Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1406
Peer-review started: December 12, 2016
First decision: December 19, 2016
Revised: December 29, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 28, 2017
Processing time: 77 Days and 19.4 Hours
To evaluate the efficiency and safety of hepatic artery infusion chemotherapy (HAIC) using raltitrexed or 5-fluorouracil for colorectal cancer (CRC) liver metastasis (CRCLM).
A retrospective analysis of patients with unresectable CRCLM who failed systemic chemotherapy and were subsequently treated with HAIC at our institute from May 2013 to April 2015 was performed. A total of 24 patients were treated with 5-fluorouracil, and 18 patients were treated with raltitrexed.
The median survival time (MST) from diagnosis of CRC was 40.8 mo in the oxaliplatin plus raltitrexed (TOMOX) arm and 33.5 mo in the oxaliplatin plus 5-fluorouracil (FOLFOX) arm (P = 0.802). MST from first HAIC was 20.6 mo in the TOMOX arm and 15.4 mo in the FOLFOX arm (P = 0.734). Median progression-free survival (PFS) from first HAIC was 4.9 mo and 6.6 mo, respectively, in the TOMOX arm and FOLFOX arm (P = 0.215). Leukopenia (P = 0.026) was more common in the FOLFOX arm, and hepatic disorder (P = 0.039) was more common in the TOMOX arm. There were no treatment-related deaths in the TOMOX arm and one treatment-related death in the FOLFOX arm. Analysis of prognostic factors indicated that response to HAIC was a significant factor related to survival.
No significant difference in survival was observed between the TOMOX and FOLFOX arms. HAIC treatment with either TOMOX or FOLFOX was demonstrated as an efficient and safe alternative choice.
Core tip: Our study shows that hepatic artery infusion chemotherapy (HAIC) with either TOMOX (oxaliplatin plus raltitrexed) or FOLFOX (oxaliplatin plus 5-fluorouracil) was proven to be an efficient and safe alternative choice for patients with chemotherapy refractory colorectal cancer liver metastasis and no significant difference in survival was found between these two treatments. Cox univariate analysis shows that response to HAIC was a significant predictive factor.
- Citation: Guo JH, Zhang HY, Gao S, Zhang PJ, Li XT, Chen H, Wang XD, Zhu X. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol 2017; 23(8): 1406-1411
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1406
Colorectal cancer is the third leading cause of cancer death and has the third leading incidence of new cases in Western countries[1]. The situation in China is similar; there were 376.3 thousand new colorectal cancer cases in 2015, and colorectal cancer was the fifth leading cause of cancer death[2]. Approximately 30%-50% of patients develop liver metastasis, and no more than 20% of liver metastasis patients are candidates for liver resection[3,4]. Chemotherapy is the primary treatment for advanced colorectal cancer. The efficiency and survival benefit of standard first- or second-line systemic therapy have been improved by the combination of targeted therapy[5,6], and the overall survival (OS) after effective first-line therapy is nearly 30 mo[7-9]. However, the survival of chemotherapy refractory patients, who failed previous systemic treatment, is expected to improve. Third-line chemotherapy could result in an OS period of 9.3 mo[10]. Alternative treatments, such as transarterial chemoembolization (TACE) and hepatic artery infusion chemotherapy (HAIC), are greatly needed.
HAIC with FOLFOX [oxaliplatin plus 5-fluorouracil (5-Fu)] in patients with CRCLM has also been demonstrated as a feasible and low-toxicity treatment, with a local overall disease control rate of 50%-79.2%[11,12]. However, 5-Fu should be administered intra-arterially for approximately 44 h, and a higher incidence of catheter thrombosis and catheter-associated infection is reported[13]. As a specific inhibitor of thymidylate synthase, raltitrexed has been used in CRC patients and could be infused in approximately 1 h. Several previous studies have shown that TOMOX (oxaliplatin plus raltitrexed) showed efficiency similar to other traditional first-line treatments in CRC patients and was associated with less neutropenia and gastrointestinal toxicity and uncommon cardiotoxicity[14-16]. However, studies concerning HAIC with TOMOX are rare. Khouri et al[3] examined 17 patients who underwent HAIC with TOMOX, and the treatment was demonstrated as a safe alternative choice. The goal of this retrospective study was to report a head-to-head study comparing the TOMOX and FOLFOX arms in CRCLM patients treated at our center.
From May 2013 to April 2015, 42 patients were treated with oxaliplatin-based HAIC at our center. All of the patients were histologically confirmed with colorectal adenocarcinoma with unresectable liver metastasis and failed two lines of systemic chemotherapy. The treatment criteria for HAIC were: ECOG performance status no more than 2 points; life expectancy ≥ 3 mo; tumor involvement less than 70% of liver volume; and adequate liver and renal dysfunction (total bilirubin serum levels < 3 mg/dL, serum albumin level > 20 g/L, and serum creatinine level < 2 mg/dL). Patients with extrahepatic metastases were included if their main lesion remained in the liver.
The Seldinger technique was used to access the femoral artery after the achievement of local anesthesia. Then, arteriography was routinely performed prior to chemoembolization to gather information for the abdominal aorta and celiac trunk. Subsequently, a coaxial catheter (Renegade Hi Flo, Boston Scientific, United States/Stride ASAHI INTECC, Japan) was inserted into the hepatic artery and subsegmental arteries. According to tumor stain, Spongostan particles (Jinling, Nanjing, China) and iodized oil (Lipiodol; Laboratoire Andre Guerbet, Aulnaysous- Bois, France) mixed with 20-40 mg epirubicin hydrochloride (Main Luck Pharmaceutical, Shenzhen, China) were injected. The temporary indwelling catheter was inserted into the hepatic artery until the end of HAIC. HAIC was performed via the catheter with oxaliplatin (Hengrui Medicine Co., Ltd., Jiangsu, China) administered at 85 mg/m2 in 4 h, 5-Fu (Jinyao aminoacid Co., Ltd., Tianjing, China) administered at 2000 mg/m2 in approximately 44 h, CF (Hengrui Medicine Co., Ltd. Jiangsu China) administered at 200 mg/m2 in 2-4 h via the peripheral vein, and raltitrexed (Tianqing Pharmaceutical Co., Ltd., Nanjing, China) administered at 3 mg/m2 in approximately 1 h. At the end of perfusion, the catheter was removed every cycle.
HAIC was regularly applied every 3 wk, until the patient died or liver function was Child-Pugh C or disease progressed. Enhanced computed tomography or magnetic resonance imaging and laboratory tests were regularly performed, and all patients were followed until death or loss to follow-up. Objective response rate (ORR) was evaluated using Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1, and adverse reactions were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) 2.0. Peripheral neuropathy was graded according to a modified Levi Scale.
OS after diagnosis was calculated from the date of diagnosis of CRC to the date of death or last follow-up time, OS after first HAIC was calculated from the date of first HAIC to the date of death or last follow-up time, and PFS was calculated from the date of the initiation of therapy to the date of disease progression. A biomedical statistician conducted the statistical review in the present study. The SPSS software program (version 19; SPSS, Chicago, Illinois) was used for the analyses. GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) was used to generate the charts. For all tests, a P value < 0.05 was defined as significant. Student’s t-test was used to analyze continuous variables. These variables were reported as the means ± SD if normally distributed or as a median and range if skewed. The χ2 test was used to analyze categorical variables. These variables were reported as a proportion (%) of the overall cohort. The Kaplan-Meier method was used to approximate the PFS and OS, and the significance of survival differences between the TOMOX and FOLFOX arms was determined using the log-rank test.
There were 18 patients in the TOMOX arm and 24 patients in the FOLFOX arm. The baseline characteristics of the patients are shown in Table 1. The baseline demographics were similar between the two treatment groups, with no significant imbalances in sex, age, primary tumor site, time of liver metastasis, KRAS mutation rate, extrahepatic metastasis, or additional radiofrequency ablation. Patients in the TOMOX arm received a median of 2.2 cycles of treatment, and those in the FOLFOX arm received a median of 2.1 cycles of treatment.
| Overall cohort (n = 42) | TOMOX (n = 18) | FOLFOX (n = 24) | P value | |
| Gender | 0.700 | |||
| Male | 29 | 13 | 16 | |
| Female | 13 | 5 | 8 | |
| Age at first TACE (yr) | 59 ± 10.7 | 60 ± 9.1 | 58 ± 11.8 | 0.473 |
| Primary tumor site | 0.601 | |||
| Right hemicolon | 10 | 5 | 5 | |
| Left hemicolon | 32 | 19 | 13 | |
| Time to liver metastasis | 0.508 | |||
| Synchronous | 28 | 11 | 17 | |
| Metachronous | 14 | 7 | 7 | |
| Primary tumor grade | 0.639 | |||
| Poor | 6 | 3 | 3 | |
| Well to moderate | 36 | 15 | 21 | |
| Genetic condition | 0.459 | |||
| KRAS mutation | 8 | 5 | 3 | |
| KRAS wild type | 21 | 8 | 13 | |
| Unknown | 13 | 5 | 8 | |
| Extrahepatic metastasis | 0.927 | |||
| Present | 27 | 12 | 15 | |
| Absent | 15 | 6 | 9 | |
| Combined with other local treatments | 0.209 | |||
| Yes | 10 | 6 | 4 | |
| No | 32 | 12 | 20 |
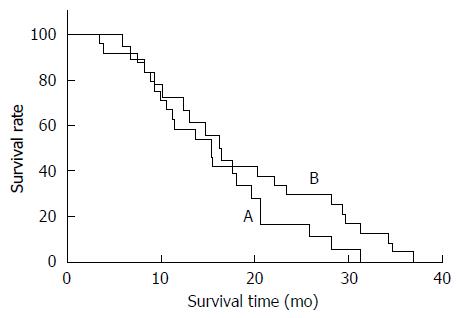
With a median follow-up period of 18 mo, the OS after the first HAIC in the FOLFOX and TOMOX arms was 15.4 and 20.6 mo (P = 0.734), respectively. The PFS in the FOLFOX and TOMOX arms was 6.6 and 4.0 mo (P = 0.215), respectively (Figure 1). The response rates of the two different treatment groups are shown in the Table 2. The overall response rate was 29.2% in the FOLFOX arm and 11.1% in the TOMOX arm, and no significant difference was observed between the FOLFOX and TOMOX groups (P = 0.158).
| Response | Treatment group | P value | |
| FOLFOX (n = 24) | TOMOX (n = 18) | ||
| Partial response | 7 (29.2) | 2 (11.1) | 0.158 |
| Stable disease | 14 (58.3) | 11 (61.1) | 0.856 |
| Progressive disease | 3 (12.5) | 5 (27.8) | 0.734 |
Cox univariate analysis (Table 3) showed that the response to HAIC was a predictive factor for prognosis. However, age, histology grade, primary tumor site, serum tumor markers, and extrahepatic metastasis showed no significance as predictive factors.
| Factor | Univariate analysis | ||
| HR | 95%CI | P value | |
| TOMOX/FOLFOX | 0.877 | 0.410-1.876 | 0.736 |
| Male sex | 0.915 | 0.411-2.035 | 0.827 |
| Age (> 60/60 yr) | 0.758 | 0.353-1.627 | 0.477 |
| Histology (poor/well and moderate) | 1.768 | 0.686-4.554 | 0.238 |
| Primary tumor site (left/right hemicolon) | 0.715 | 0.285-1.797 | 0.476 |
| Serum CA19-9 (high/normal) | 1.725 | 0.803-3.706 | 0.162 |
| Serum CA72-4 (high/normal) | 1.325 | 0.536-3.278 | 0.542 |
| Serum CEA (high/normal) | 1.339 | 0.463-3.873 | 0.590 |
| Extrahepatic metastasis (present/absent) | 1.220 | 0.550-2.706 | 0.624 |
| Time to liver metastasis (synchronous/metachronous) | 1.281 | 0.560-2.932 | 0.558 |
| Response to TACE | 0.047 | ||
| PD | 1.000 | 1.000 | |
| SD | 0.275 | 0.081-0.931 | |
| PR | 0.272 | 0.095-0.783 | |
All patients were evaluated for toxicity. The toxicity of the two groups is shown in Table 4. The most common adverse events were transient elevation of serum liver enzymes and bilirubin and abdominal pain. The transient elevation of serum liver enzymes was more frequent in the TOMOX arm than in the FOLFOX arm (100% vs 79%, P = 0.039). Hematologic adverse events were more frequent in the FOLFOX arm than in the TOMOX arm (leukopenia: 16% vs 50%, P = 0.026; anemia: 39% vs 46%, P = 0.212; and thrombocytopenia: 44% vs 54%, P = 0.533). No significant differences were observed in fever, asthenia, nausea and vomiting and neuropathy between these two treatment groups. Treatment associated cardiotoxicity was not observed in either group. One treatment-related death, diagnosed as neutropenic sepsis, occurred in the FOLFOX arm. No treatment-related death was observed in the TOMOX arm.
| Adverse event | TOMOX (n = 18) | FOLFOX (n = 24) | P value | ||
| All grade | Severe | All grade | Severe | ||
| Hematological | |||||
| Anemia | 7 (39) | 11 (46) | 0.212 | ||
| Leucopenia | 3 (16) | 12 (50) | 1 (4) | 0.026 | |
| Neutropenia | 1 (5) | 6 (25) | 1 (4) | 0.094 | |
| Thrombocytopenia | 8 (44) | 13 (54) | 3 (12) | 0.533 | |
| Nonhematological | |||||
| Elevation of liver enzymes | 18 (100) | 9 (50) | 19 (79) | 7 (29) | 0.039 |
| Elevation of bilirubin | 17 (94) | 3 (17) | 23 (95) | 4 (17) | 0.834 |
| Nausea/vomiting | 14 (78) | 17 (71) | 0.839 | ||
| Asthenia | 13 (72) | 12 (50) | 0.414 | ||
| Neuropathy | 5 (28) | 7 (29) | 1 (4) | 0.921 | |
| Pain | 14 (78) | 7 (39) | 19 (79) | 13 (54) | 0.914 |
| Fever | 6 (33) | 11 (46) | 0.558 | ||
Without an efficient treatment, systemic chemotherapy refractory patients show a median OS of 3.5 mo[17]. HAIC has been demonstrated as an alternative choice for advanced CRC patients. Most studies report the efficiency and survival data of HAI with FOLFOX, while reports concerning HAI with TOMOX are rare. Raltitrexed has been demonstrated as a considerable first-line treatment for patients with advanced CRC. Herein, we present the first head-to-head study comparing HAI with TOMOX or FOLFOX in systemic chemotherapy refractory CRC patients.
The median OS after first HAIC in the present study was 15.4 mo in the FOLFOX arm and 20.6 mo in the TOMOX arm, which was favorable compared with that of the third-line systemic chemotherapy, which achieved a median OS of 9.3 mo[10]. When TOMOX was used as a first-line treatment, the ORR was 16%-50%, and the median PFS was 5-11 mo[18-20]. Among all patients in the present study who failed in previous systemic chemotherapy, the ORR (11.1%) and median PFS (4.9 mo) were relatively low. The ORR in the FOLFOX arm was 29.2% with a median PFS of 6.6 mo, consistent previous studies[11,21,22]. Similarly, the median OS of 15.4 mo in the present study is consistent with the 11 and 18.3 mo reported in two previous studies[11,21].
The most common adverse events were the transient elevation of serum liver enzymes and bilirubin and abdominal pain. These common adverse events could be sufficiently controlled by efficient treatments. Similar to previous studies, the incidence of leukopenia grade was significantly higher in the FOLFOX arm, and the elevation of transient hepatic enzymes was significantly higher in the TOMOX arm. The TOMOX arm had no treatment- related deaths, while the FOLFOX arm had one case of neutropenic sepsis. These findings suggest that HAIC with TOMOX could represent tolerable treatments for refractory CRC patients. Survival predictor analysis suggested that early tumor response is a meaningful predictor for patients receiving oxaliplatin-based HAIC. Other factors, including age, primary tumor site, and serum tumor markers, did not show significant difference, partly reflecting the limited sample size in the present study.
The limitation of the present study is a single-center retrospective study with a limited sample size. We could not avoid some bias for the evaluation of clinical outcome and the incomplete patient data. However, the present study was the first to compare the efficiency, survival data, and toxicity of HAIC with TOMOX and FOLFOX in advanced CRC patients, and the results will provide new directions for clinical practice.
Although liver metastasis develops in approximately 30%-50% of colorectal cancer patients, efficient treatments for advanced colorectal cancer are rare. Third-line chemotherapy confers only a survival period of 9.3 mo. Alternative treatment, such as hepatic artery infusion, is greatly needed. Previous studies have shown that hepatic artery infusion with oxaliplatin and 5-Fu is a safe and efficient choice for these patients; however, 5-Fu should be administered intra-arterially for approximately 44 h and is associated with a higher incidence of catheter thrombosis and infection. Raltitrexed, which could be infused in one hour, is a specific inhibitor of thymidylate synthase and has been reported as an efficient agent in colorectal cancer.
The authors propose that hepatic artery infusion with raltitrexed and oxaliplatin (TOMOX) is a safe and efficient treatment for patients with colorectal cancer liver metastasis. Herein, we provide support for this hypothesis, showing similar response rates and survival data between the FOLFOX and TOMOX arms.
Previous studies have shown that raltitrexed is a considerable first-line treatment for patients with advanced colorectal cancer. The present study is the first head-to-head study comparing hepatic artery infusion (HAI) with TOMOX or FOLFOX in systemic chemotherapy refractory colorectal cancer patients.
Patients with colorectal cancer liver metastasis who failed systemic chemotherapy were treated with hepatic artery infusion with TOMOX or FOLFOX.
HAI chemotherapy is designed to improve the chemotherapy benefits for liver cancer by increasing the amount of chemotherapy delivered to the site of the tumor. Chemotherapy is dispensed from a specialized infusion system in which a catheter is placed into the hepatic artery to directly deliver the chemotherapy to the liver.
This study, concerning hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis, is interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim ES, Raff E S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 3. | Khouri C, Guiu B, Cercueil JP, Chauffert B, Ladoire S, Ghiringhelli F. Raltitrexed and oxaliplatin hepatic arterial infusion for advanced colorectal cancer: a retrospective study. Anticancer Drugs. 2010;21:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, Adam R, Arvidsson D, Carrato A, Georgoulias V. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037-2045. [PubMed] |
| 5. | Masi G, Salvatore L, Boni L, Loupakis F, Cremolini C, Fornaro L, Schirripa M, Cupini S, Barbara C, Safina V. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. 2015;26:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Pantelic A, Markovic M, Pavlovic M, Jancic S. Cetuximab in third-line therapy of patients with metastatic colorectal cancer: A single institution experience. J BUON. 2016;21:70-79. [PubMed] |
| 7. | Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866-4875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2200] [Article Influence: 104.8] [Reference Citation Analysis (1)] |
| 9. | Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 795] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 10. | Chong G, Dickson JL, Cunningham D, Norman AR, Rao S, Hill ME, Price TJ, Oates J, Tebbutt N. Capecitabine and mitomycin C as third-line therapy for patients with metastatic colorectal cancer resistant to fluorouracil and irinotecan. Br J Cancer. 2005;93:510-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Melichar B, Ferko A, Krajina A, Rousková L, Dvorák J, Svébisova H, Neoral C, Köcher M, Malirová E, Paral J. Hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin in patients with liver metastases from colorectal carcinoma. J BUON. 2012;17:677-683. [PubMed] |
| 12. | Arai Y, Aoyama T, Inaba Y, Okabe H, Ihaya T, Kichikawa K, Ohashi Y, Sakamoto J, Oba K, Saji S. Phase II study on hepatic arterial infusion chemotherapy using percutaneous catheter placement techniques for liver metastases from colorectal cancer (JFMC28 study). Asia Pac J Clin Oncol. 2015;11:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Barni S, Ghidini A, Coinu A, Borgonovo K, Petrelli F. A systematic review of raltitrexed-based first-line chemotherapy in advanced colorectal cancer. Anticancer Drugs. 2014;25:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kelly C, Bhuva N, Harrison M, Buckley A, Saunders M. Use of raltitrexed as an alternative to 5-fluorouracil and capecitabine in cancer patients with cardiac history. Eur J Cancer. 2013;49:2303-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Gravalos C, Salut A, García-Girón C, García-Carbonero R, León AI, Sevilla I, Maurel J, Esteban B, García-Rico E, Murias A. A randomized phase II study to compare oxaliplatin plus 5-fluorouracil and leucovorin (FOLFOX4) versus oxaliplatin plus raltitrexed (TOMOX) as first-line chemotherapy for advanced colorectal cancer. Clin Transl Oncol. 2012;14:606-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Tellez C, Benson AB, Lyster MT, Talamonti M, Shaw J, Braun MA, Nemcek AA, Vogelzang RL. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Chiara S, Nobile MT, Tomasello L, Acquati M, Taveggia P, Murolo C, Percivale P, Rosso R. Phase II trial of irinotecan and raltitrexed in chemotherapy-naive advanced colorectal cancer. Anticancer Res. 2005;25:1391-1396. [PubMed] |
| 19. | Martoni A, Mini E, Pinto C, Gentile AL, Nobili S, Dentico P, Marino A, Scicolone S, Angelelli B, Mazzei T. Oxaliplatin plus raltitrexed in the treatment of patients with advanced colorectal cancer: a phase II study. Anticancer Res. 2003;23:687-691. [PubMed] |
| 20. | Feliu J, Castañón C, Salud A, Mel JR, Escudero P, Pelegrín A, López-Gómez L, Ruiz M, González E, Juárez F. Phase II randomised trial of raltitrexed-oxaliplatin vs raltitrexed-irinotecan as first-line treatment in advanced colorectal cancer. Br J Cancer. 2005;93:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Neyns B, Van Nieuwenhove Y, Aerts M, Fontaine C, Vermeij J, Schallier D, Decoster L, De Mey J, Vandenbroucke F, Hoorens A. Hepatic arterial infusion of oxaliplatin and L-folinic acid-modulated 5-fluorouracil for colorectal cancer liver metastases. Anticancer Res. 2006;26:611-619. [PubMed] |
| 22. | Del Freo A, Fiorentini G, Sanguinetti F, Muttini MP, Pennucci C, Mambrini A, Pacetti P, Della Seta R, Lombardi M, Torri T. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo. 2006;20:743-746. [PubMed] |