Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1412
Peer-review started: October 25, 2016
First decision: December 19, 2016
Revised: December 29, 2016
Accepted: January 17, 2017
Article in press: January 17, 2017
Published online: February 28, 2017
Processing time: 127 Days and 12.7 Hours
To clarify which factors may influence pathological tumor response and affect clinical outcomes in patients with locally advanced rectal carcinoma treated with neo-adjuvant chemoradiotherapy and surgery.
Tumor regression grade (TRG) according to the Dworak system and yTNM stage were assessed and correlated with pre-treatment clinico-pathological variables in 215 clinically locally advanced (cTNM stage II and III) rectal carcinomas. Prognostic value of all pathological and clinical factors on disease free survival (DFS) and cancer specific survival (CSS) was analyzed by Kaplan Meier and Cox-regression analyses.
cN+ status, mucinous histotype or poor differentiation in the pre-treatment biopsy were significantly associated with lower pathological response (low Dworak grade and TNM remaining unchanged/upstaging). Cases showing acellular mucin pools in surgical specimens all had unremarkable clinical courses with no deaths or recurrences during follow-up. Dworak grade had prognostic significance for DFS and CSS. However, compared to the 5-tiered system, a simplified two-tiered grading system, in which grades 0, 1 and 2 were grouped as absent/partial regression and grades 3 and 4 were grouped as total/subtotal regression, was more reproducible and prognostically informative. The two-tiered Dworak system, yN stage, craniocaudal extension of the tumor and radial margin status were significant independent prognostic variables.
Our data suggest that caution should be applied in using a conservative approach in rectal carcinomas with cN+ status, extensive/lower involvement of the rectum and mucinous histotype or poor differentiation. Although Dworak TRG is prognostically significant, a simplified two-tiered system could be preferable. Finally, cases with acellular mucin pools should be carefully evaluated to definitely exclude residual mucinous carcinoma.
Core tip: This study evaluates the prognostic significance of clinico-pathological variables in patients with locally advanced rectal carcinoma treated with neo-adjuvant chemo-radiotherapy (CRT) and surgery. Our data show that tumors with cN+ status, extensive/lower involvement of the rectum, mucinous histotype and poor differentiation have a lower response to pre-operative CRT. Dworak tumor regression grade was prognostically informative; however, a simplified two-tiered system was more reproducible and prognostically significant. Acellular mucin pools were found in a percentage of cases with excellent outcomes. Although acellular mucin pools should be considered as complete pathological responses, careful histological examination is mandatory to exclude residual mucinous carcinoma.
- Citation: Reggiani Bonetti L, Lionti S, Domati F, Barresi V. Do pathological variables have prognostic significance in rectal adenocarcinoma treated with neoadjuvant chemoradiotherapy and surgery? World J Gastroenterol 2017; 23(8): 1412-1423
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1412
Neo-adjuvant chemo-radiotherapy (CRT) currently represents the standard of care for locally advanced (clinical T stage 3/4 or N+) rectal cancer[1,2]. Indeed, CRT improves resectability and sphincter preservation and decreases the probability of local recurrence in patients affected by rectal carcinoma[3,4]. However, tumor response to CRT is highly heterogeneous and governed by unclear mechanisms[5]. Post-treatment Tumor Node Metastasis (yTNM) stage and Tumor Regression Grade (TRG) are currently used to measure tumor response in surgical specimens obtained after CRT. According to several studies, TRG seems to have prognostic relevance on both disease-free survival (DFS) and overall survival in patients with rectal cancer[6-9]. Several systems have been proposed to score TRG, and all are based on the proportion of residual tumor to stromal fibrosis in the primary tumor site[10-13]. However, none of the systems currently in use is universally accepted, and all may have fair interobserver concordance due to the lack of precise and objective criteria for scoring[14]. Of note, several authors reported on the presence of mucin pools devoid of neoplastic cells in surgical rectal carcinomas pre-treated with CRT with an incidence ranging between 4.8% and 31%[15-19]. One issue in the assessment of TRG of rectal cancer relates to the interpretation of acellular mucin pools[15-19]. Indeed, only the TRG proposed by the Royal College of Pathologists clearly states that acellular mucin pools should be regarded as complete tumor regression[20], while the other grading systems do not give a precise indication on this topic. Additionally, only a few papers addressed the prognostic significance of acellular mucin pools in rectal cancer treated with neo-adjuvant therapy and these studies reported controversial findings[15-19]. Further, some authors suggest that acellular mucin deposits are associated with higher biological aggressiveness of the tumor[16], while others do not find any association with patient prognosis[15,17-19]. Finally, whether development of acellular mucin pools is associated with any of the clinico-pathological characteristics present in the tumor prior to CRT is still to be determined.
In this study, we analyzed a cohort of rectal carcinomas submitted to neo-adjuvant CRT with the aim to investigate: (1) the reproducibility and prognostic significance of Dworak TRG[11]; (2) the prognostic significance of acellular mucin pools; and (3) the possible correlation between TRG, acellular mucin pools or TNM stage variation after therapy and the various clinico-pathological characteristics present in the tumor before treatment.
A total of 238 rectal adenocarcinomas, treated by neoadjuvant CRT and surgical resection with mesorectal excision, were identified in the Tumor Registry of Colorectal Cancer of the University of Modena and Reggio Emilia, Italy, in the period between 2001 and 2012.
Twenty-three patients were excluded from this study because they had clinical TNM (cTNM) stage IV. Thus, the final cohort in this analysis was composed of 215 patients (141 males, 74 females; mean age: 66.3 years; age range: 30-85 years) with cT3/T4 or cN+ rectal cancer. cTNM staging workup had been performed by using digital rectal examination, chest radiography, total-body computed tomography (CT), magnetic resonance imaging, endorectal ultrasonography and coloscopy with biopsy.
Clinical records were reviewed to retrieve information on the localization in the rectum (upper, medium, lower or extensive), the circumferential involvement of the rectal wall (one-third, middle or complete), distance from the anal verge (more or less than 1 cm) and craniocaudal extension (more or less than 3 cm) of the tumor. The histological grade and histotype of the tumor were assessed using endoscopic biopsy and according to World Health Organization criteria[21,22].
After the histological diagnosis on endoscopic biopsy, all patients received a total dose of 50 Gy radiotherapy, which was administered in 28 fractions of 1.8 Gy each for five consecutive days per week, and a daily continuous infusion of 225 mg 5-fluorouracil per day and per square meter of body surface for the duration of radiotherapy.
Then, patients were submitted to surgical resection. Data on cancer-specific survival (CSS) and DFS were available for all patients. After surgery, patients were monitored for disease progression by using total-body CT scan, coloscopy and blood tests (including measurement of CEA and CA 19-9). Patients who died of diseases independent of rectal cancer were censored. Both local and distant recurrences were considered in the assessment of DFS. Information on eventual adjuvant therapy was available for 127 patients.
Surgical specimens were fixed in formalin for 24 h at room temperature and were grossly examined for obvious or presumable tumor remains as a mass, ulcer or fibrotic lesion. At least 3 samples were taken for paraffin embedding from specimens showing an obvious tumor mass. On the other hand, lesions with questionable residual tumors were completely embedded, and if no tumor cells were detected on first paraffin sections, three additional leveled sections were examined from each paraffin block. The total number of paraffin blocks from the primary tumor region ranged between 3 and 25; the average number was 6.4. In each case, at least 12 lymph nodes were retrieved from perirectal fat.
The histological slides of each case were retrieved from the archive of the Unit of Anatomic Pathology of the University of Modena and Reggio Emilia and reviewed by two independent pathologists (L.R.B. and V.B.) to assess the TRG and yTNM staging.
TRG was assessed in the primary tumor, but not in nodal metastases, according to Dworak scale[11]. In detail, cases were defined as follows: grade 0, no regression; grade 1, dominant tumor mass with obvious fibrosis and⁄or vasculopathy; grade 2, dominant fibrotic changes with few neoplastic cells or groups (easy to find); grade 3, evidence of very few neoplastic cells in fibrotic tissue; and grade 4, no tumor cells (pathological complete response)[11]. Cases showing acellular mucin pools were initially considered separately and then categorized as grade 4 because of the absence of tumor cells.
yTNM staging was established using the criteria of Union for International Cancer Control (UICC) (TNM 7th edition)[23].
We also evaluated the status of the radial (circumferential resection) margin, which was defined as positive when normal tissue from the edge of the tumor measured 1 mm or less[24].
In the comparison between cTNM and yTNM, we were able to establish the variation rate of T, N and TNM staging after neoadjuvant CRT and define three groups of tumors, as follows: (1) rectal cancer with no change in TNM staging; (2) rectal cancers with downstaging after therapy; and (3) rectal carcinomas with upstaging after therapy.
Fleiss-Cohen weighted κ statistics were used to establish interobserver variability in the assessment of TRG.
The χ2 test was applied to analyze the statistical correlations between Dworak TRG and the various clinico-pathological parameters and to investigate the statistical association between acellular mucin pools and clinico-pathological variables in the subgroup of Dworak grade 4 tumors.
We also used the χ2 test to establish the statistical correlation between T, N, or TNM stage changing and the other clinico-pathological parameters.
DFS and CSS were assessed by the Kaplan-Meier method, using the date of primary surgery as the entry date. The end point for the DFS analysis was the length of survival to disease progression (either local or distant). CSS was characterized as the length of survival to death from rectal cancer or to the last follow-up date. For DFS and CSS analyses, Dworak grades 0, 1 and 2 were grouped and defined as absent/partial regression, while grades 3 and 4 were grouped together and considered as total/subtotal regression.
The Mantel-Cox log-rank test was applied to assess the strength of the association between DFS or CSS and each of the parameters (age and gender of the patient as well as site, circumferential spread, distance from the anal verge, craniocaudal extension, histological grade, histotype, cT, cN, yT, yN, yM, yTNM stage, T stage variation, N stage variation, TNM stage variation, radial margin, Dworak regression grade, tumor regression, and adjuvant chemotherapy) as a single variable.
Subsequently, a stepwise multivariate analysis (Cox regression model) was utilized to determine the independent effect of each variable on survival. TNM stage variation (and not single T or N stage variation) and tumor regression (not Dworak regression grade) were included in the multivariate analyses. Adjuvant chemotherapy was not considered in the multivariate analyses because data were available in only a proportion of patients.
A probability (P) value less than 0.05 was considered significant. Statistical analysis was performed using MedCalc 12.1.4.0 statistical software (MedCalc Software, Mariakerke, Belgium).
The clinico-pathological characteristics of rectal cancer in the study are summarized in Table 1. The median follow-up period of the patients was 70 mo (range: 3-183 mo). During the follow-up 73 (34%) patients developed recurrences, and 58 died of disease.
| Variables | Category | n | Death due to disease | Recurrence |
| Sex | M | 141 | 35 (25) | 43 (30) |
| F | 74 | 23 (31) | 30 (40) | |
| Age | ≤ 67 yr | 106 | 24 (23) | 32 (30) |
| > 67 yr | 109 | 34 (31) | 41 (38) | |
| Site | Upper | 93 | 23 (25) | 30 (32) |
| Medium | 32 | 10 (31) | 13 (41) | |
| Lower | 77 | 21 (27) | 26 (34) | |
| Extensive | 13 | 4 (31) | 4 (31) | |
| Circumferential spread | One-third | 94 | 24 (26) | 32 (34) |
| One middle | 62 | 17 (27) | 21 (34) | |
| Complete | 59 | 17 (29) | 20 (34) | |
| Craniocaudal extension | < 3 cm | 51 | 7 (14) | 10 (20) |
| ≥ 3 cm | 164 | 51 (31) | 63 (38) | |
| Distance from the anal verge | ≥ 1 cm | 204 | 56 (27) | 2 (18) |
| < 1 cm | 11 | 2 (18) | 71 (35) | |
| Histological grade | 1 | 6 | 0 (0) | 0 (0) |
| 2 | 179 | 47 (26) | 61 (34) | |
| 3 | 30 | 11 (37) | 12 (40) | |
| Histotype | NOS | 200 | 53 (26) | 67 (33) |
| Mucinous | 15 | 5 (33) | 6 (40) | |
| cT | cT2 | 12 | 1 (8) | 2 (17) |
| cT3 | 170 | 43 (25) | 54 (32) | |
| cT4 | 33 | 14 (42) | 17 (51) | |
| cN | cN0 | 98 | 21 (21) | 28 (28) |
| cN+ | 117 | 37 (32) | 45 (38) | |
| yT | yT0 | 35 | 3 (9) | 8 (23) |
| yT1 | 19 | 1 (5) | 1 (5) | |
| yT2 | 61 | 13 (21) | 16 (26) | |
| yT3 | 83 | 32 (39) | 36 (43) | |
| yT4 | 17 | 9 (53) | 12 (70) | |
| yN | yN0 | 146 | 28 (91) | 38 (26) |
| yN+ | 69 | 30 (43) | 35 (51) | |
| yM | yM0 | 212 | 56 (20) | 70 (33) |
| yM+ | 3 | 2 (67) | 3 (100) | |
| y stage | T0N0M0 | 33 | 3 (9) | 8 (24) |
| 1 | 63 | 10 (16) | 13 (21) | |
| 2 | 50 | 15 (30) | 17 (34) | |
| 3 | 66 | 28 (42) | 32 (48) | |
| 4 | 3 | 2 (67) | 3 (100) | |
| TNM Stage variation | None | 80 | 33 (41) | 38 (47) |
| Downstaging | 119 | 19 (16) | 28 (23) | |
| Upstaging | 16 | 6 (38) | 7 (44) | |
| T stage variation | None | 86 | 34 (40) | 41 (48) |
| Downstaging | 122 | 22 (18) | 29 (24) | |
| Upstaging | 7 | 2 (29) | 3 (43) | |
| N stage variation | None | 141 | 43 (31) | 55 (39) |
| Downstaging | 61 | 11 (18) | 14 (23) | |
| Upstaging | 13 | 4 (31) | 4 (31) | |
| Dworak Regression grade | 0 | 34 | 16 (47) | 19 (56) |
| 1 | 76 | 20 (26) | 26 (24) | |
| 2 | 50 | 16 (32) | 16 (32) | |
| 3 | 21 | 3 (14) | 4 (19) | |
| 4 | 34 | 3 (9) | 8 (23) | |
| Regression | Absent/partial | 160 | 52 (33) | 61 (38) |
| Total/subtotal | 55 | 6 (11) | 12 (22) | |
| Radial margin | Negative | 201 | 51 (25) | 65 (32) |
| Positive | 14 | 7 (50) | 8 (57) | |
| Adjuvant chemotherapy | No | 47 | 16 (34) | 18 (38) |
| Yes | 80 | 24 (30) | 31 (39) |
The Dworak grade could be assessed in all 215 cases. Interobserver concordance in the assessment of Dworak TRG was good (K: 0,74) and increased to very good (K: 0.82) when cases were subdivided into total/subtotal regression (Dworak grades 3 and 4) and absent/partial regression (Dworak grades 0, 1 and 2).
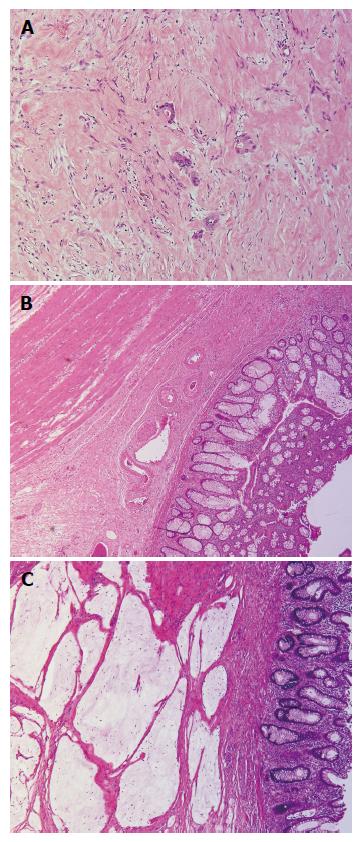
In detail, 34 (16%) rectal carcinomas were classified as Dworak grade 0, 76 (35%) as grade 1, 50 (23%) as grade 2 (Figure 1A), 21 (10%) as grade 3 and 34 (16%) as grade 4 (Figure 1B and Table 1). Lower Dworak grade (0/1/2) was significantly more frequent among cN+ tumors (cTNM stage III) (P = 0.0008) (Figure 2) and was also significantly associated with death from rectal cancer (P = 0.0047) and recurrence (P = 0.0254) (Table 2).
| Variable | Dworak regression grade | P value | ||||
| 0 | 1 | 2 | 3 | 4 | ||
| Sex | ||||||
| M | 23 | 52 | 29 | 11 | 26 | 0.285 |
| F | 11 | 24 | 21 | 10 | 8 | |
| Age | ||||||
| ≤ 67 yr | 14 | 35 | 32 | 9 | 16 | |
| > 67 yr | 20 | 41 | 18 | 12 | 18 | 0.202 |
| Site | ||||||
| Upper | 9 | 37 | 22 | 8 | 17 | |
| Medium | 7 | 11 | 7 | 1 | 6 | |
| Lower | 13 | 23 | 19 | 11 | 11 | |
| Extensive | 5 | 5 | 2 | 1 | 0 | 0.2506 |
| Circumferential spread | ||||||
| One-third | 14 | 30 | 28 | 8 | 14 | |
| Middle | 12 | 19 | 10 | 9 | 12 | |
| Complete | 8 | 27 | 12 | 4 | 8 | 0.31 |
| Craniocaudal extension | ||||||
| < 3 cm | 8 | 15 | 13 | 6 | 9 | |
| ≥ 3 cm | 26 | 61 | 37 | 15 | 25 | 0.873 |
| Distance from the anal verge | ||||||
| ≥ 1 cm | 2 | 4 | 2 | 2 | 1 | |
| < 1 cm | 32 | 72 | 48 | 19 | 33 | 0.853 |
| Histological grade | ||||||
| 1 | 1 | 3 | 2 | 0 | 0 | |
| 2 | 28 | 61 | 36 | 20 | 34 | |
| 3 | 5 | 12 | 12 | 1 | 0 | 0.0732 |
| Histotype | ||||||
| NOS | 28 | 71 | 49 | 20 | 32 | |
| Mucinous | 6 | 5 | 1 | 1 | 2 | 0.0875 |
| cT | ||||||
| 2 | 2 | 2 | 5 | 3 | ||
| 3 | 23 | 63 | 37 | 18 | 29 | |
| 4 | 9 | 11 | 8 | 3 | 2 | 0.219 |
| cN | ||||||
| cN0 | 14 | 28 | 21 | 10 | 25 | |
| cN+ | 20 | 48 | 29 | 11 | 9 | 0.008 |
| Death due to disease | ||||||
| No | 18 | 56 | 34 | 18 | 31 | |
| Yes | 16 | 20 | 16 | 3 | 3 | 0.0047 |
| Recurrence | ||||||
| No | 15 | 50 | 34 | 17 | 26 | |
| Yes | 19 | 26 | 16 | 4 | 8 | 0.0254 |
A total of 15 cases in our cohort were classified as mucinous on the endoscopic biopsy; 11 (73%) were classified as Dworak grade 0 or 1; 1 (7%) was classified as Dworak grade 2; 1 (7%) was classified as Dworak grade 3 and 2 (13%) were classified as Dworak grade 4 on the surgical specimen (Table 2). A low Dworak grade (0-1-2) was more frequently observed in rectal carcinomas with mucinous histotype or high histological grade on the endoscopic biopsy, although statistical significance was not achieved (Table 2). Among the patients with mucinous carcinoma, 5 (33%) died of disease, and 6 (40%) experienced disease recurrence (Table 1).
Among the cases with Dworak grade 4, 7 (21%) had acellular mucin pools (Figure 1C); 5 (72%) originated from cTNM stage II cases; and 2 (28%) originated from cTNM stage III tumors. All of these cases were classified as yT0N0M0 stage and having an uneventful clinical course (no evidence of recurrence or death from rectal cancer in a median follow-up period of 71 mo; follow-up ranged between 71 and 183 mo). The presence of acellular mucin pools in Dworak 4 rectal cancer was significantly associated with mucinous histotype (P = 0.004) (Table 3). Indeed, 2/34 cases had been classified as mucinous on endoscopic biopsy, and both had acellular mucin pools in the surgical specimen (Table 3). However, the remaining five cases with acellular mucin pools showed no evidence of mucin in the endoscopic sample taken prior to CRT and surgery.
| Variable | Acellular mucin pools | P value | |
| Absent | Present | ||
| Sex | |||
| M | 20 | 6 | |
| 7 | 1 | 0.523 | |
| Age | |||
| ≤ 67 yr | 13 | 3 | |
| > 67 yr | 14 | 4 | 0.805 |
| Site | |||
| Upper | 14 | 3 | |
| Medium | 4 | 2 | |
| Lower | 9 | 2 | |
| Extensive | 0 | 0 | 0.695 |
| Circumferential spread | |||
| One-third | 11 | 3 | |
| Middle | 10 | 2 | |
| Complete | 6 | 2 | 0.898 |
| Craniocaudal extension | |||
| < 3 cm | 7 | 2 | |
| ≥ 3 cm | 20 | 5 | 0.889 |
| Distance from the anal verge | |||
| ≥ 1 cm | 1 | 0 | |
| < 1 cm | 26 | 7 | 0.61 |
| Histotype | |||
| NOS | 27 | 5 | |
| Mucinous | 0 | 2 | 0.004 |
| cT | |||
| 2 | 2 | 1 | |
| 3 | 23 | 6 | |
| 4 | 2 | 0 | 0.664 |
| cN | |||
| cN0 | 20 | 5 | |
| cN+ | 7 | 2 | 0.889 |
| Death due to disease | |||
| No | 24 | 7 | |
| Yes | 3 | 0 | 0.362 |
| Recurrence | |||
| No | 19 | 7 | |
| Yes | 8 | 0 | 0.1047 |
In the comparison between cT and yT staging, we noticed that 86 (40%) cases showed no variation of T stage after CRT, 122 (57%) tumors underwent T downstaging, while 3% had T upstaging (Table 2). T remaining unchanged/upstaging was significantly more frequent in female patients (P = 0.001) and in tumors with mucinous histotype (P = 0.01), cN+ stage (P = 0.014), high histological grade (P < 0.0001) and low Dworak grade (0-1-2) (P < 0.0001) and was significantly associated with death from rectal cancer (P = 0.002) and development of recurrence (P = 0.002) (Table 4).
| Variable | T stage variation | P value | ||
| None | Downstaging | Upstaging | ||
| Sex | ||||
| M | 58 | 83 | 0 | |
| F | 28 | 39 | 7 | 0.001 |
| Age | ||||
| ≤ 67 yr | 40 | 64 | 2 | |
| > 67 yr | 46 | 58 | 5 | 0.375 |
| Site | ||||
| Upper | 33 | 56 | 4 | |
| Medium | 12 | 19 | 1 | |
| Lower | 35 | 41 | 1 | |
| Extensive | 6 | 6 | 1 | 0.692 |
| Circumferential spread | ||||
| One-third | 41 | 51 | 2 | |
| Middle | 22 | 37 | 3 | |
| Complete | 23 | 34 | 2 | 0.792 |
| Craniocaudal extension | ||||
| < 3 cm | 21 | 29 | 1 | |
| ≥ 3 cm | 65 | 93 | 6 | 0.832 |
| Distance from the anal verge | ||||
| ≥ 1 cm | 5 | 6 | 0 | |
| < 1 cm | 81 | 116 | 7 | 0.789 |
| Histotype | ||||
| NOS | 77 | 118 | 5 | |
| Mucinous | 9 | 4 | 2 | 0.01 |
| cN | ||||
| cN0 | 29 | 66 | 3 | |
| cN+ | 57 | 56 | 4 | 0.0145 |
| Histological grade | ||||
| 1 | 0 | 4 | 2 | |
| 2 | 68 | 107 | 4 | |
| 3 | 18 | 11 | 1 | < 0.0001 |
| Dworak grade | ||||
| 0 | 23 | 7 | 0 | |
| 1 | 38 | 35 | 7 | |
| 2 | 23 | 27 | 0 | |
| 3 | 2 | 19 | 0 | |
| 4 | 0 | 34 | 0 | < 0.0001 |
| Death due to disease | ||||
| No | 52 | 100 | 5 | |
| Yes | 34 | 22 | 2 | 0.0027 |
| Recurrence | ||||
| No | 45 | 93 | 4 | |
| Yes | 41 | 29 | 3 | 0.0014 |
Furthermore, 56 (48%) cases with cN+ status showed no change in N stage after CRT, while 61 (52%) had N downstaging. On the other hand, 13 cN0 (13%) rectal carcinomas underwent N upstaging after CRT (Table 3). N remaining unchanged/upstaging was significantly more frequent in female patients (P = 0.0347) and in tumors with extensive involvement of the rectum or localization in the lower rectum (P = 0.0183), and low Dworak grade (0-1-2) (P = 0.0013) and was significantly associated with death from rectal cancer (P = 0.0043) and development of recurrence (P = 0.0013) (Table 5).
| Variable | N stage variation | P value | ||
| None | Downstaging | Upstaging | ||
| Sex | ||||
| M | 19 | 35 | 7 | |
| F | 37 | 26 | 6 | 0.0347 |
| Age | ||||
| ≤ 67 yr | 27 | 34 | 5 | |
| > 67 yr | 29 | 27 | 7 | 0.565 |
| Site | ||||
| Upper | 16 | 33 | 7 | |
| Medium | 10 | 11 | 3 | |
| Lower | 25 | 16 | 1 | |
| Extensive | 5 | 1 | 2 | 0.0183 |
| Circumferential spread | ||||
| One-third | 25 | 28 | 8 | |
| Middle | 17 | 17 | 1 | |
| Complete | 14 | 16 | 4 | 0.582 |
| Craniocaudal extension | ||||
| < 3 cm | 10 | 14 | 5 | |
| ≥ 3 cm | 46 | 47 | 8 | 0.27 |
| Distance from the anal verge | ||||
| ≥ 1 cm | 1 | 4 | 1 | |
| < 1 cm | 55 | 57 | 12 | 0.402 |
| Histotype | ||||
| NOS | 49 | 59 | 13 | |
| Mucinous | 7 | 2 | 0 | 0.0851 |
| cT | ||||
| 2 | 4 | 8 | 0 | |
| 3 | 38 | 42 | 12 | |
| 4 | 14 | 11 | 1 | 0.265 |
| Histological grade | ||||
| 1 | 3 | 2 | 0 | |
| 2 | 39 | 50 | 11 | |
| 3 | 14 | 9 | 2 | 0.512 |
| Dworak grade | ||||
| 0 | 14 | 6 | 3 | |
| 1 | 25 | 23 | 8 | |
| 2 | 16 | 13 | 2 | |
| 3 | 0 | 11 | 0 | |
| 4 | 1 | 8 | 0 | 0.0013 |
| Death due to disease | ||||
| No | 30 | 50 | 9 | |
| Yes | 26 | 11 | 4 | 0.0043 |
| Recurrence | ||||
| No | 25 | 47 | 9 | |
| Yes | 31 | 14 | 4 | 0.0013 |
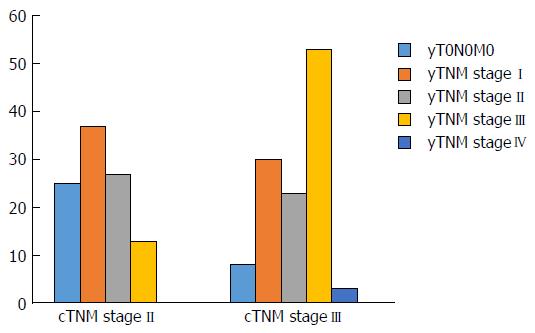
On the whole, 80 (37%) cases showed no change in TNM stage, and 119 (55%) exhibited TNM downstaging, while 16 (8%) underwent TNM upstaging (Figure 3). In 13 cases, upstaging was due to development of nodal metastases, while, in 3 cases, upstaging was related to development of liver (2 cases) and peritoneal (1 case) metastases. Unchanged/increased TNM stage was significantly more frequent in cases showing extensive involvement of the rectum (P = 0.036), mucinous histotype (P = 0.04), cN+ stage (P = 0.001), and low Dworak grade (0-1-2) (P < 0.0001) and was significantly associated with death from rectal cancer (P < 0.0001) and development of recurrence (P = 0.001) (Table 6).
| pTNM stage variation | P value | |||
| Variable | None | Downstaging | Upstaging | |
| Sex | ||||
| M | 53 | 81 | 7 | |
| F | 27 | 41 | 6 | 0.655 |
| Age | ||||
| ≤ 67 yr | 36 | 65 | 5 | |
| > 67 yr | 44 | 57 | 8 | 0.372 |
| Site | ||||
| Upper | 26 | 60 | 7 | |
| Medium | 11 | 18 | 3 | |
| Lower | 35 | 41 | 1 | |
| Extensive | 8 | 3 | 2 | 0.0194 |
| Circumferential spread | ||||
| One-third | 31 | 55 | 8 | |
| Middle | 25 | 36 | 1 | |
| Complete | 24 | 31 | 4 | 0.396 |
| Craniocaudal extension | ||||
| < 3 cm | 17 | 29 | 5 | |
| ≥ 3 cm | 63 | 93 | 8 | 0.4 |
| Distance from the anal verge | ||||
| ≥ 1 cm | 3 | 7 | 1 | |
| < 1 cm | 77 | 115 | 12 | 0.747 |
| Histotype | ||||
| NOS | 70 | 117 | 13 | |
| Mucinous | 10 | 5 | 0 | 0.043 |
| Histological grade | ||||
| 1 | 4 | 2 | 0 | |
| 2 | 60 | 108 | 11 | |
| 3 | 16 | 12 | 2 | 0.135 |
| cT | ||||
| 2 | 4 | 8 | 0 | |
| 3 | 58 | 100 | 12 | |
| 4 | 18 | 14 | 1 | 0.185 |
| cN | ||||
| cN0 | 27 | 58 | 13 | |
| cN+ | 53 | 64 | 0 | < 0.0001 |
| Dworak grade | ||||
| 0 | 20 | 11 | 3 | |
| 1 | 35 | 33 | 8 | |
| 2 | 22 | 26 | 3 | |
| 3 | 2 | 19 | 0 | |
| 4 | 1 | 33 | 0 | < 0.0001 |
| Death due to disease | ||||
| No | 47 | 101 | 9 | |
| Yes | 33 | 11 | 4 | < 0.0001 |
| Recurrence | ||||
| No | 42 | 91 | 9 | |
| Yes | 38 | 31 | 4 | 0.0051 |
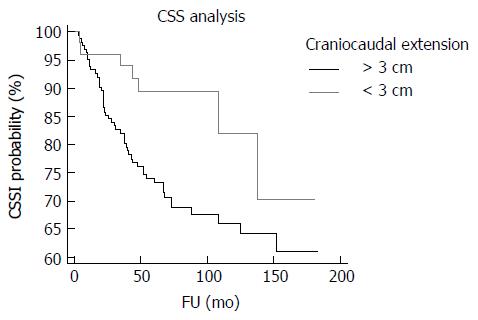
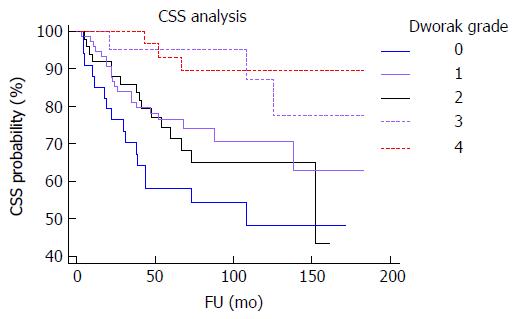
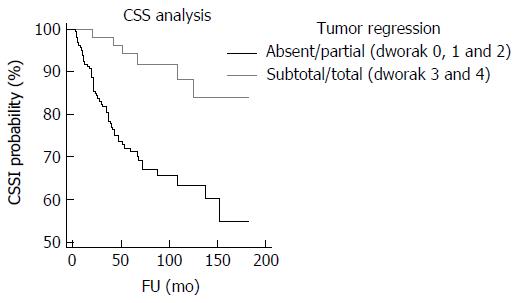
Univariate analyses showed that craniocaudal extension (P = 0.0225; P = 0.022) (Figure 4), cT (P = 0.0215; P = 0.021), yT (P < 0.0001; P = 0.0001), yN (P < 0.0001; P < 0.0001) (Figure 5), yM (P = 0.0164; P = 0.005), ystage (P < 0.0001; P = 0.0001), TNM stage variation (P < 0.0001; P = 0.0005), T stage variation (P < 0.0001; P = 0.001), Dworak regression grade (P = 0.0023; P = 0.023) (Figure 6), tumor regression (P = 0.0006; P = 0.01) (Figure 7) and radial margin (P = 0.0069; P = 0.025) were significant prognostic factors for CSS and DFS, respectively. Craniocaudal extension, yN status, radial margin status and tumor regression were independent variables in the multivariate analyses for CSS. On the other hand, craniocaudal extension (P = 0.0189), yT stage (P = 0.0327) and yN stage (P = 0.001) were significant prognostic parameters for DFS.
In this study, we aimed to identify clinico-pathological variables, which may have significance for predicting recurrence risk and outcome in patients with rectal cancer submitted to neo-adjuvant CRT. Our findings can be summarized as follows. First, we confirmed previous evidence[7-9] that Dworak TRG is a reproducible histological parameter able to discriminate rectal carcinomas at the increased risk of recurrence or adverse outcome. However, the comparison of Kaplan-Meier curves showed that DFS and CSS were only slightly different between cases classified as Dworak 4 and 3 and among Dworak 0, 1 and 2 rectal carcinomas. These results suggested that a two-tiered rather than a five-tiered system could be used to grade tumor regression in surgical specimens. Accordingly, the use of a simplified dichotomized system, in which Dworak 0, 1 and 2 were considered as absent/partial regression and Dworak 3 and 4 as total/subtotal regression not only raised the inter-observer reproducibility but also increased the prognostic relevance of TRG; furthermore, two-tiered TRG was an independent and significant prognostic variable for CSS in the multivariate analysis. Moreover, the advantages of a simplified Dworak system were already demonstrated by Elezkurtaj et al[25] who showed that compared to Dworak’s five tier system, dichotomized TRG has higher correlations with nodal disease and UICC stage. Finally, grouping Dworak grades 3 and 4 together would avoid the need to determine complete pathological response by using step sections, which is highly time consuming and whose benefits appear to be questionable[26]. Of note, a lower response to CRT (corresponding to Dworak TRG 0, 1 or 2) was observed in tumors showing mucinous histotype or poor differentiation in pre-treatment biopsy. Some authors recently suggested a “watch and wait” approach to avoid the side-effects of surgery in patients with rectal cancer showing complete clinical tumor regression after CRT[27]. Our findings indicate that caution should be used when applying a “watch and wait” policy to rectal cancer with mucinous or poorly differentiated histology, as these features are associated with a higher possibility of absent /incomplete tumor regression and T/TNM remaining unchanged/upstaging after CRT.
Seven (3.2%) rectal surgical specimens in our cohort showed acellular mucin pools upon histological examination. Two of those cases were derived from tumors with a mucinous histotype in the pre-treatment endoscopic biopsy. In those cases, acellular mucin deposits could be interpreted as a complete tumor response of mucinous rectal carcinoma. However, in 5 cases we could not find extracellular mucin in the pre-treatment sample, and this finding suggests that acellular mucin deposits in surgical specimens after CRT may represent a radiation effect, as was already hypothesized[28]. Of note, all 7 patients with acellular mucin pools had an unremarkable clinical course, with no recurrence or death from rectal cancer. This outcome supports the College of American Pathologists’ consensus recommendation that acellular mucin pools should not be regarded as residual disease in rectal cancer treated with neo-adjuvant CRT[29]. Accordingly, we did not observe any significant difference in CSS and DFS between cases with acellular mucin pools and cases scored as Dworak 4. Hence, although acellular mucin pools are not mentioned in Dworak TRG[11], we may presume that due to the absence of neoplastic cells, they should be considered as complete tumor regression (Dworak 4). Our results are in line with those reported by other authors[15,17-19]. However, some of the previous analyses could be flawed by the short follow-up of the patients, which was limited to 3 or 5 years[16-18,30], or by sampling restricted to the macroscopically abnormal areas in the rectum[16]. In our cases with acellular mucin pools, the median follow-up time was 71 mo. Additionally, we overcame the possibility of the incorrect assessment of tumor response by embedding the entire surgical specimen and by cutting three additional leveled sections from each paraffin block in cases with questionable residual tumor. In our opinion, cutting step levels is mandatory when seeing acellular mucin pools after CRT. Indeed, this histological feature might represent either a treatment effect or residual tumor. Only careful histological examination of the entire surgical specimen with leveled sections allows for the exclusion of residual tumor. Moreover, a pre-treatment biopsy might not be representative of the entire tumor and may not show mucinous histology. Additionally, mucinous adenocarcinoma may show only rare foci of neoplastic cells that can be hard to identify.
Our results also showed that yN status is an independent predictor of shorter DFS and CSS in patients with rectal cancer submitted to neo-adjuvant CRT. Interestingly, 13% of patients with cN0 status had yN+ after therapy. This result highlights the importance of pathological examination after CRT to ensure appropriate staging and therapy of patients, as was already underscored[31]. Since N remaining unchanged/upstaging was significantly more frequent in tumors showing extensive involvement of the rectum or localization in the lower rectum, surgical resection and follow-up histological examination might have particular relevance in rectal carcinomas showing those features.
Tumor craniocaudal extension higher than 3 cm was an additional significant and independent prognostic parameter associated with worse DFS and CSS in our analyses. Since this feature was not associated with TNM variation or with tumor regression after therapy, we may speculate that cancers with higher craniocaudal extension might have had occult metastases undetectable at the moment of yTNM staging. However, our data suggest that particular attention should be given in the treatment and follow up of tumors existing at a higher extent in the rectum.
Finally, T and N remaining unchanged/upstaging was significantly more frequent in female patients. Although we are not able to explain this phenomenon, the role of hormones in the biological aggressiveness of rectal cancer should be better investigated in the future.
In conclusion, we confirmed the reproducibility and prognostic value of Dworak TRG in rectal cancer treated with neo-adjuvant CRT; however, our data suggested that a simplified two-tiered system may be used with better results. Acellular mucin pools in the surgical specimen may be considered as complete tumor regression, but step levels are warranted in those cases to exclude residual mucinous carcinoma. Finally, particular attention should be paid to rectal carcinomas showing the mucinous histotype, poor differentiation and extensive/lower involvement of the rectum due to the higher probability of absent/partial regression and TNM remaining unchanged/upstaging in those cases.
Patients with locally advanced rectal cancer are currently submitted to neo-adjuvant chemo-radiotherapy (CRT) to improve resectability and sphincter preservation and to decrease the probability of local recurrence. Post-treatment Tumor Node Metastasis stage (yTNM) and Tumor Regression Grade (TRG) are used to measure tumor response in surgical specimens obtained after CRT. Several systems, such as the Dworak system, are used to score tumor regression. However, all these types of systems suffer from low interobserver reproducibility.
A major issue in the interpretation of tumor histological response to CRT is related to the presence of acellular mucin pools. It is not clear whether these pools should be interpreted as complete regression. Finally, the value of clinico-pathological factors in predicting tumor regression after neo-adjuvant CRT remains to be determined.
This article shows that, compared to the currently used four-tiered system a simplified two-tiered, Dworak TRG is preferable for determining score response to CRT because of its higher reproducibility and prognostic significance. Mucinous histotype and poor differentiation are significantly associated with lower response to neo-adjuvant CRT, as highlighted by higher frequency of absent/limited histological regression and TNM remaining unchanged/upstaging in those tumors.
A simplified two-tiered, Dworak TRG could be used in routine practice to increase reproducibility and prognostic relevance of TRG. A watch and wait approach should be used with caution in rectal carcinomas showing mucinous histotype and poor differentiation in endoscopic pre-treatment biopsy due to their significant association with low response to pre-operative CRT.
TRG: measure of the histological response to neo-adjuvant chemoradiotherapy, and it can be assessed by using different systems, such as that proposed by Dworak and colleagues in 1997, which is based on the presence of tumor cells and fibrosis in a surgical specimen. Acellular mucin pools: the presence of mucin deposits devoid of neoplastic cells in rectal surgical specimen after neo-adjuvant CRT that need to be differentiated from residual mucinous carcinoma.
A major finding reported in this study is the possible use of a two-tiered system to score TRG after neo-adjuvant chemoradiotherapy. Strength of this study is the high number (215 cases) of rectal carcinomas analyzed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu XJ, Popp CS, Shi XY S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] |
| 2. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. [PubMed] |
| 3. | Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027-1038. [PubMed] |
| 4. | Stipa F, Chessin DB, Shia J, Paty PB, Weiser M, Temple LK, Minsky BD, Wong WD, Guillem JG. A pathologic complete response of rectal cancer to preoperative combined-modality therapy results in improved oncological outcome compared with those who achieve no downstaging on the basis of preoperative endorectal ultrasonography. Ann Surg Oncol. 2006;13:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121-1130. [PubMed] |
| 6. | Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, Paty PB, Goodman KA, Wu A, Gollub M. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106:pii: dju248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Kim SH, Chang HJ, Kim DY, Park JW, Baek JY, Kim SY, Park SC, Oh JH, Yu A, Nam BH. What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy? Cancer Res Treat. 2016;48:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Zhang LN, Xiao WW, Xi SY, OuYang PY, You KY, Zeng ZF, Ding PR, Zhang HZ, Pan ZZ, Xu RH. Pathological Assessment of the AJCC Tumor Regression Grading System After Preoperative Chemoradiotherapy for Chinese Locally Advanced Rectal Cancer. Medicine (Baltimore). 2016;95:e2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 11. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [PubMed] |
| 12. | Edge S, Byrd DR, Carducci MA, Compton CA. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2009. . |
| 13. | MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J Clin Pathol. 2012;65:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Chetty R, Gill P, Govender D, Bateman A, Chang HJ, Deshpande V, Driman D, Gomez M, Greywoode G, Jaynes E. International study group on rectal cancer regression grading: interobserver variability with commonly used regression grading systems. Hum Pathol. 2012;43:1917-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Smith KD, Tan D, Das P, Chang GJ, Kattepogu K, Feig BW, Skibber JM, Rodriguez-Bigas MA. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010;251:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | de Campos-Lobato LF, Dietz DW, Stocchi L, Vogel JD, Lavery IC, Goldblum JR, Skacel M, Pelley RJ, Kalady MF. Clinical implications of acellular mucin pools in resected rectal cancer with pathological complete response to neoadjuvant chemoradiation. Colorectal Dis. 2012;14:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Shia J, McManus M, Guillem JG, Leibold T, Zhou Q, Tang LH, Riedel ER, Weiser MR, Paty PB, Temple LK. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2011;35:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Bhatti AB, Akbar A, Khattak S, Kazmi AS, Jamshed A, Syed AA. Impact of acellular mucin pools on survival in patients with complete pathological response to neoadjuvant treatment in rectal cancer. Int J Surg. 2014;12:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Cienfuegos JA, Baixauli J, Rotellar F, Arredondo J, Sola JJ, Arbea L, Pastor C, Hernández-Lizoáin JL. Clinical significance of cellular and acellular mucin pools in rectal carcinoma following preoperative chemoradiotherapy. Clin Transl Oncol. 2016;18:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Williams JT, Quirke P, Shepherd NA. Dataset for Colorectal Cancer. 2nd edn. London: The Royal College of Pathologists, 2007. Updated September 2007. Available from: http://www.rcpath.org/Resources/RCPath/MigratedResources/Documents/G/G049-ColorectalDataset-Sep07.pdf. |
| 21. | Hamilton SR, Volgelstein B, Kudo S, Riboli E, Nakamura S, Hainaut P, Rubio CA, Sobin LH, Fogt F, Winawer SJ. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000: 110-111. . |
| 22. | Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI, Quirke P, Riboli E, Sobin LH Bosman FT, Boffetta P. Carcinoma of the colon and rectum. In: Bosman T, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2010: 138-139. . |
| 23. | Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. Chichester, West Sussex, UK: Wiley Blackwell, 2009: 100¨C105. . |
| 24. | DeCaria K, Rahal R, Niu J, Lockwood G, Bryant H. Rectal cancer resection and circumferential margin rates in Canada: a population-based study. Curr Oncol. 2015;22:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Elezkurtaj S, Moser L, Budczies J, Müller AJ, Bläker H, Buhr HJ, Dietel M, Kruschewski M. Histopathological regression grading matches excellently with local and regional spread after neoadjuvant therapy of rectal cancer. Pathol Res Pract. 2013;209:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Park SY, Chang HJ, Kim DY, Jung KH, Kim SY, Park JW, Oh JH, Lim SB, Choi HS, Jeong SY. Is step section necessary for determination of complete pathological response in rectal cancer patients treated with preoperative chemoradiotherapy? Histopathology. 2011;59:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 28. | Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol. 2004;204:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, Halling K, Frankel W, Jessup J, Kakar S. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 30. | Lim SB, Hong SM, Yu CS, Hong YS, Kim TW, Park JH, Kim JH, Kim JC. Prevalence and clinical significance of acellular mucin in locally advanced rectal cancer patients showing pathologic complete response to preoperative chemoradiotherapy. Am J Surg Pathol. 2013;37:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Swellengrebel HA, Bosch SL, Cats A, Vincent AD, Dewit LG, Verwaal VJ, Nagtegaal ID, Marijnen CA. Tumour regression grading after chemoradiotherapy for locally advanced rectal cancer: a near pathologic complete response does not translate into good clinical outcome. Radiother Oncol. 2014;112:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |