Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1497
Peer-review started: November 6, 2016
First decision: December 2, 2016
Revised: December 9, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 28, 2017
Processing time: 119 Days and 5.1 Hours
To investigate the prevalence and association of Helicobacter pylori (H. pylori) with end-stage renal disease (ESRD).
SA comprehensive literature search was completed from inception until October 2016. Studies that reported prevalence, relative risks, odd ratios, hazard ratios or standardized incidence ratio of H. pylori among ESRD patients were included. Participants without H. pylori were used as comparators to assess the association between H. pylori infection and ESRD. Pooled risk ratios and 95%CI was calculated using a random-effect model. Adjusted point estimates from each study were combined by the generic inverse variance method of DerSimonian and Laird.
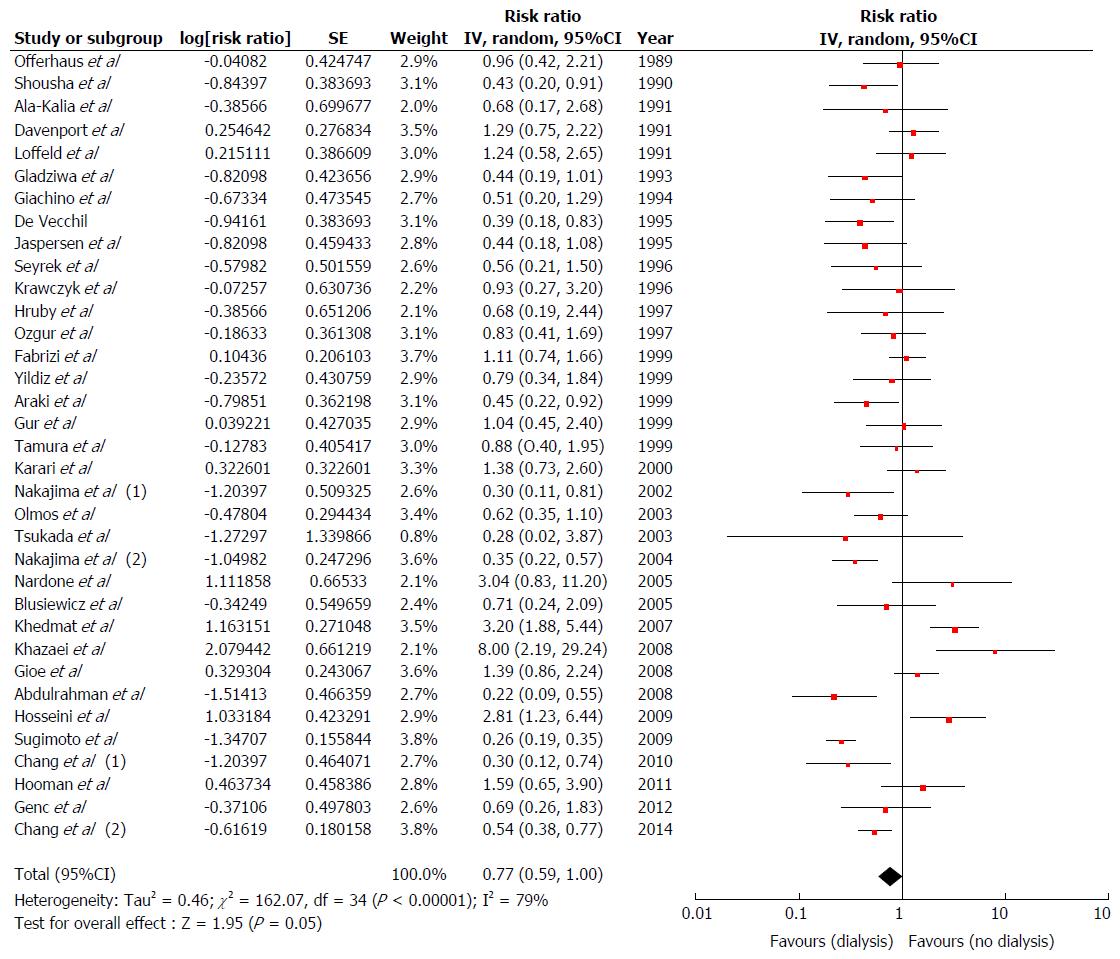
Of 4546 relevant studies, thirty-seven observational studies met all inclusion criteria. Thirty-five cross-sectional studies were included in the analyses to assess the prevalence and association of H. pylori with ESRD. The estimated prevalence of H. pylori among ESRD patients was 44% (95%CI: 40%-49%). The pooled RR of H. pylori in patients with ESRD was 0.77 (95%CI: 0.59-1.00) when compared with the patients without ESRD. Subgroup analysis showed significantly reduced risk of H. pylori in adult ESRD patients with pooled RR of 0.71 (95%CI: 0.55-0.94). The data on the risk of ESRD in patients with H. pylori were limited. Two cohort studies were included to assess the risk of ESRD in patients with H. pylori. The pooled risk RR of ESRD in patients with H. pylori was 0.61 (95%CI: 0.03-12.20).
The estimated prevalence of H. pylori in ESRD patients is 44%. Our meta-analysis demonstrates a decreased risk of H. pylori in adult ESRD patients.
Core tip:Helicobacter pylori (H. pylori) is the most common chronic bacterial infection in gastrointestinal tract of humans. The prevalence and association of H. pylori with end-stage renal disease (ESRD), however, are still unclear. To further investigate this potential relationship, we conducted this systematic review and meta-analysis of observational studies reporting the association between H. pylori infection and ESRD and prevalence in ESRD patients. We found an estimated prevalence of H. pylori in ESRD patients of 44%. In addition, our meta-analysis demonstrates a 0.71-fold decreased risk of H. pylori in adult ESRD patients.
- Citation: Wijarnpreecha K, Thongprayoon C, Nissaisorakarn P, Lekuthai N, Jaruvongvanich V, Nakkala K, Rajapakse R, Cheungpasitporn W. Association between Helicobacter pylori and end-stage renal disease: A meta-analysis. World J Gastroenterol 2017; 23(8): 1497-1506
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1497
Helicobacter pylori (H. pylori) is the most common chronic bacterial infection in the gastrointestinal tract of humans[1]. It has been estimated that the prevalence of H. pylori infection is up to thirty percent in adult aged 18 to 30 years and to fifty percent in those older than 60 years old[2]. Many studies demonstrated that H. pylori infection is associated with a peptic and duodenal ulcer, chronic gastritis, and gastric cancer[3,4]. Recently, epidemiologic studies have demonstrated associations between H. pylori infection and extra-gastrointestinal organ involvements including coronary artery disease, dyslipidemia, insulin resistance, and hematologic disorders[5-7].
End-stage renal disease (ESRD) is a common and serious chronic disease worldwide that continues to increase in prevalence by approximately 21000 cases per year in the United States[8]. Although there is no visible evidence demonstrated that H. pylori infection is directly associated with renal disease, patients with ESRD usually have gastrointestinal problems such as gastritis, dyspeptic symptoms or ulcers[9-11]. Interestingly, recent investigations have demonstrated an association between H. pylori infection and ESRD[12-14]. In addition, an increase in renal resistance index due to systemic inflammation state H. pylori infection was also described[15-18]. However, many studies reported the conflict data regarding the association between H. pylori infection in ESRD and also the prevalence of H. pylori infection in ESRD patients[19-42]. Thus, we conducted the systematic review and meta-analysis that summarized all available evidence to determine the prevalence of H. pylori infection among ESRD patients and the association between H. pylori infection and ESRD.
Three investigators (Wijarnpreecha K, Thongprayoon C and Cheungpasitporn W) independently reviewed published studies indexed in MEDLINE and EMBASE database from their inception to October 2016 using the search strategy that included the terms for “Helicobacter”, “hemodialysis”, and “renal disease” as described in Item S1 in online Supplementary Data 1. A search for additional articles utilizing references from included studies was also performed. There was no confinement on language in the literature search. We conducted this systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.
The inclusion criteria were: (1) observational studies appraising the association between H. pylori and ESRD and prevalence in hemodialysis; (2) prevalence, odds ratios, relative risks, or hazard ratios with 95%CI were presented; and (3) individuals without H. pylori were used as comparators in cohort studies while individuals without ESRD were used as comparators in the cross-sectional and case-control studies. Wijarnpreecha K, Thongprayoon C and Cheungpasitporn W individually examined the titles and abstracts of the studies. After the first phase, the full text of the included studies was subsequently examined to ascertain if they met the inclusion criteria. Discrepancies were also settled by discussion with all investigators.
A structured data collection form was utilized to obtain the data from included studies including title of the study, year of publication, country where the study was conducted, name of the first author, demographic of subjects, method used to diagnose H. pylori, prevalence of H. pylori, effect estimates (hazard ratios, odds ratios, relative risks) with 95%CI, and factors adjusted in the multivariate analysis. To ensure the certainty, this data extraction process was reviewed by all investigators. The quality of each study was individually appraised by each investigator. We utilized the validated Newcastle-Ottawa quality assessment scale for cohort and case-control studies[43] and modified Newcastle-Ottawa scale[44] for the cross-sectional study.
MetaXL software (EpiGear International Pty Ltd)[45] was used for meta-analysis of prevalence. Otherwise, data analysis was performed using the Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates from each study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study based on its variance[46]. We used a random-effect model due to the high likelihood of between-study variance from different study designs, populations, and H. pylori testing. Cochran’s Q test and I2 statistic were used to ascertain the between-study heterogeneity. A value of I2 of 0%-25%, 25%-50%, 50%-75%, and > 75% embodied insignificant, low, moderate and high heterogeneity, respectively[47].
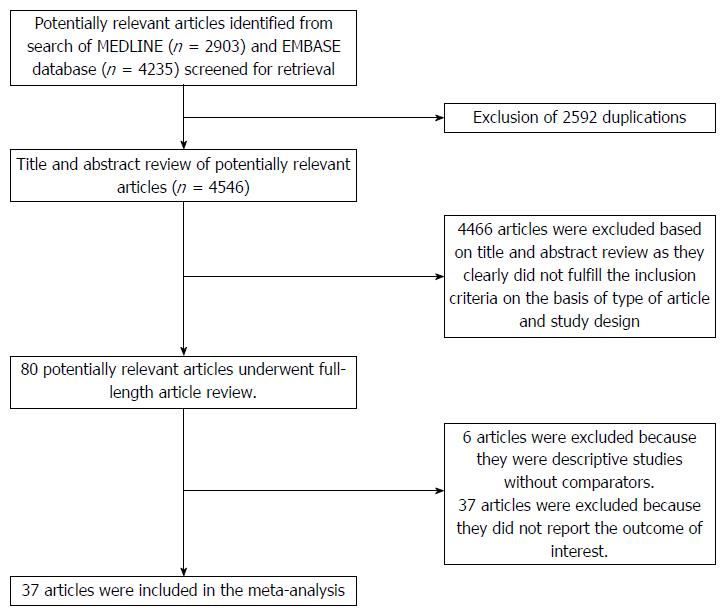
Of 4546 potentially relevant articles, 4466 articles were excluded due to the title and abstract not meeting inclusion criteria. Subsequently, 43 articles were excluded (6 articles were not observational studies, and 37 articles did not describe the outcomes of interest). Finally, thirty-seven observational studies (2 cohort[14,48] and 35 cross-sectional studies[12,13,16,19-42,49-56]) met all inclusion criteria. The literature retrieval, review, and selection process are shown in Figure 1. The characteristics and quality assessment of the included cross-sectional studies are presented in Table 1 while the characteristics of the included cohort studies are shown in Table 2.
| Study | Country | Year | Study sample | H. pylori testing | H. pylori prevalence (%) | OR | Study quality |
| Offerhaus et al[36] | The Netherland | 1989 | Dialysis | Antibody | 22/50 (44%) | 0.96 (0.42-2.22) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Shousha et al[55] | United Kingdom | 1990 | Dialysis | Histology | 12/50 (24%) | 0.43 (0.20-0.90) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Loffeld et al[34] | The Netherland | 1991 | HD | Antibody | 13/30 (43%) | 1.24 (0.58-2.64) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Davenport et al[22] | United Kingdom | 1991 | HD | Antibody | 27/76 (36%) | 1.29 (0.75-2.22) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Ala-Kaila et al[16] | Finland | 1991 | HD | Histology | 3/23 (13%) | 0.68 (0.17-2.64) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Gladziwa et al[27] | Germany | 1993 | HD | Cumulative evaluation (urease, test, histology, culture and direct examiniation) | 12/35 (34%) | 0.44 (0.19 -1.00) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Giachino et al[25] | Italy | 1994 | HD | Urease test, histology and culture | 13/40 (32%) | 0.51 (0.20-1.28) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| De Vecchi et al[51] | Italy | 1995 | HD and PD | Antibody | HD and PD | HD and PD | S 3 |
| 37/67 (55%) | 0.39 (0.18-0.81) | C 1 | |||||
| HD | HD | O 2 | |||||
| 17/29 (59%) | 0.54 (0.18-1.62) | ||||||
| PD | PD | ||||||
| 20/38 (53%) | 0.30 (0.11-0.81) | ||||||
| Jaspersen et al[31] | Germany | 1995 | HD | Urease test and histology | 7/34 (21%) | 0.44 (0.18-1.09) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Seyrek et al[39] | Turkey | 1996 | HD | Antibody | 13/91 (14%) | 0.56 (0.21-1.50) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Krawczyk et al[33] | Poland | 1996 | HD | Urease test and histology | 13/21 (62%) | 0.93 (0.27-3.20) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Ozgür et al[38] | Turkey | 1997 | HD | Urease test | 28/47 (60%) | 0.83 (0.41-1.69) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Hruby et al[30] | Poland | 1997 | HD | Antibody, culture | 9/26 (35%) by culture | 0.68 (0.19-2.44) by culture | S 3 |
| 16/26 (62%) by antibody | 0.53 (0.13-2.12) | C 0 | |||||
| O 2 | |||||||
| Yildiz et al[42] | Turkey | 1999 | HD | Antibody | 31/47 (66%) | 0.79 (0.34-1.84) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Fabrizi et al[23] | United States | 1999 | HD | Antibody | 127/228 (56%) | 1.11 (0.74-1.66) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Tamura et al[40] | Japan | 1999 | HD and PD | Urease test, histology, and culture | 25/49 (51%) | 0.88 (0.40-1.96) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Gür et al[28] | Turkey | 1999 | HD | Urease test and histology | 25/45 (56%) | 1.04 (0.45-2.40) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Araki et al[50] | Japan | 1999 | HD and PD | Histology and culture | 29/63 (46%) | 0.45 (0.22-0.91) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Karari et al[32] | Kenya | 2000 | CRF | Urease test and histology | 41/77 (53%) | 0.90 (0.48-1.70) | S 3 |
| (HD - 36%) | C 1 | ||||||
| O 2 | |||||||
| Nakajima et al[53] | Japan | 2002 | HD | Urease test, histology, and culture | 14/51 (28%) | 0.30 (0.11-0.81) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Tsukada et al[41] | Japan | 2003 | HD | Histology | 9/36 (25%) | 0.28 (0.02-3.82) | S 3 |
| C 2 | |||||||
| O 2 | |||||||
| Olmos et al[37] | Argentina | 2003 | HD | Antibody | 44/93 (47%) | 0.62 (0.35-1.11) | S 3 |
| C 2 | |||||||
| O 2 | |||||||
| Nakajima et al[54] | Japan | 2004 | HD | Antibody | 51/138 (37%) | 0.35 (0.22-0.58) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Nardone et al[35] | Italy | 2005 | HD | Urease test, histology, urea breath test and stool antigen | 7/11 (64%) | 3.04 (0.82-11.13) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Blusiewicz et al[19] | Poland | 2005 | HD | Urease, histology | 19/30 (63%) | 0.71 (0.24-2.07) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Khedmat et al[13] | Iran | 2007 | HD | Urease test | 46/73 (63%) | 3.20 (1.88-5.44) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Khazaei et al[52] | Iran | 2008 | HD - children | Urease test, and histology | 16/24 (67%) | 8.00 (2.19-29.25) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Gioè et al[26] | Italy | 2008 | HD | Urease test, and histology | 75/142 (53%) | 1.39 (0.86-2.23) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Abdulrahman et al[49] | Saudi Arabia | 2008 | ESRD | Histology | 16/40 (40%) | 0.22 (0.09-0.56) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Asl et al[12] | Iran | 2009 | HD | Histology | 23/40 (58%) | 2.81 (1.13-6.99) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Sugimoto et al[56] | Japan | 2009 | HD | Antibody | 262/539 (49%) | 0.26 (0.19-0.35) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Chang et al[21] | South Korea | 2010 | HD | Urease test and histology | 12/33 (36%) | 0.30 (0.12-0.74) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Hooman et al[29] | Iran | 2011 | HD - children | Histology | 19/68 (28%) | 1.59 (0.65-3.92) | S 3 |
| C 0 | |||||||
| O 2 | |||||||
| Genç et al[24] | Turkey | 2013 | HD and PD - children | Antibody | 17/33 (52%) | 0.69 (0.26-1.83) | S 3 |
| C 1 | |||||||
| O 2 | |||||||
| Chang et al[20] | Taiwan | 2014 | ESRD | Urease test and histology | 81/144 (56%) | 0.54 (0.38-0.77) | S 4 |
| C 2 | |||||||
| O 3 |
| Study | Lo et al[48] | Lin et al[14] |
| Country | Hong Kong | Taiwan |
| Study design | Cohort study | Cohort study |
| Year | 2004 | 2015 |
| Study sample | Type 2 diabetic patients with clinical proteinuria and renal insufficiency | H. pylori-infected and non-infected patients without ESRD |
| H. pylori testing | Antibody | Diagnosis of H. pylori infection (ICD-9 041.86) was used from inpatient database of The Taiwan National Health Insurance Research Database |
| Positive H. pylori (Titer > 1.1 U/mL) | ||
| ESRD definition | Doubling of baseline serum creatinine concentration or need for dialysis or serum creatinine ≥ 500 μmol/L | ESRD was identified from Registry for Catastrophic Illness Patient Database |
| Adjusted HR | 0.12 (0.03, 0.52) | 2.58 (2.33, 2.86) |
| Confounder adjustment | Sex, H. pylori status, serum creatinine, hemoglobin, systolic blood pressure, ACE inhibitors, Hepatitis B surface antigen status | Age, sex, comorbidity |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 | Selection: 4 |
| Comparability: 2 | Comparability: 2 | |
| Outcome: 3 | Outcome: 3 |
Thirty-five cross-sectional studies were included in the analyses to assess the prevalence and association of H. pylori with ESRD. The estimated prevalence of H. pylori among ESRD patients was 44% (95%CI: 40%-49%, I2 = 80%), as demonstrated in Figure 2. Subgroup analysis was also performed on thirty-two studies[12,13,16,19-23,25-28,30-42,49-51,53-56] that provided prevalence on adult subjects and three studies[24,29,52] that provided prevalence on pediatric patients and showed estimated prevalences of H. pylori among adult ESRD patients of 44% (95%CI: 39%-49%, I2 = 81%), and 47% (95%CI: 24%-71%, I2 = 84%) among ESRD children, respectively as demonstrated in Supplementary Figures 1 and 2.
We found a marginal but not significantly decreased risk of H. pylori infection in overall ESRD subjects compared with non-ESRD subjects[12,13,16,19-42,49-56] with pooled RR of 0.77 (95%CI: 0.59-1.00, I2 = 79%) (Figure 3). Subgroup analysis based on ageing as described above, we found a significant decreased risk of H. pylori infection among adult ESRD patients[12,13,16,19-23,25-28,30-42,49-51,53-56] with pooled RR of 0.71 (95%CI: 0.55-0.94, I2 = 79%) compared with non-ESRD patients (Supplementary Figure 3). Nevertheless, we did not find a significant association between H. pylori infection and ESRD among ESRD children[24,29,52]; pooled RR = 1.93 (95%CI: 0.55-6.82, I2= 77%), (Supplementary Figure 4).
The data on the risk of ESRD in patients with H. pylori were limited. Two cohort[14,48] studies were included to assess the risk of ESRD in patients with H. pylori. The pooled risk RR of ESRD in patients with H. pylori was 0.61 (95%CI: 0.03-12.20).
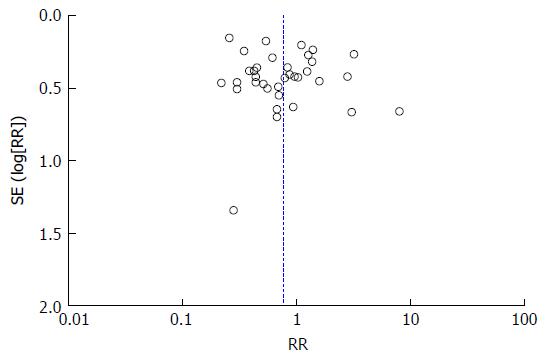
A funnel plot assessing publication bias for the association between H. pylori infection in overall ESRD subjects was demonstrated in Figure 4. The funnel plot of the association between H. pylori infection in overall ESRD subjects was symmetric and suggested no publication bias.
In this meta-analysis summarizing all presently available data on the prevalence of H. pylori infection among ESRD patients and the association between H. pylori infection and ESRD, we demonstrated an estimated prevalence of H. pylori in ESRD patients of 44%. In addition, we found a 0.71-fold decreased risk of H. pylori in adult ESRD patients.
Although the precise explanation of reduced risk of H. pylori among adult ESRD patients is still unclear, there are several plausible explanations for this association. First, it has been postulated in previous studies that administering antibiotics and antacid more frequently in ESRD patients may contribute to lower the prevalence of H. pylori infection[39,53]. Previous study proposed that ESRD patients may have a lower risk of H. pylori infection from routinely used of antacids to prevent renal osteodystrophy by reducing intestinal phosphate absorption[16]. Second, patients with ESRD have higher levels of inflammatory cytokines including tumor necrotic factor, interleukin-6 and -8 from infiltrative inflammatory cells in gastric mucosa[57] and chronic circulatory failure[58,59] could lead to gastric mucosal damage and progress to gastric atrophy or atrophic gastritis, increased in gastric pH mucosa, and eventually eradication of H. pylori infection[60-62].
Although the included studies in this meta-analysis are almost of good quality, there are several limitations to this study that need to be addressed. Firstly, there was a statistical heterogeneity in the completed analysis. Possible sources of this heterogeneity include differences in confounder-adjusted methods (e.g., age, gender, ethnicity and socioeconomic status), different test to detect H. pylori infection in each study, various grades of uremia. Secondly, our subgroup analysis revealed significantly decreased the risk of H. pylori infection among adult subjects with ESRD but not in children likely due to a limitation in some studies. Although the number of study assessing H. pylori in children was limited and the insignificant finding in ESRD children could be from the lack of power, further studies are required to determine the role of aging in the underlying pathogenesis of H. pylori infection among ESRD patients. Lastly, this study is a meta-analysis of observational studies. Thus, our study demonstrated an association, but could not establish causality as unknown confounders could play a role in the association between prevalence of H. pylori among hemodialysis and association between H. pylori and ESRD.
In conclusion, our meta-analysis demonstrated an estimated prevalence of H. pylori in ESRD patients of 44%. In addition, our meta-analysis demonstrates a decreased risk of H. pylori in adult ESRD patients. ESRD could be a potential protective factor for H. pylori infection.
Helicobacter pylori (H. pylori) is the most common chronic bacterial infection in the gastrointestinal tract of humans. Epidemiologic studies showed the link between H. pylori infection and extra-gastrointestinal tract including end-stage renal disease (ESRD). However, many studies reported the conflict data regarding the association between H. pylori infection in ESRD and also the prevalence of H. pylori infection in ESRD patients.
The results of those epidemiologic studies were inconsistent. To further investigate this possible association of H. pylori infection and ESRD and determine the prevalence of H. pylori among ESRD patients, the authors conducted this systematic review and meta-analysis of observational studies reporting the association between H. pylori and ESRD and prevalence of H. pylori among ESRD patients.
The authors found an estimated prevalence of H. pylori in ESRD patients of 44% (95%CI: 40%-49%). Moreover, the authors also found a decreased risk of H. pylori infection among adult ESRD patients with pooled RR of 0.71 (95%CI: 0.55-0.94).
This study demonstrated a significantly decreased risk of H. pylori infection among ESRD patients. This finding suggests that ESRD may be an independent potential protective factor for H. pylori infection.
This meta-analysis investigated the prevalence and association of H. pylori with end-stage renal diseases and demonstrated a decreased risk of H. pylori in adult ESRD patients. The context is well organized and the conclusion is of interest.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Telkes G, Vorobjova T, Zhu YL S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Cave DR. Transmission and epidemiology of Helicobacter pylori. Am J Med. 1996;100:12S-17S; discussion 17S-18S. [PubMed] |
| 2. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. [PubMed] |
| 3. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 4. | Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233-1235. [PubMed] |
| 5. | Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21 Suppl 1:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Sun J, Rangan P, Bhat SS, Liu L. A Meta-Analysis of the Association between Helicobacter pylori Infection and Risk of Coronary Heart Disease from Published Prospective Studies. Helicobacter. 2016;21:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Upala S, Jaruvongvanich V, Riangwiwat T, Jaruvongvanich S, Sanguankeo A. Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis. J Dig Dis. 2016;17:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Onuigbo MA. The CKD enigma with misleading statistics and myths about CKD, and conflicting ESRD and death rates in the literature: results of a 2008 U.S. population-based cross-sectional CKD outcomes analysis. Ren Fail. 2013;35:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ala-Kaila K. Upper gastrointestinal findings in chronic renal failure. Scand J Gastroenterol. 1987;22:372-376. [PubMed] |
| 10. | Musola R, Franzin G, Mora R, Manfrini C. Prevalence of gastroduodenal lesions in uremic patients undergoing dialysis and after renal transplantation. Gastrointest Endosc. 1984;30:343-346. [PubMed] |
| 11. | Sotoudehmanesh R, Ali Asgari A, Ansari R, Nouraie M. Endoscopic findings in end-stage renal disease. Endoscopy. 2003;35:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Asl MK, Nasri H. Prevalence of Helicobacter pylori infection in maintenance hemodialysis patients with non-ulcer dyspepsia. Saudi J Kidney Dis Transpl. 2009;20:223-226. [PubMed] |
| 13. | Khedmat H, Ahmadzad-Asl M, Amini M, Lessan-Pezeshki M, Einollahi B, Pourfarziani V, Naseri MH, Davoudi F. Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc. 2007;39:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lin SY, Lin CL, Liu JH, Yang YF, Huang CC, Kao CH. Association between Helicobacter pylori infection and the subsequent risk of end-stage renal disease: a nationwide population-based cohort study. Int J Clin Pract. 2015;69:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Afsar B, Ozdemir FN, Elsurer R, Sezer S. Helicobacter pylori infection may increase renal resistive index. Med Hypotheses. 2007;69:956-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ala-Kaila K, Vaajalahti P, Karvonen AL, Kokki M. Gastric Helicobacter and upper gastrointestinal symptoms in chronic renal failure. Ann Med. 1991;23:403-406. [PubMed] |
| 17. | Hsu WY, Lin CH, Lin CC, Sung FC, Hsu CP, Kao CH. The relationship between Helicobacter pylori and cancer risk. Eur J Intern Med. 2014;25:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Lai CY, Yang TY, Lin CL, Kao CH. Helicobacter pylori infection and the risk of acute coronary syndrome: a nationwide retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2015;34:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Blusiewicz K, Rydzewska G, Rydzewski A. Gastric juice ammonia and urea concentrations and their relation to gastric mucosa injury in patients maintained on chronic hemodialysis. Rocz Akad Med Bialymst. 2005;50:188-192. [PubMed] |
| 20. | Chang SS, Hu HY. Lower Helicobacter pylori infection rate in chronic kidney disease and end-stage renal disease patients with peptic ulcer disease. J Chin Med Assoc. 2014;77:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Chang WC, Jo YI, Park HS, Jegal J, Park JH, Lee JH, Jin CJ. Helicobacter pylori eradication with a 7-day low-dose triple therapy in hemodialysis patients. Clin Exp Nephrol. 2010;14:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Davenport A, Shallcross TM, Crabtree JE, Davison AM, Will EJ, Heatley RV. Prevalence of Helicobacter pylori in patients with end-stage renal failure and renal transplant recipients. Nephron. 1991;59:597-601. [PubMed] |
| 23. | Fabrizi F, Martin P, Dixit V, Quan S, Brezina M, Abbey H, Gerosa S, Kaufman E, DiNello R, Polito A. Epidemiology of Helicobacter pylori in chronic haemodialysis patients using the new RIBA H. pylori SIA. Nephrol Dial Transplant. 1999;14:1929-1933. [PubMed] |
| 24. | Genç G, Çaltepe G, Özkaya O, Nalçacıoğlu H, Hökelek M, Kalayci AG. [Helicobacter pylori infection in children on dialysis because of chronic renal failure]. Haseki Tip Bulteni. 2013;51:1-4. |
| 25. | Giachino G, Sallio-Bruno F, Chiappero F, Saltarelli M, Rosati C, Mazzucco D, Pallante C, Forneris G, Suriani R. [Helicobacter pylori in patients undergoing periodic hemodialysis]. Minerva Urol Nefrol. 1994;46:213-215. [PubMed] |
| 26. | Gioè FP, Cudia B, Romano G, Cocchiara G, Li Vecchi V, Gioè MA, Calì C, Lo Coco L, Li Vecchi M, Romano M. Role and clinical importance of Helicobacter pylori infection in hemodialysis patients. G Chir. 2008;29:81-84. [PubMed] |
| 27. | Gladziwa U, Haase G, Handt S, Riehl J, Wietholtz H, Dakshinamurty KV, Glöckner WM, Sieberth HG. Prevalence of Helicobacter pylori in patients with chronic renal failure. Nephrol Dial Transplant. 1993;8:301-306. [PubMed] |
| 28. | Gür G, Boyacioglu S, Gül C, Turan M, Gürsoy M, Baysal C, Ozdemir N. Impact of Helicobacter pylori infection on serum gastrin in haemodialysis patients. Nephrol Dial Transplant. 1999;14:2688-2691. [PubMed] |
| 29. | Hooman N, Mehrazma M, Talachian E, Otukesh H, Nakhaii S. Helicobacter pylori infection in pediatric candidates for kidney transplantation. Iran J Kidney Dis. 2011;5:124-129. [PubMed] |
| 30. | Hruby Z, Myszka-Bijak K, Gościniak G, Błaszczuk J, Czyz W, Kowalski P, Falkiewicz K, Szymańska G, Przondo-Mordarska A. Helicobacter pylori in kidney allograft recipients: high prevalence of colonization and low incidence of active inflammatory lesions. Nephron. 1997;75:25-29. [PubMed] |
| 31. | Jaspersen D, Fassbinder W, Heinkele P, Kronsbein H, Schorr W, Raschka C, Brennenstuhl M. Significantly lower prevalence of Helicobacter pylori in uremic patients than in patients with normal renal function. J Gastroenterol. 1995;30:585-588. [PubMed] |
| 32. | Karari EM, Lule GN, McLigeyo SO, Amayo EO. Endoscopic findings and the prevalence of Helicobacter pylori in chronic renal failure patients with dyspepsia. East Afr Med J. 2000;77:406-409. [PubMed] |
| 33. | Krawczyk W, Górna E, Suwała J, Rózyc P, Pawłowski L, Krzywicka A, Wieczerza B, Król A. Frequency of Helicobacter pylori infection in uremic hemodialyzed patients with antral gastritis. Nephron. 1996;74:621-622. [PubMed] |
| 34. | Loffeld RJ, Peltenburg HG, vd Oever H, Stobberingh E. Prevalence of Helicobacter pylori antibodies in patients on chronic intermittent haemodialysis. Nephron. 1991;59:250-253. [PubMed] |
| 35. | Nardone G, Rocco A, Fiorillo M, Del Pezzo M, Autiero G, Cuomo R, Sarnelli G, Lambiase A, Budillon G, Cianciaruso B. Gastroduodenal lesions and Helicobacter pylori infection in dyspeptic patients with and without chronic renal failure. Helicobacter. 2005;10:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Offerhaus GJ, Kreuning J, Valentijn RM, Salvador Peña A, Endtz PH, van Duyn W, Lamers CB. Campylobacter pylori: prevalence and significance in patients with chronic renal failure. Clin Nephrol. 1989;32:239-241. [PubMed] |
| 37. | Olmos JA, Rosa Diez G, Higa R, Algranati S, Ríos H, De Paula JA, Dos Ramos Farías E, Dávolos J. [Helicobacter pylori seroprevalence in dialysis patients]. Acta Gastroenterol Latinoam. 2003;33:139-144. [PubMed] |
| 38. | Ozgür O, Boyacioğlu S, Ozdoğan M, Gür G, Telatar H, Haberal M. Helicobacter pylori infection in haemodialysis patients and renal transplant recipients. Nephrol Dial Transplant. 1997;12:289-291. [PubMed] |
| 39. | Seyrek N, Kocabas E, Hazar S, Paydas S, Aksaray N, Sagliker Y. Helicobacter pylori antibodies in patients on chronic hemodialysis. Nephron. 1996;72:725-726. [PubMed] |
| 40. | Tamura H, Tokushima H, Murakawa M, Matsumura O, Itoyama S, Mitarai T, Isoda K. Influences of Helicobacter pylori on serum pepsinogen concentrations in dialysis patients. Nephrol Dial Transplant. 1999;14:113-117. [PubMed] |
| 41. | Tsukada K, Miyazaki T, Katoh H, Yoshikawa M, Masuda N, Ojima H, Tajima K, Fukai Y, Nakajima M, Kamiyama Y. Helicobacter pylori infection in hemodialysis patients. Hepatogastroenterology. 2003;50:2255-2258. [PubMed] |
| 42. | Yildiz A, Beşişik F, Akkaya V, Sever MS, Bozfakioğlu S, Yilmaz G, Ark E. Helicobacter pylori antibodies in hemodialysis patients and renal transplant recipients. Clin Transplant. 1999;13:13-16. [PubMed] |
| 43. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12667] [Article Influence: 844.5] [Reference Citation Analysis (0)] |
| 44. | Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 962] [Cited by in RCA: 1147] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 45. | Barendregt J, Doi S. MetaXL User Guide: Version 1.0. Wilston, Australia: EpiGear International Pty Ltd 2010; . |
| 46. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 47. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46547] [Article Influence: 2115.8] [Reference Citation Analysis (3)] |
| 48. | Lo MK, Lee KF, Chan NN, Leung WY, Ko GT, Chan WB, So WY, Ng MC, Ho CS, Tam JS. Effects of gender, Helicobacter pylori and hepatitis B virus serology status on cardiovascular and renal complications in Chinese type 2 diabetic patients with overt nephropathy. Diabetes Obes Metab. 2004;6:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Abdulrahman IS, Al-Quorain AA. Prevalence of gastroesophageal reflux disease and its association with Helicobacter pylori infection in chronic renal failure patients and in renal transplant recipients. Saudi J Gastroenterol. 2008;14:183-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Araki H, Miyazaki R, Matsuda T, Gejyo F, Koni I. Significance of serum pepsinogens and their relationship to Helicobacter pylori infection and histological gastritis in dialysis patients. Nephrol Dial Transplant. 1999;14:2669-2675. [PubMed] |
| 51. | De Vecchi AF, Quatrini M, Boni F, Castelnovo C, Viganó E, Baldassarri AR, Tenconi L, Bianchi P. Epidemiology of Helicobacter pylori in dialysis patients. Perit Dial Int. 1995;15:178-179. [PubMed] |
| 52. | Khazaei MR, Imanieh MH, Hosseini Al-Hashemi G. Gastrointestinal evaluation in pediatric kidney transplantation candidates. Iran J Kidney Dis. 2008;2:40-45. [PubMed] |
| 53. | Nakajima F, Sakaguchi M, Amemoto K, Oka H, Kubo M, Shibahara N, Ueda H, Katsuoka Y. Helicobacter pylori in patients receiving long-term dialysis. Am J Nephrol. 2002;22:468-472. [PubMed] |
| 54. | Nakajima F, Sakaguchi M, Oka H, Kawase Y, Shibahara N, Inoue T, Ueda H, Katsuoka Y. Prevalence of Helicobacter pylori antibodies in long-term dialysis patients. Nephrology (Carlton). 2004;9:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Shousha S, Arnaout AH, Abbas SH, Parkins RA. Antral Helicobacter pylori in patients with chronic renal failure. J Clin Pathol. 1990;43:397-399. [PubMed] |
| 56. | Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 59. | Nakamura S, Sasaki O, Nakahama H, Inenaga T, Kawano Y. Clinical characteristics and survival in end-stage renal disease patients with arteriosclerosis obliterans. Am J Nephrol. 2002;22:422-428. [PubMed] |
| 60. | Wesdorp RI, Falcao HA, Banks PB, Martino J, Fischer JE. Gastrin and gastric acid secretion in renal failure. Am J Surg. 1981;141:334-338. [PubMed] |
| 61. | Tamadon MR, Saberi Far M, Soleimani A, Ghorbani R, Semnani V, Malek F, Malek M. Evaluation of noninvasive tests for diagnosis of Helicobacter pylori infection in hemodialysis patients. J Nephropathol. 2013;2:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 62. | Jalalzadeh M, Ghadiani MH, Mousavinasab N. Association between helicobacter pylori infection and body mass index, before and after eradication of infection in hemodialysis batients. J Nephropathol. 2012;1:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |