Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1469
Peer-review started: October 7, 2016
First decision: November 21, 2016
Revised: December 2, 2016
Accepted: February 8, 2017
Article in press: February 8, 2017
Published online: February 28, 2017
Processing time: 146 Days and 0.6 Hours
To investigate death for liver failure and for tumor recurrence as competing events after hepatectomy of hepatocellular carcinoma.
Data from 864 cirrhotic Child-Pugh class A consecutive patients, submitted to curative hepatectomy (1997-2013) at two tertiary referral hospitals, were used for competing-risk analysis through the Fine and Gray method, aimed at assessing in which circumstances the oncological benefit from tumour removal is greater than the risk of dying from hepatic decompensation. To accomplish this task, the average risk of these two competing events, over 5 years of follow-up, was calculated through the integral of each cumulative incidence function, and represented the main comparison parameter.
Within a median follow-up of 5.6 years, death was attributable to tumor recurrence in 63.5%, and to liver failure in 21.2% of cases. In the first 16 mo, the risk of dying due to liver failure exceeded that of dying due to tumor relapse. Tumor stage only affects death from recurrence; whereas hepatitis C infection, Model for End-stage Liver Disease score, extent of hepatectomy and portal hypertension influence death from liver failure (P < 0.05 in all cases). The combination of these clinical and tumoral features identifies those patients in whom the risk of dying from liver failure did not exceed the tumour-related mortality, representing optimal surgical candidates. It also identifies those clinical circumstances where the oncological benefit would be borderline or even where the surgery would be harmful.
Having knowledge of these competing events can be used to weigh the risks and benefits of hepatic resection in each clinical circumstance, separating optimal from non-optimal surgical candidates.
Core tip: Optimal candidates for hepatectomy should benefit from the tumour removal that encompasses the risk of dying from post-operative liver function worsening and failure. This means that when evaluating patients for surgery, the competing risks of tumour-related death and of liver failure have to be weighed against each other, and considered from the point of view of available alternative therapies. In the present study, a large cohort of Child-Pugh class A cirrhotic patients submitted to curative (R0) hepatic resection for hepatocellular carcinoma was analysed to provide a competing-risk analysis of these two competing events.
- Citation: Cucchetti A, Sposito C, Pinna AD, Citterio D, Cescon M, Bongini M, Ercolani G, Cotsoglou C, Maroni L, Mazzaferro V. Competing risk analysis on outcome after hepatic resection of hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol 2017; 23(8): 1469-1476
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1469
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide[1]. It often arises on the background of cirrhosis, making its treatment completely different from other liver malignancies because of the conflicting needs of being oncologically appropriate and of preserving hepatic function. In this regard, a careful preoperative evaluation of both tumour burden and functional reserve is essential in order to select candidates that will most benefit from surgery[2]. The balance between tumour stage, hepatic curtailment needed to curatively remove the tumour, and the hepatic reserve is critical to obtain a survival benefit, avoiding a pointless oncological outcome, postoperative liver failure, progressive hepatic deterioration and, ultimately, patient premature death. Having these aspects as determinants, hepatic resection is typically indicated in patients able to achieve a significant oncologic benefit from surgery, with low or null probabilities of experiencing liver function worsening[3]. Cirrhotic patients resected for HCC mainly die due to recurrence of the tumour consequences, and/or complications of end-stage liver disease. This means that when evaluating patients for surgery, the competing risks of tumour-related death and of liver failure have to be weighed against each other, and considered from the point of view of available alternative therapies. Having knowledge of these aspects can help in identifying optimal surgical candidates as well as in recognizing sub-optimal and/or non-optimal candidates who will most benefit from other therapies.
Common statistical techniques for time-to-event analysis, including cancer-specific survival, focus on failure-time data with a single type of failure (even if composite such as disease-free survival), are not able to capture competing risks arising when a failure can result from one of several causes and one cause precludes the others[4,5]. Competing risk analysis can more adequately capture the real cause-specific survivals of HCC cirrhotic patients submitted to hepatectomy. However, at present, no data are currently available on the competing risk of these two main end-events. The aim of the present study was to analyze the competing-risk of dying from tumour recurrence or liver failure in a cohort of cirrhotic patients, belonging to Child-Pugh class A, submitted to curative hepatic resection, and to investigate prognostic factors, taking into consideration the competing nature of these two events.
Prospectively collected data from two Western centres with similar volumes, expertise and management strategy for HCC (Fondazione IRCCS - Istituto Nazionale Tumori, Milan, Italy and S.Orsola-Malpighi Hospital, Bologna, Italy) were reviewed, and patients submitted to curative resection (R0) of a pathologically proven HCC between 1997 and 2013 were identified (patients with R1 or R2 margin positive resection were excluded). Approval for conducting the study was obtained from the institutional review board at both centres. The study population selection was focused on patients with well-preserved liver function, since they commonly represent typical candidates for hepatic resection. Consequently, only patients belonging to Child-Pugh class A were retained for the analysis. None of the patients in the study group was treated as an emergency; none had macroscopic tumour portal vein invasion, invasion of adjacent organs or spread to the lymph nodes of the hepatic hilum. The final study population consisted of 864 consecutive Child-Pugh class A cirrhotic patients submitted to curative (R0) hepatectomy. Presence of clinical signs of portal hypertension (PHT) was not considered an absolute contraindication for hepatectomy[6]. Thus, the study cohort also includes patients with total bilirubin > 1 mg/dL and/or with a platelet count < 100000/mL and/or with oesophageal varices at endoscopy, namely, when tumour resection was judged to provide a greater benefit than other available options such as liver transplantation, and loco-regional or systemic therapies.
The following variables were recorded for each patient: age, sex, aetiology of underlying liver disease, presence of oesophageal varices, main serological parameters (total bilirubin, creatinine, international normalized ratio, albumin, platelets count), and main tumour radiological characteristics (number and size of lesions). Presence of PHT was defined as the presence of oesophageal varices or a platelet count < 100000/mL[3]. The extent of the hepatectomy was based on the International Hepato-Pancreato-Biliary Association Classification[7]. Tumours were staged on the basis of preoperative imaging, according to the United Network for Organ Sharing (UNOS)-TNM classification[8].
Following discharge, all patients were observed periodically at follow-up to exclude possible recurrence of HCCs: biochemical liver function tests, serum α-fetoprotein level measurement, and ultrasound were conducted 3 and 6 mo after discharge and then according to an annual or semi-annual surveillance program in the more recent period[9]. Recurrence was diagnosed on the basis of HCC diagnosis guidelines released during the study period. None of the patients in this study group received adjuvant chemotherapy. Patients presenting recurrence were managed with various therapeutic modalities, including re-resection, when possible, and salvage liver transplantation, for selected patients with transplantable recurrence. The patient selection criteria for second hepatic resection were the same as for primary resection. Patients with non-resectable recurrence, and not suitable for liver transplant, were submitted to loco-regional therapies. From the end of 2008, Sorafenib therapy was also adopted, either alone or in combination with loco-regional approaches.
Continuous data are reported as median and interquartile ranges; categorical data as counts and percentages. Patient survival was measured from the date of hepatic resection until death or the date of the last follow-up. The cause of death was recorded considering that patients would have died because of the tumour in the presence of a disseminated extra- and/or intra-hepatic tumour recurrence, including also those cases where liver function worsened as a consequence of tumour spread (i.e., liver involvement > 50% and/or development of tumour portal vein invasion). On the contrary, patients would have likely died because of liver failure (and/or its complications) in the absence of tumour recurrence and in the presence of clinical signs of end-stage liver disease, or, in cases where recurrence was diagnosed, in all those cases where the burden of tumour relapse did not justify liver failure (i.e., progressive liver worsening in the presence of small recurrences). Controversial cases were discussed between authors Cucchetti A and Sposito C. Cases not fulfilling these criteria were recorded as other causes of death. Follow-up ended at June 2015. Patients submitted to salvage transplantation were censored the day prior to transplant. Survival rates, observed after surgery, were obtained by plotting Kaplan-Meier curves. Cumulative incidences of the competing events of interest were calculated using the Fine and Gray competing risks approach using the STATA syntax stcrreg (StataCorp. Stata Statistical Software: Release 12.)[10]. Factors identified having a P < 0.10 on simple (univariate) competing risk analysis were entered into a multivariable regression model. A backward stepwise variable-selection process was adopted to identify independent predictors of death for tumour recurrence or for liver failure. A P value < 0.05 was considered statistically significant in all the analyses. The cumulative incidence of death for tumour relapse or hepatic failure was thus calculated for each combination of independent variables. The area under the curve of cumulative incidence over time was then calculated using trapezoidal rule and divided by time so it was expressed as average risk within the first 5 years from surgery. Differences between these incidences were compared through standardized differences (d) calculation, a measure of the effect size[11]. In particular, d values < |0.1| indicated very small differences between the means; d values between |0.1| and |0.3| indicated small differences, d values between |0.3| and |0.5| indicated moderate differences, and d values > |0.5| indicated considerable differences[12].
Baseline characteristics of the 864 cirrhotic patients, all belonging to Child - Pugh class A forming the study population, are reported in Table 1. The median age was 66.7 years, ranging between 18 and 85 years, most of the patients were hepatitis C positive (62.3%), had a single tumour (78.2%), were within UNOS-T1/T2 stage (70.6%), and were submitted to the removal of one Couinaud segment or less (sub-segmentectomy) (72.3%).
| n = 864 | |
| Age (yr) | 67 (61- 72) |
| ≥ 67 yr | 432 (50.0) |
| Gender male | 678 (78.5) |
| Anti-HCV positive | 538 (62.3) |
| HBsAg positive | 197 (22.8) |
| Alcohol/other | 120 (13.9) |
| Creatinine (mg/dL) | 0.90 (0.80-1.04) |
| Albumin (g/L) | 4.0 (3.7-4.3) |
| Bilirubin (mg/dL) | 0.85 (0.64-1.19) |
| INR | 1.13 (1.07-1.22) |
| Platelet count (× 103/mL) | 142 (102-186) |
| < 100.000/mL | 202 (23.4) |
| MELD score | 9 (8-10) |
| < 9 | 428 (49.5) |
| 9-10 | 298 (34.5) |
| > 10 | 138 (16.0) |
| Oesophageal varices | 216 (25.0) |
| Tumour size (cm) | 3.5 (2.3-5.0) |
| Single nodule | 676 (78.2) |
| UNOS Stage | |
| T1 | 84 (9.7) |
| T2 | 527 (61.0) |
| T3 | 237 (27.4) |
| T4a | 16 (1.9) |
| Extension of hepatectomy | |
| Wedge/segmentectomy | 625 (72.3) |
| Bisegmentectomy | 146 (16.9) |
| Three or more segments | 93 (10.8) |
During a median follow-up of the whole cohort of 5.6 years (range: 1 d-13 years), 489 patients experienced tumour recurrence (56.6%) and 334 patients died (38.7%). Death was attributable to tumour recurrence in 212 patients (63.5% of causes of death) and to liver failure in 71 patients (21.2%). The median time between tumor recurrence and death was 1.4 years. In 51 patients, the cause of death was not attributable to either of these two events (15.3%). The 30-d and 90-d post-operative death rates were 1.0% and 2.4%, respectively. The 1-, 3-, 5- and 10-year patient survival probabilities were 90.9%, 70.9%, 54.9% and 29.0%.
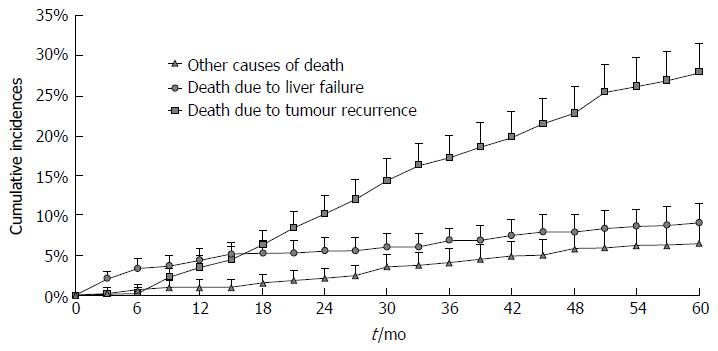
Competing risk analysis on cause of death for the entire study population is reported in Figure 1. As can be noted, in the first 16 mo, the predicted risk of dying due to liver failure exceeds that of dying because of tumour recurrence. Afterwards, most deaths were attributable to tumour relapse whereas liver failure mortality remained roughly stable through subsequent years, namely between 4.4% at 1 year and 9.1% at 5 years.
Relationships between clinical variables and the cumulative incidence of death due to tumour recurrence or due to liver failure are reported in Table 2. Tumour stage and extension of hepatectomy were predictors of death due to tumour relapse (P = 0.002 and 0.042, respectively). Regarding the cumulative incidence of death attributable to liver failure, hepatitis C infection (P = 0.032), Model for End-stage Liver Disease (MELD) score (P = 0.001) and PHT (P = 0.029) were significantly related to the predicted risk of dying from liver failure.
| Death from tumour recurrence | Death from liver failure | |||||
| 1-year (%) | 3-year (%) | 5-year (%) | 1-year (%) | 3-year (%) | 5-year (%) | |
| Age (yr) | P = 0.915 | P = 0.267 | ||||
| < 67 | 3.8 (0.9) | 19.9 (2.2) | 28.1 (2.6) | 4.5 (1.0) | 6.2 (1.2) | 7.8 (1.4) |
| ≥ 67 | 3.2 (0.8) | 14.7 (1.9) | 28.1 (2.8) | 4.3 (1.0) | 6.7 (1.3) | 10.5 (1.8) |
| Gender | P = 0.738 | P = 0.287 | ||||
| Male | 3.6 (0.7) | 17.7 (1.7) | 27.7 (2.1) | 4.2 (0.8) | 6.6 (1.0) | 8.5 (1.2) |
| Female | 3.4 (1.4) | 15.4 (2.9) | 28.2 (4.1) | 5.0 (1.6) | 7.2 (1.9) | 11.1 (2.8) |
| Hepatitis C infection | P = 0.838 | P = 0.032 | ||||
| Positive | 3.7 (0.8) | 15.9 (1.8) | 27.4 (2.4) | 5.3 (1.0) | 7.5 (1.2) | 10.9 (1.6) |
| Negative | 3.2 (1.0) | 19.3 (2.5) | 28.3 (3.1) | 2.8 (0.9) | 4.7 (1.2) | 5.9 (1.5) |
| Portal hypertension1 | P = 0.515 | P = 0.029 | ||||
| Absent | 3.6 (0.8) | 17.6 (1.8) | 27.2 (2.3) | 3.3 (0.8) | 5.1 (1.0) | 6.9 (1.2) |
| Present | 3.3 (1.0) | 16.5 (2.3) | 28.8 (3.2) | 6.2 (1.4) | 8.9 (1.7) | 12.9 (2.2) |
| MELD score | P = 0.760 | P = 0.001 | ||||
| < 9 | 4.2 (1.0) | 16.7 (2.0) | 27.7 (2.8) | 1.9 (0.7) | 3.3 (0.9) | 5.2 (1.3) |
| 9-10 | 2.5 (0.9) | 17.7 (2.5) | 27.8 (3.2) | 4.7 (1.2) | 6.0 (1.4) | 10.2 (2.1) |
| > 10 | 3.6 (1.6) | 17.3 (3.6) | 27.7 (4.7) | 11.0 (2.7) | 16.6 (3.2) | 17.9 (3.4) |
| UNOS T-stage | P = 0.002 | P = 0.597 | ||||
| T1 | 1.0 (0.0) | 7.6 (0.2) | 14.5 (0.7) | 1.2 (1.0) | 3.2 (2.3) | 10.6 (4.6) |
| T2 | 3.0 (0.7) | 15.4 (1.8) | 25.0 (2.4) | 4.1 (0.9) | 6.7 (1.2) | 8.7 (1.4) |
| T3-T4a | 5.8 (1.5) | 26.3 (3.0) | 34.6 (3.6) | 6.0 (1.5) | 7.8 (1.7) | 9.3 (2.0) |
| Hepatectomy extension | P = 0.042 | P = 0.052 | ||||
| Wedge/segmentectomy | 3.2 (0.7) | 15.2 (1.6) | 26.7 (2.3) | 3.0 (0.7) | 5.5 (1.0) | 8.7 (1.4) |
| Two or more segments | 4.4 (1.4) | 22.3 (2.9) | 30.7 (3.5) | 8.1 (1.8) | 9.0 (1.9) | 10.3 (2.1) |
For completeness of results, the same analysis was conducted for other causes of death but none of the variables analysed was found to be significantly related to death for causes other than tumour recurrence or liver failure; thus, detailed results were omitted.
Results from multivariable competing risk analysis are reported in Table 3. Tumour stage only affects death due to recurrence (P = 0.001) whereas hepatitis C positivity (P = 0.046), MELD score (P = 0.001), extension of hepatectomy (P = 0.019) and PHT (P = 0.024) influence death as a result of liver failure.
| Sub-Hazard ratio | 95%CI | P value | |
| Death for tumour recurrence | |||
| UNOS T-stage (T1 vs T2 vs T3-4a) | 1.59 | 1.21-2.09 | 0.001 |
| Removal of more than one segment | 1.08 | 0.76-1.51 | 0.667 |
| Death for liver failure | |||
| Hepatitis C (positive vs negative) | 1.79 | 1.01-3.17 | 0.046 |
| Portal hypertension (present vs absent) | 1.84 | 1.08-3.12 | 0.024 |
| MELD class (< 9 vs 9-10 vs > 10) | 2.21 | 1.59-3.07 | 0.001 |
| Removal of more than one segment | 1.89 | 1.11-3.21 | 0.019 |
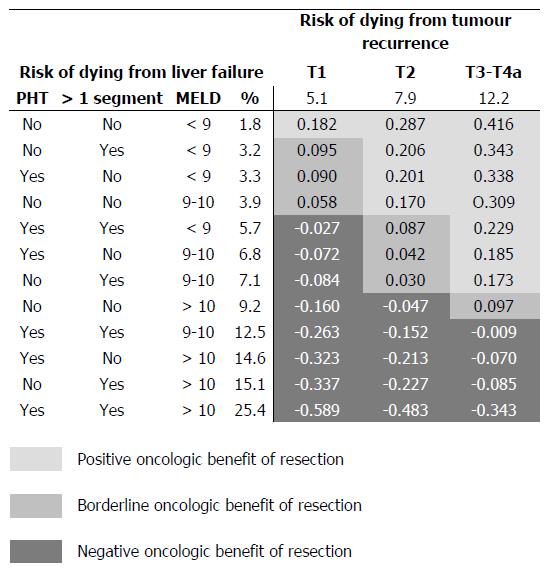
A summary of possible combinations of clinical and tumoral variables is reported in the Figure 2. Results are reported as average risk over 5 years of follow-up, predicted by the multivariable regression model (the integer of the cumulative incidences over time divided by time) and were adjusted for the distribution of hepatitis C positive patients in the present study population (62.3%). The threshold for positive oncologic surgical benefit of liver resection (positive d-values) widens as the T stage progresses, while HCC resectability of HCC in cirrhosis becomes less achievable with the progression of liver dysfunction. Some examples can help to clarify this figure. A patient with a T1 tumour has an average annual risk of dying from cancer of 5.1% and a corresponding risk of dying from liver failure of 1.8%, provided there are no clinical signs of PHT, the patient has a MELD score < 9 and the tumour is removable with a wedge or a segmentectomy (d = 0.182: positive benefit). If the removal of the tumour requires a more extended hepatectomy and/or there are clinical signs of PHT, the risk of dying from liver failure increases to 3.2/3.3% (d < 0.10: borderline benefit), thus nullifying the oncologic surgical benefit. A patient with a T2 tumour has an annual average risk of dying from cancer of 7.9% and, in the presence of a MELD score > 10, clinical signs of PHT and the need for a wedge or a segmentectomy, a corresponding risk of dying of liver failure of 14.6%; thus, the risk of dying from liver failure exceeds the oncologic surgical benefit (d = -0.213: negative effect size).
Despite the increasing incidence of HCC, the curative approach of hepatic resection remains underused and at times ignored. Recent data from the American College of Surgeons National Cancer Data Base showed that even if hepatic resection was associated with a significant increase in survival among patients with AJCC stage I/II HCC, only less than 40% of such patients were treated surgically[13]. This proportion increases when patients are managed in academic centres, probably because of a better knowledge of risks and benefits obtainable with hepatic resection[13]. The main concern in offering surgical resection to cirrhotic patients is represented by the need to avoid post-hepatectomy liver failure and persistent function worsening[2,3,6]. Considering that the main causes of death after hepatic resection are tumour relapse and liver failure, a comprehensive knowledge of these two distinct risks can help in daily clinical practice, especially when comparing surgical and non-surgical therapies[2]. The present results can help in fill this gap of knowledge.
The first result showed that the risk course of the two competing end-events varies with the passage of time (Figure 1). In the mid-term following liver resection, namely in the first 16 mo, the risk of death from tumour relapse was lower than that from liver failure (that does not exceed 5%), confirming the curative value of surgery. The surgical benefit is clinically supported by the fact that diagnosis of recurrence is not associated with dismal prognosis as it used to be, since the improved ability to treat recurrence (even curatively) can significantly prolong survival[9]. These aspects have to be weighed against the risk of liver failure, which, in the absence of a liver transplant, still represents a diagnosis of imminent death. From the present results, it can be said that, starting from the first year onwards, the healing ability of surgery begins to decline and long-term risks and benefits should be evaluated from this time point onwards.
One main clinical indication that can be derived from the present study regards the identification of optimal and non-optimal candidates for liver surgery, even beyond conventional recommendations (Figure 2). It can be suggested that optimal surgical candidates are those patients having a long-term risk of dying due to tumour relapse constantly higher than that of dying from liver failure. In cirrhotic patients with a T1 tumour (single nodule < 2 cm), in the absence of clinical signs of PHT, MELD < 9 and limited extent of liver resection, namely a wedge or a segmentectomy, the average annual risk of dying from cancer is considerably higher (5.1%) than that of dying of liver failure (1.8%). The magnitude of the effect size (d = 0.182) supports the indication for surgery in this kind of patient, who has a tumour that is superficially located and easily removable with limited removal of liver parenchyma. In cases in which the presence of initial signs of liver function worsening (PHT or increased MELD score) or where there is a need for greater parenchymal removal, the risk of dying from liver failure starts to increase, nullifying the oncological benefit (effect size < 0.1) and becoming potentially harmful in more advanced degrees of liver dysfunction (negative effect size). In these cases, patients become non-ideal candidates for surgery, supporting the role of loco-regional treatments.
Patients with a T2 tumour (single nodule 2-3 cm or 2-3 nodules all < 3 cm) represent the majority of surgical candidates[14] and it is worthy of note that in cases of multiple lesions these patients fall beyond conventional recommendations built on the Barcelona Clinic Liver Cancer (BCLC) staging system[3,15,16]. T2 patients are burdened by an average annual risk of dying from tumour recurrence of 7.9%. From the comparison of the two competing risks, it can be noted that the oncological benefit obtainable from surgery, allows acceptance of a higher risk of liver failure (Figure 2). That is, in the presence of a T2 tumour, clinical signs of PHT should not represent an absolute contraindication to surgery (effect size > 0.1), provided there is a substantial normal liver function (MELD < 9-10)[6,16,17]. In these cases, the risk of tumour-related death was permanently higher than that of death due to liver failure, supporting the concept that the benefit obtainable with surgery is considerably greater than the risk of liver failure. Conversely, a more advanced degree of liver dysfunction and/or the need for more extensive hepatectomies may turn surgery into a harmful treatment that can be justified by the possibility of a subsequent salvage transplantation: a resource-consuming alternative that, on top of poorly predictable outcome, transforms an elective strategy into an emergency procedure.
Tumours still of surgical interest while belonging to UNOS-T3-T4a stages mostly fall within the intermediate stage of BCLC algorithm[3,15,18]. Resectability in these patients should be assessed by experienced surgical groups before offering trans-arterial chemo-embolization (TACE)[18] as a (non-curative) alternative treatment option. These tumour stages are burdened by the highest risk of dying from tumour relapse, thus making it possible to prioritise the surgical indication, including also patients with PHT and MELD scores around 10. In fact, surgically-resectable T3-T4a patients are non-ideal candidates for resection but, as recently outlined in a large analysis, a patient who may not be an ideal candidate for resection may still have a better outcome than what is expected when alternative modalities recommended by the current guidelines are applied[2]. Thus, the recommended treatment modality for the intermediate stage represented by TACE[18] can be challenged by liver resection, not in all cases but under the specific circumstances specified above. Notably, in pertinent literature benchmarks, TACE has, in the best-case scenario, a median survival of about 2 years[3,15,18,19], corresponding to an average risk of dying during the first 5 years of about 48%, which is always higher than present figures after resection (Figure 2). In other words, the present results support patients with intermediate stage HCC being offered liver resection when this is judged technically feasible in experienced centres and when the risk of dying from liver cancer exceeds the risk of liver failure. In all other instances, T3-T4a patients should remain with the conventional approach and be considered for TACE.
In determining the risk of dying from liver failure, hepatitis C infection deserves special consideration. The present study population encompasses a time period when direct antiviral agents (DAA) were not available, and only a small proportion of the most recently resected patients are currently receiving DAA. The low probabilities of achieving a cure for hepatitis C infection with standard interferon-based regimens of the past decades, and consequently the low probabilities of slowing down (or stopping) the progression of cirrhosis, are the reasons for its strong impact in the liver failure-related deaths observed in the present study. Although somewhat optimistic, it is reasonable to think that DAA can, in the future, achieve control of the progression of cirrhosis: presuming an improvement in the progression of cirrhosis, the competing role of tumour-related death will increase the benefit obtainable from hepatic resection.
Some limitations of the present study deserve appropriate discussion. First, it reports the experience from a surgical series and a comparison with non-surgical therapies would be the ultimate goal. Such a comparison is advisable for future studies that consider the competing risks of dying from cancer or liver failure in relationship with other therapeutic modalities. However, it is not strictly necessary in the current analysis to have this control group: these surgical results can be seen as a reference point for other studies of TACE or ablation without all being assessed in the same study. Nevertheless, future comparative studies using a competing risk approach are warranted. Second, the retrospective nature of the present study and the policies adopted in our centres may have determined a surgical population selection not completely representative of all patients suffering from resectable HCC. However, as previously outlined[13], patients managed in academic centres are more frequently offered such a potentially curative treatment, and the present data can be considered representative of a tertiary level hospital experience. Finally, limitations of the multivariable competing risk regression model have also to be taken into account. Even if it comes from a relatively large sample size, the risk of over-fitting the model cannot be excluded and the present results require further external or prospective validations to confirm their validity.
In conclusion, the present study provided a first competing risk analysis of causes of death after hepatic resection of HCC that could be used as a reference for similar analyses conducted on alternative treatments such as ablation or TACE. Ideal candidates for hepatic resection should be represented by those patients having a risk of dying from tumour cancer that is significantly greater than the risk of dying from liver failure.
The main concern in offering surgical resection to cirrhotic patients with hepatocellular carcinoma (HCC) is represented by the need to avoid post-hepatectomy function worsening. Considering that the main causes of death after surgery are represented by tumour relapse and liver failure, a comprehensive knowledge of these two distinct competing risks can help in clinical practice to distinguish optimal from non-optimal surgical candidates.
Ideal candidates for hepatic resection should be represented by those patients having a risk of dying from tumour cancer that is significantly greater than the risk of dying from liver failure.
This study is the first one to provide a first competing risk analysis of causes of death after hepatic resection of HCC. The combination of tumour size and number, Model for End-stage Liver Disease score, extension of hepatectomy required to curatively remove the tumour and the absence or presence of clinical signs of portal hypertension, identifies those patients in whom the risk of dying from liver failure did not exceed the tumour-related mortality, representing optimal surgical candidates.
Having knowledge of these competing events can be used to weigh risks and benefits of hepatic resection in each clinical circumstance, providing a benchmark to also assess the benefit achievable from non-surgical therapies, such as ablation or embolization.
Survival analysis is the analysis of data measured from a specific time of origin until an event of interest or a specified endpoint occurs, where every patient provides two pieces of information: follow-up time and status (i.e., event or censored endpoint). However, a patient may experience an event different from the event of interest. For example, a patient with HCC may die due to causes unrelated to his/her cancer. Such events are termed competing risk events.
A very interesting observation study provided a first competing risk analysis of causes of death after hepatic resection of HCC particular on the patients with Child’ A functional class. This manuscript is well written and analyzing. It should benefit to kind in mild that those patients having a risk of dying from cancer resection that significantly overcome the risk of dying from liver failure.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chamuleau RA, Chiu KW S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 2. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 3. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 4. | Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. 1995. John Wiley & Sons, New York. . |
| 5. | Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated. Data Pharmaceut. Statist. 2003;3:303-304. |
| 6. | Cucchetti A, Cescon M, Golfieri R, Piscaglia F, Renzulli M, Neri F, Cappelli A, Mazzotti F, Mosconi C, Colecchia A. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol. 2016;64:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2: 333-39. HPB (Oxford). 2002;4:99; author reply 99-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | United Network for Organ Sharing. Policy 3.6. Available from: http://www.unos.org. |
| 9. | Cucchetti A, Zanello M, Cescon M, Ercolani G, Del Gaudio M, Ravaioli M, Grazi GL, Pinna AD. Improved diagnostic imaging and interventional therapies prolong survival after resection for hepatocellular carcinoma in cirrhosis: the university of bologna experience over 10 years. Ann Surg Oncol. 2011;18:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing Risk. J Am Stat Assoc. 1999;94:496-509. |
| 11. | Burnand B, Kernan WN, Feinstein AR. Indexes and boundaries for “quantitative significance” in statistical decisions. J Clin Epidemiol. 1990;43:1273-1284. [PubMed] |
| 12. | Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. 1988. . |
| 13. | Mohanty S, Rajaram R, Bilimoria KY, Salem R, Pawlik TM, Bentrem DJ. Assessment of non-surgical versus surgical therapy for localized hepatocellular carcinoma. J Surg Oncol. 2016;113:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 16. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 17. | Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) group. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013;33:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 361] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 19. | Cappelli A, Cucchetti A, Cabibbo G, Mosconi C, Maida M, Attardo S, Pettinari I, Pinna AD, Golfieri R. Refining prognosis after trans-arterial chemo-embolization for hepatocellular carcinoma. Liver Int. 2016;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |