | Oostenbrug LE, Dijkstra G, Nolte IM, van Dullemen HM, Oosterom E, Faber KN, de Jong DJ, van der Linde K, te Meerman GJ, van der Steege G. Absence of association between the multidrug resistance (MDR1) gene and inflammatory bowel disease. Scand J Gastroenterol. 2006;41:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Fischer S, Lakatos PL, Lakatos L, Kovacs A, Molnar T, Altorjay I, Papp M, Szilvasi A, Tulassay Z, Osztovits J. ATP-binding cassette transporter ABCG2 (BCRP) and ABCB1 (MDR1) variants are not associated with disease susceptibility, disease phenotype response to medical therapy or need for surgeryin Hungarian patients with inflammatory bowel diseases. Scand J Gastroenterol. 2007;42:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Dudarewicz M, Barańska M, Rychlik-Sych M, Trzciński R, Dziki A, Skrętkowicz J. C3435T polymorphism of the ABCB1/MDR1 gene encoding P-glycoprotein in patients with inflammatory bowel disease in a Polish population. Pharmacol Rep. 2012;64:343-350. [PubMed] |
| 69. | Zhao JJ, Wang D, Yao H, Sun DW, Li HY. CTLA-4 and MDR1 polymorphisms increase the risk for ulcerative colitis: A meta-analysis. World J Gastroenterol. 2015;21:10025-10040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Wang J, Guo X, Yu S, Zhang J, Song J, Ji M, Cao Z, Wang J, Liu Y, Dong W. MDR1 C3435T polymorphism and inflammatory bowel disease risk: a meta-analysis. Mol Biol Rep. 2014;41:2679-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Brant SR, Panhuysen CI, Nicolae D, Reddy DM, Bonen DK, Karaliukas R, Zhang L, Swanson E, Datta LW, Moran T. MDR1 Ala893 polymorphism is associated with inflammatory bowel disease. Am J Hum Genet. 2003;73:1282-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 72. | Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248-24252. [PubMed] |
| 73. | Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077-6080. [PubMed] |
| 74. | Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O'Toole D, Mahmud N, Keeling PW, Weir DG, Kelleher D. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Hirano T, Onda K, Toma T, Miyaoka M, Moriyasu F, Oka K. MDR1 mRNA expressions in peripheral blood mononuclear cells of patients with ulcerative colitis in relation to glucocorticoid administration. J Clin Pharmacol. 2004;44:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Farrell RJ, Menconi MJ, Keates AC, Kelly CP. P-glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther. 2002;16:1021-1031. [PubMed] |
| 77. | Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946-2025. [PubMed] |
| 78. | Syed SB, Coumar MS. P-Glycoprotein Mediated Multidrug Resistance Reversal by Phytochemicals: A Review of SAR & amp; Future Perspective for Drug Design. Curr Top Med Chem. 2016;16:2484-2508. [PubMed] |
| 79. | Klünemann M, Schmid M, Patil KR. Computational tools for modeling xenometabolism of the human gut microbiota. Trends Biotechnol. 2014;32:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |