Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1375
Peer-review started: October 13, 2016
First decision: December 2, 2016
Revised: December 20, 2016
Accepted: January 17, 2017
Article in press: January 17, 2017
Published online: February 28, 2017
Processing time: 138 Days and 5.4 Hours
To investigate the therapeutic effect of hydrogen-rich water (HRW) on inflammatory bowel disease (IBD) and to explore the potential mechanisms involved.
Male mice were randomly divided into the following four groups: control group, in which the mice received equivalent volumes of normal saline (NS) intraperitoneally (ip); dextran sulfate sodium (DSS) group, in which the mice received NS ip (5 mL/kg body weight, twice per day at 8 am and 5 pm) for 7 consecutive days after IBD modeling; DSS + HRW group, in which the mice received HRW (in the same volume as the NS treatment) for 7 consecutive days after IBD modeling; and DSS + HRW + ZnPP group, in which the mice received HRW (in the same volume as the NS treatment) and ZnPP [a heme oxygenase-1 (HO-1) inhibitor, 25 mg/kg] for 7 consecutive days after IBD modeling. IBD was induced by feeding DSS to the mice, and blood and colon tissues were collected on the 7th d after IBD modeling to determine clinical symptoms, colonic inflammation and the potential mechanisms involved.
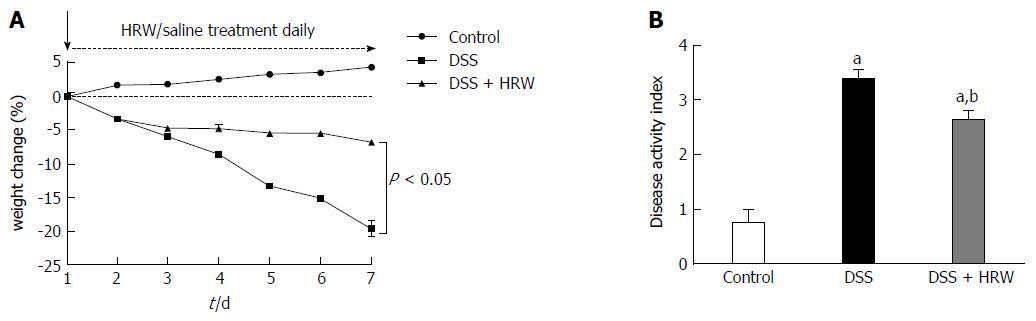
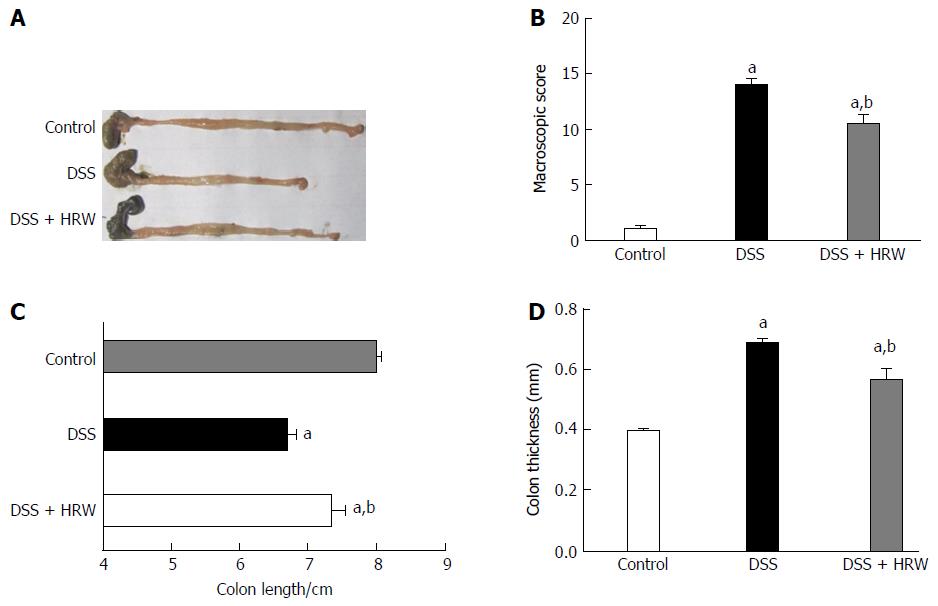
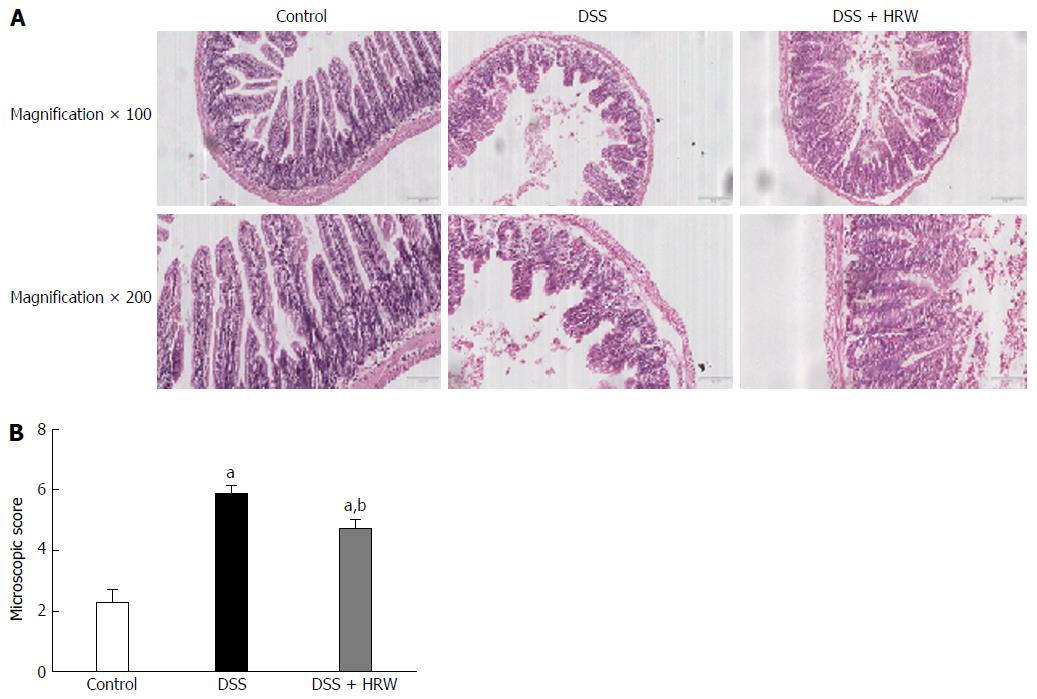
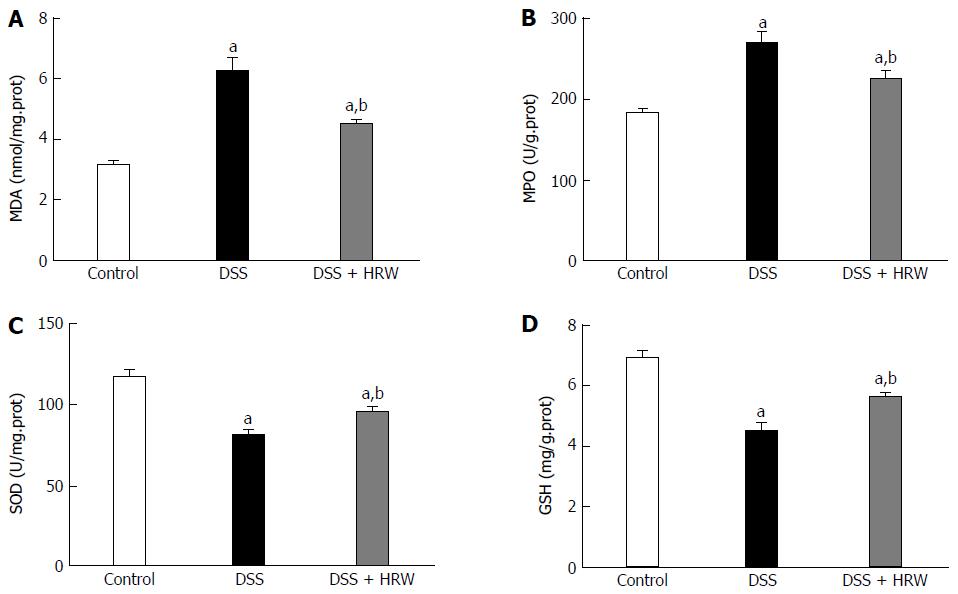
The DSS + HRW group exhibited significantly attenuated weight loss and a lower extent of disease activity index compared with the DSS group on the 7th d (P < 0.05). HRW exerted protective effects against colon shortening and colonic wall thickening in contrast to the DSS group (P < 0.05). The histological study demonstrated milder inflammation in the DSS + HRW group, which was similar to normal inflammatory levels, and the macroscopic and microcosmic damage scores were lower in this group than in the DSS group (P < 0.05). The oxidative stress parameters, including MDA and MPO in the colon, were significantly decreased in the DSS + HRW group compared with the DSS group (P < 0.05). Simultaneously, the protective indicators, superoxide dismutase and glutathione, were markedly increased with the use of HRW. Inflammatory factors were assessed, and the results showed that the DSS + HRW group exhibited significantly reduced levels of TNF-α, IL-6 and IL-1β compared with the DSS group (P < 0.05). In addition, the pivotal proteins involved in endoplasmic reticulum (ER) stress, including p-eIF2α, ATF4, XBP1s and CHOP, were dramatically reduced after HRW treatment in contrast to the control group (P < 0.05). Furthermore, HRW treatment markedly up-regulated HO-1 expression, and the use of ZnPP obviously reversed the protective role of HRW. In the DSS + HRW + ZnPP group, colon shortening and colonic wall thickening were significantly aggravated, and the macroscopic damage scores were similar to those of the DSS + HRW group (P < 0.05). The histological study also showed more serious colonic damage that was similar to the DSS group.
HRW has a significant therapeutic potential in IBD by inhibiting inflammatory factors, oxidative stress and ER stress and by up-regulating HO-1 expression.
Core tip: Inflammatory bowel disease (IBD) is a chronic and relapsing disease primarily caused by the production of pro-inflammatory cytokines and leukocyte infiltration, resulting in structural and functional damage to the bowel. Hydrogen has obvious anti-oxidative and anti-inflammatory effects. We launched a study to investigate the protective role of hydrogen-rich water (HRW) on IBD in mice. The present study found that HRW has a significant therapeutic potential in IBD by inhibiting inflammatory factors, oxidative stress and endoplasmic reticulum stress and by up-regulating heme oxygenase-1 expression.
- Citation: Shen NY, Bi JB, Zhang JY, Zhang SM, Gu JX, Qu K, Liu C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J Gastroenterol 2017; 23(8): 1375-1386
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1375
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic and relapsing disease primarily caused by the production of pro-inflammatory cytokines and leukocyte infiltration, resulting in structural and functional damage to the bowel. It is associated with environmental factors, genetics, microbial factors and so on[1-3]. The major symptoms of IBD include inflammation of the colon, abdominal pain, altered visceral sensation, diarrhea, rectal bleeding, weakness and weight loss[4]. CD is often located in the terminal ileum and/or the colon and characterized by formation of non-caseating granulomas, which are involved in transmural and discontinuous inflammation in the mucosa. In contrast, UC is a colon disorder in which inflammation is restricted to the mucosal and submucosal areas, initially affecting the rectum, but it may extend continuously and diffusely throughout the colon[5].
The major therapeutic goals in IBD patients are the alleviation of inflammation and the attenuation of IBD symptoms, mainly abdominal pain and altered bowel movements. The current range of treatments for IBD covers both conventional and biological therapies. Conventional therapy includes the use of anti-inflammatory drugs, immunosuppressive agents, antibiotics, and probiotics; biological therapy mainly includes the use of different anti-TNF-α agents, and a plethora of other novel biological agents[6]. Dextran sulfate sodium (DSS)-induced IBD in mice is a classical mouse IBD model that is accepted worldwide. The mechanism of DSS-induced colitis is mainly due to the direct toxicity to the colonic epithelial cells, subsequently increasing the permeability of the intestinal mucosa and allowing the transport of luminal bacterial products from the bowel lumen to the submucosal tissue[7,8].
Molecular hydrogen, which has been explored as a new medical gas over the last ten years, is a potent anti-oxidative, anti-apoptotic, and anti-inflammatory agent and an ideal therapy for many diseases[9]. The benefit of hydrogen as a novel anti-oxidant is that it can penetrate cell membrane, diffuse into the cytosol and target organelles easily, and selectively reduce hydroxide radicals and peroxynitrite without affecting physiological reactive oxygen species (ROS) involved in normal cell signaling[10]. Moreover, hydrogen therapy has been proven to be safe and effective in many clinical trials[11,12]. With respect to intestinal diseases, previous studies have shown that hydrogen may alleviate intestinal ischemia-reperfusion injury, UC, and colon inflammation[13-15]. However, the detailed mechanism responsible for this effect is not yet well illustrated. Hydrogen-rich water (HRW) is an effective, convenient way to deliver molecular hydrogen, which has the same effectiveness as inhaled hydrogen gas and is more suitable for clinical applications. Therefore, the main aim of our study was to assess the protective effect of HRW on IBD in mice and explore the detailed mechanisms involved.
This study was conducted using male C57BL/6J mice (4-5 wk old, 21-26 g) (Animal Feeding Center of Xi’an Jiaotong University Medical School). The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark cycle, 50% humidity, and ad libitum access to food and water) for one week prior to experimentation. All mice were housed (5 per cage) in clear, pathogen-free polycarbonate cages in the animal care facility, and they were fed a standard animal diet (No. 120161128007, Jiangsu Xietong Pharmaceutical Bio-technology Co., Ltd.) and water ad libitum under controlled temperature conditions with 12-h light-dark cycles. They were cared in accordance with the Ethical Committee, Xi’an Jiaotong University Health Science Center. The study was reviewed and approved by the Xi’an Jiaotong University Health Science Center Institutional Review Board. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Xi’an Jiaotong University Health Science Center. The animal protocol was designed to minimize pain and discomfort to the animals. All animals were euthanized with isoflurane gas for tissue collection. HRW was produced by Naturally Plus Japan International Co., Ltd. and was stored under atmospheric pressure at 4 °C in an aluminum bag with no dead volume, as performed in our previous studies[16-18].
IBD was induced by DSS feeding. Male C57BL/6J mice were provided with drinking water containing 5% (wt/vol) DSS (35-50 kDa, Sigma-Aldrich, Steinheim, Germany) ad libitum from day 0 to day 5. On days 6 to 7, the animals received tap water (without DSS). Control animals received tap water throughout the entire experiment.
Mice in the present study were divided into the following four groups: (1) control group, in which the mice received equivalent volumes of normal saline (NS) intraperitoneally (ip); (2) DSS group, in which the mice received NS i.p (5 mL/kg body weight, twice per day at 8 a.m. and 5 p.m.) for 7 consecutive days after IBD modeling; (3) DSS + HRW group, in which the mice received HRW (in the same volume as the NS treatment) for 7 consecutive days after IBD modeling; and (4) DSS + HRW + ZnPP group, in which the mice received HRW (in the same volume as the NS treatment) and ZnPP [a heme oxygenase-1 (HO-1) inhibitor, 25 mg/kg] for 7 consecutive days after IBD modeling. Six mice were used per group in this study. The weight, presence of blood in stool, and gross stool consistency of all mice were monitored daily. Each score was determined as follows: (1) change in weight (0: < 1%, 1: 1%-5%, 2: 5%-10%, 3: 10%-15%, 4: > 15%); (2) blood in stool (0-1: negative; 2-3: hemoccult positive; 4: gross bleeding); and (3) stool consistency (0: normal; 1-2: loose stools; 3-4: diarrhea). The disease activity index was determined by combining the scores from these three categories and dividing that number by three (Supplementary Table 1)[19].
Mice were sacrificed after being anesthetized with isoflurane gas on the 7th d after IBD modeling, and blood samples were collected from the periorbital plexus. The serum was separated by centrifugation at 3000 g for 15 min at 4 °C. The colon without the cecum was removed immediately from each mouse and stored at -80 °C until further analysis.
After the mice in all groups were sacrificed, the colon from each mouse was rapidly isolated and weighed with fecal content. The colon was then opened along the mesenteric border, and the fecal material removed. The total macroscopic damage score was calculated for each animal based on the following parameters: fecal blood (0: absence; 1: presence), presence of diarrhea (0: no diarrhea; 1: loosely shaped moist pellets; 2: amorphous, moist, sticky pellets; 3: diarrhea), the extent of colon damage (0: no inflammation; 1: reddening, mild inflammation; 2: moderate or widely distributed inflammation; 3: severe and/or extensively distributed inflammation), colon length (0: < 5% shortening; 1: 5%-14%; 2: 15%-24%; 3: 25%-35%; 4: > 35%) and weight (0: < 5% weight loss; 1: 5%-14%; 2: 15%-24%; 3: 25%-35%; 4: > 35%) (Supplementary Table 2)[20].
Samples from the distal colon were fixed in 10% formalin solution for 24 h, dehydrated and embedded in paraffin. Serial sections of 5-μm thickness were obtained and stained with hematoxylin and eosin to evaluate the morphology. Two researchers examined the results in a blinded fashion. The microscopic total damage score was assessed using the following parameters: the depletion of goblet cells (0: absence; 1: presence), crypt abscesses (0: absence; 1: presence), the destruction of mucosal architecture (1: normal; 2: moderate; 3: extensive), the extent of muscle thickening (1: normal; 2: moderate; 3: extensive), and the presence and degree of cellular infiltration (1: normal; 2: moderate; 3: transmural) (Supplementary Table 3)[21].
The levels of serum TNF-α, IL-6 and IL-1β were measured with commercial ELISA kits according to the instructions from the manufacturer (Dakewe, Shenzhen, China).
The concentrations of malonaldehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) in colon tissue were measured as markers of oxidative stress of colon tissue. Colon tissues were homogenized on ice in 10 volumes (w/v) of NS. The homogenates were centrifuged at 4000 rpm at 4 °C for 15 min for MDA, SOD and GSH detection by using assay kits purchased from Nanjing Jiancheng Corp., China. MDA levels in the supernatants were determined by measurement of thiobarbituric acid (TBA)-reactive substance levels using an MDA assay kit according to the manufacturer’s instructions. The samples were heated with TBA under acidic conditions and the pink color formed was read at 532 nm. The results are calculated as nmol/mg protein. SOD activity in the supernatants of colon tissue was evaluated by inhibition of nitroblue tetrazolium (NBT) reduction by O2- generated by the xanthine/xanthine oxidase system in accordance with the manufacturer’s instructions. The rate of NBT reduction was measured at 560 nm. The results were expressed as U/mg protein. For GSH assay, 5,5’-Dithiobis-2-nitrobenzoicacid (DTNB) was used to develop color. The development of yellow color was monitored at 412 nm on a spectrophotometer. The results are expressed as mg/g protein. For MPO assay, colon samples were homogenized in 5 volumes (w/v) of phosphate buffered saline containing 0.5% hexadecyltrimethylammonium hydroxide. Samples were measured on a spectrophotometry at 460 nm absorbance. One unit of MPO activity is defined as degrading 1 μmol of hydrogen peroxide at 37 °C, and MPO activity of tissue is expressed as U/g protein.
Proteins were extracted from the colon according to the manufacturer’s instructions. BCA protein assay kit was used to detect the concentration of extracted proteins. Equal amounts of protein were loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then the proteins were transferred onto poly-vinylidene difluoride (PVDF) membranes, which were immunoblotted with the appropriate primary antibody at 4 °C overnight. Then the membranes were incubated with secondary antibodies. The anti-p-eIF2α, ATF4, XBP1s, CHOP, HO-1 and β-actin monoclonal antibodies were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. The protein concentration was determined by the BCA method. Western blot analysis was performed as previously described[22].
Measurement data are expressed as the mean ± standard error of mean (SEM). Differences between the experimental and control groups were assessed by either the analysis of variance or t test, as applicable, using SPSS 18.0 (SPSS, 165 Inc.). A P value less than 0.05 was considered statistically significant.
To investigate the effect of HRW treatment on IBD, the weight change and disease activity index were assessed on the 7th d after IBD modeling (Figure 1). The results showed that the weight of the mice showed a downward trend on day 7 after IBD induction. However, the DSS + HRW group had significantly less weight loss compared with the DSS group on the 7th d (P < 0.05). Considering the change in weight, blood in stool, and stool consistency, the disease activity index was calculated. The disease activity index observably increased after DSS treatment, and the DSS + HRW group exhibited a lower extent of disease than the DSS group (P < 0.05).
Mice were sacrificed, and the colons were assessed on the 7th d after IBD modeling. We discovered that the average length of the colons exhibited a significant reduction after DSS administration. More importantly, HRW exerted a protective effect against the shortening of the colon in the DSS + HRW group, in which the colon was markedly longer than that in the DSS group (P < 0.05). In addition, the colonic wall thickening was alleviated in the DSS + HRW group in comparison to the DSS group (P < 0.05). The macroscopic damage score assessed by diarrhea, colon damage and colon length showed that the DSS + HRW group also received a lower score than the DSS group (P < 0.05) (Figure 2).
For the further study of the alterations of the colon, we conducted the experiments in microcosmic aspect. The histological study revealed that the mice in the DSS group developed severe colonic inflammation including mucosal hyperemia, inflammatory cell infiltration, formation of crypt abscesses, destruction of the mucosal architecture, and the depletion of goblet cells. Conversely, the DSS + HRW group exhibited mild inflammation that was much closer to normal. The microcosmic scores based on the goblet cell depletion, crypt abscesses, destruction of the mucosal architecture, muscle thickening, and cellular infiltration in the DSS + HRW group were also lower than those in the DSS group (P < 0.05) (Figure 3). This evidence indicated that HRW could improve colonic damage in DSS-induced IBD.
Oxidative stress and inflammation play an initial and crucial role in the process of IBD. The oxidative stress parameters in the colon, including MDA and MPO, were significantly decreased in the DSS + HRW group compared with the DSS group (P < 0.05). The protective indicator, SOD, was markedly increased with the use of HRW. Additionally, HRW also reversed the depletion of GSH caused by DSS administration (Figure 4). These facts demonstrated that HRW could indeed inhibit oxidative stress.
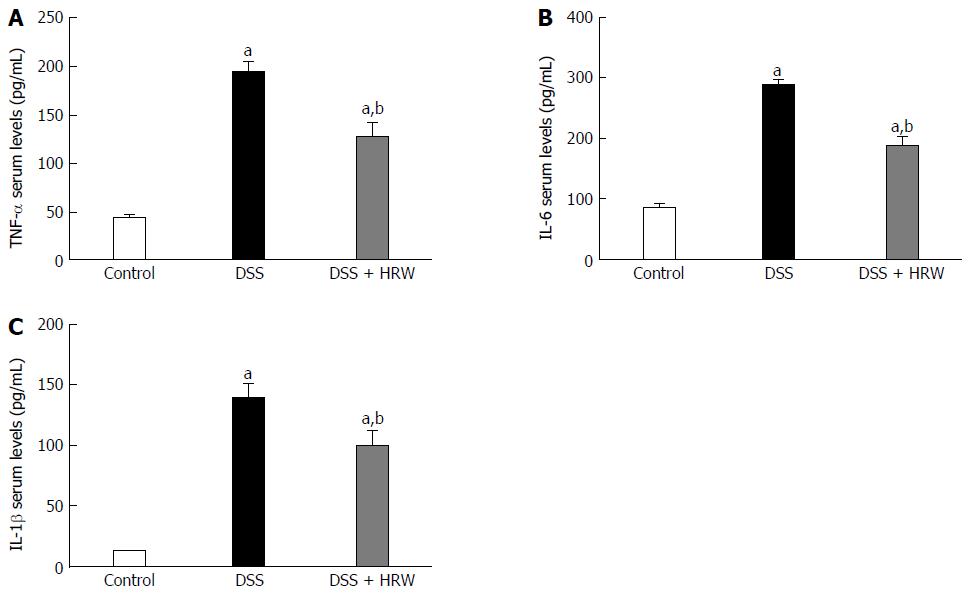
To explore the anti-inflammatory mechanism of HRW, inflammatory factors were assessed on the 7th d after IBD modeling by determining the plasma levels of TNF-α, IL-6 and IL-1β. A marked increase in TNF-α, IL-6 and IL-1β secretion was observed after DSS treatment. Moreover, the DSS + HRW group had significantly reduced levels of TNF-α, IL-6 and IL-1β compared with the DSS group (P < 0.05) (Figure 5).
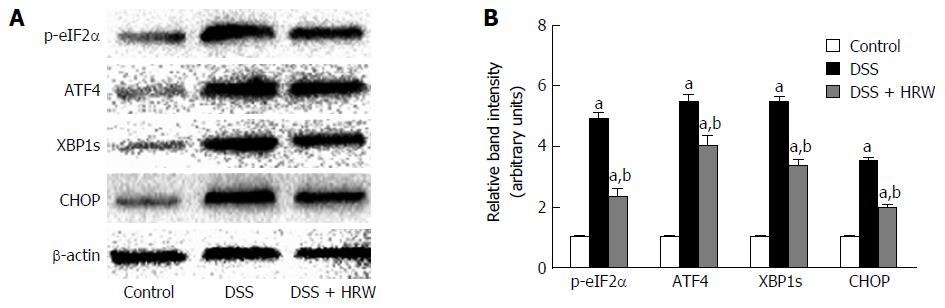
Endoplasmic reticulum (ER) stress participating in a cellular process triggered by a variety of conditions that disturb the folding of proteins in the ER also aggravates the progress of IBD. The pivotal proteins involved in ER stress, including p-eIF2α, ATF4, XBP1s and CHOP, were detected to assess the effect of HRW on ER stress in DSS-induced IBD (Figure 6). The results demonstrated that the expression of these proteins was significantly increased after DSS administration. Moreover, the p-eIF2α, ATF4, XBP1s and CHOP proteins were dramatically reduced after HRW treatment in contrast to the control group. These findings indicated that HRW may ameliorate the manifestation of IBD by inhibiting the process of ER stress.
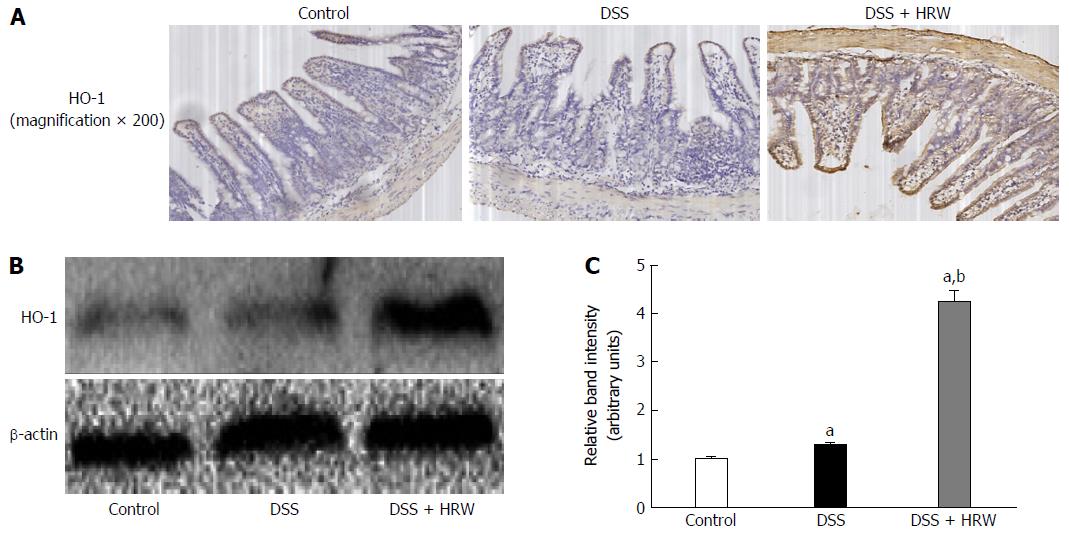
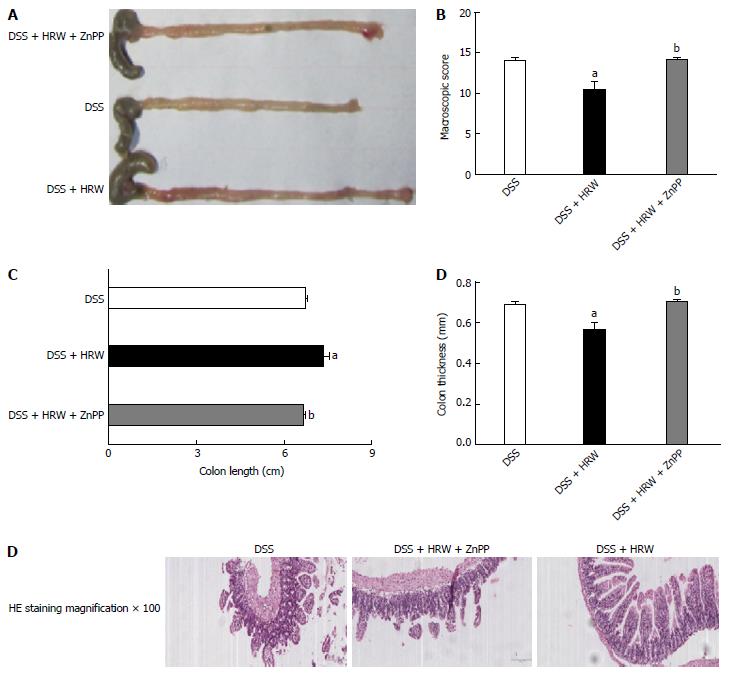
HO-1 has anti-inflammatory and anti-oxidative effects protecting against many diseases. On the 7th d after IBD modeling, the mice were sacrificed, and the colon tissues were obtained to detect the HO-1 expression (Figure 7). The results revealed that HRW treatment markedly accelerated HO-1 expression compared with the DSS group. For the further study of the role that HO-1 played in IBD, ZnPP, an HO-1 inhibitor, was used. We discovered that colon length was visibly reduced in the DSS + HRW + ZnPP group compared with the DSS + HRW group (P < 0.05). The colonic wall in the DSS + HRW + ZnPP group was also thicker than that in the DSS + HRW group (P < 0.05). In addition, the DSS + HRW + ZnPP group got a higher macroscopic damage score in comparison to the DSS + HRW group (P < 0.05). The histological study showed that the DSS + HRW + ZnPP group exhibited more serious colonic damage that was similar to that observed in the DSS group (Figure 8). Based on these findings, we confirmed that HO-1 plays a key role in the mechanisms for HRW to alleviate IBD.
Hydrogen has anti-oxidant, anti-inflammatory, anti-apoptotic and other protective effects, and great progress has been achieved in the research of hydrogen therapy for diseases such as metabolic disorders, tissue ischemia reperfusion injury, myocardial injury, and hepatic injury[23-26]. In this study, a model of IBD was established in mice by DSS feeding, and the therapeutic role of HRW was assessed. We demonstrated that treatment with HRW significantly alleviated the symptoms and colonic damage in DSS-induced IBD. The mechanisms by which HRW alleviates DSS-induced IBD may include the following: (1) inhibiting the secretion of inflammatory factors, such as TNF-α, IL-6 and IL-1β, to ameliorate the inflammatory response; (2) inhibiting oxidative stress, such as reducing MPO and ROS as well as increasing SOD and GSH; (3) inhibiting ER stress such as decreasing the expression of p-eIF2α, ATF4, XBP1s and CHOP; and (4) up-regulating HO-1 expression to ease oxidative stress and decrease inflammation. All the evidence revealed that HRW is a potential new method for the treatment of IBD.
IBD is an enteric disorder characterized by acute and chronic intestinal inflammation. The etiology and precise pathogenesis of IBD are still unclear. However, several possible causes, including genetic, infectious, immunological factors and dysfunction of the adaptive and innate immune systems in response to the fecal microbiome, have been recognized[27-30]. DSS is a physical agent with an intrinsic capacity to disrupt the epithelial barrier and activate macrophages, causing inflammation and tissue damage[8]. The related histological changes include ulceration and inflammation of the intestinal mucosa with leukocyte infiltration. The clinical presentation includes weight loss, blood in stool, and diarrhea. In the present study, we chose to evaluate changes in weight, the disease activity index, colon shortening, colonic wall thickening, histological study, and macroscopic and microcosmic scores to assess the severity of the DSS-induced IBD.
Oxidative stress is an imbalance of oxidation and anti-oxidation systems in the body and may be caused by excessive detrimental ROS, depletion of GSH, etc. In IBD, the production of ROS and MPO exceeds anti-oxidant defenses and leads to a state of oxidative stress that fuels inflammation and causes direct mitochondrial damage[31,32]. Hydrogen selectively quenches detrimental ROS, such as hydroxyl radicals and peroxynitrite, but it does not damage physiological ROS, such as superoxide anion radicals, hydrogen peroxide, and nitric oxide[33]. In this study, we found that HRW significantly reduced the levels of MDA and MPO and facilitated the protective indicators SOD and GSH. Furthermore, we measured the levels of inflammatory factors, and the results revealed that HRW markedly inhibited the release of TNF-α, IL-6 and IL-1β. TNF-α is one of the most important pro-inflammatory cytokines, and it stimulates the production of downstream cytokines such as IL-6 and IL-8 and plays a significant role in activating the cytokine cascade[34,35]. The researchers reported that anti-TNF-α monoclonal antibodies and other drugs had dramatically improved the treatment of IBD[36,37]. In summary, our study revealed that HRW could quench detrimental oxidative stress and exert an anti-TNF-α role to alleviate IBD.
ER stress participates in a cellular process triggered by a variety of conditions that disturb the folding of proteins in the ER. ER stress further triggers the unfolded protein response (UPR) by activating the PKR and PERK signals and phosphorylating eIF2α, which is required by the initiation phase of polypeptide chain synthesis[38,39]. ATF4 is a UPR-dependent transcriptional factor, and its sustained expression may up-regulate CHOP expression and induce apoptosis[40]. XBP1s is also a UPR-dependent transcriptional factor induced by AFT6[41]. ER stress exerts important roles in many diseases such as ischemia/reperfusion injury in the liver, diabetes, and cardiac myocyte injury[42-44]. A previous study also proved that epithelial ER stress participated in CD and UC[45]. Moreover, studies have found that hydrogen has anti-apoptotic and anti-inflammatory functions[46,47]. In this study, we discovered that HRW dramatically reduced the expression of p-eIF2α, ATF4, XBP1s and CHOP proteins and conclude that HRW protects against IBD by inhibiting ER stress.
To determine the deeper mechanism of the protective effect of HRW against IBD, we focused on the effect of hydrogen on HO-1 expression. Heme oxygenases (HOs) catalyze the rate-limiting step in heme degradation, which can produce bilirubin, iron, and carbon monoxide (CO). HO-1, increased by stimuli that induce cellular stress, reduces the secretion of inflammatory cytokines in many diseases, such as sepsis and LPS-stimulated macrophages[48,49]. Additionally, HO-1 conferred its cytoprotective effects by increasing anti-oxidative capacity and inhibiting oxidative stress[50,51]. In addition, recent studies have shown that HO-1 was involved in the downstream effect of Treg cells[52]. Based on these facts, we speculated that hydrogen may confer its cytoprotective role by up-regulating HO-1. We measured the level of HO-1 and used ZnPP, an HO-1 inhibitor, for the further study. Not surprisingly, HRW treatment markedly up-regulated HO-1 expression, and the use of ZnPP clearly reversed the protective role of HRW. We verified that HO-1 indeed plays a key role in the mechanisms by which HRW alleviates IBD. The detailed mechanism may be that HO-1 inhibits the secretion of inflammatory cytokines and oxidative stress to alleviate IBD.
In this study, we have proven that HRW has a significant therapeutic potential in the treatment of IBD by inhibiting inflammatory factors and oxidative stress. More importantly, we discovered that HRW could inhibit ER stress to prevent apoptosis and up-regulate HO-1 expression. The high level of HO-1 further exerted anti-oxidative and anti-inflammatory functions in the process of IBD. Additionally, due to its advantageous distribution characteristics, hydrogen can penetrate biomembranes and diffuse into the cytosol, mitochondria, and nucleus, successfully targeting the organelles. All of these effects make HRW a potential new treatment method against DSS-induced IBD. However, our study is based on animal experiments, and prospective clinical studies are needed to evaluate whether HRW is fit for the clinical treatment of IBD.
In conclusion, the results of the present study demonstrate that HRW can alleviate the symptoms and colonic damage in DSS-induced IBD, most likely due to its unique cytoprotective properties such as its anti-oxidant and anti-inflammatory activities. More importantly, HRW can inhibit ER stress and up-regulate HO-1 expression. All of these findings indicate that HRW can be a potential therapy for DSS-induced IBD.
Inflammatory bowel disease (IBD) is a chronic and relapsing disease, and therapeutic goals include controlling inflammation and ameliorating clinical symptoms. Hydrogen-rich water (HRW) is a potent anti-oxidative, anti-apoptotic, and anti-inflammatory agent and an ideal therapy for many diseases.
Effective therapeutic schemes for IBD are lacking. Research of mechanisms and new therapeutic approaches for IBD has received increasing attention from scientists and clinicians. Hydrogen therapy is a new medical approach that has recently gained much appreciation. HRW exerts considerable anti-oxidative, anti-apoptotic, and anti-inflammatory effects. More importantly, drinking HRW is very convenient in the course of daily life. To explore the effect of HRW on different types of diseases and promote its clinical usage is currently an important goal in hydrogen medicine.
The present study concluded that HRW can significantly prevent IBD in mice by inhibiting inflammatory factors, oxidative stress, and endoplasmic reticulum stress and by up-regulating HO-1 expression. Moreover, based on the use of the pharmaceutical inhibition of HO-1, we can conclude that HO-1 may be a key effective protein in HRW function.
Hydrogen therapy may be a safe and effective treatment for IBD. Moreover, the application of drinking HRW is very convenient and acceptable for usage.
Hydrogen is the lightest gas in nature, which has powerful anti-oxidant and anti-inflammatory effects. It has therapeutic effects in many diseases, which is proven by many basic research and clinical studies. HRW is produced by forcing hydrogen gas into water by a specific device under high pressure.
Congratulations. It is a very well designed work with very interesting results. Some suggestions: Was there any examination or histological study of the puncture site? It would have been interesting to know if there is any reaction in that place.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Madrid AM, Perse M S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 2. | Shanahan F. Crohn’s disease. Lancet. 2002;359:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 353] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1003] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 4. | Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol. 2012;18:3806-3813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 421] [Article Influence: 38.3] [Reference Citation Analysis (2)] |
| 6. | Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (5)] |
| 8. | Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (1)] |
| 9. | Xia C, Liu W, Zeng D, Zhu L, Sun X, Sun X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clin Transl Sci. 2013;6:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Ostojic SM. Targeting molecular hydrogen to mitochondria: barriers and gateways. Pharmacol Res. 2015;94:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | McCarty MF. Potential ghrelin-mediated benefits and risks of hydrogen water. Med Hypotheses. 2015;84:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Ohta S. [Initiation, development and potential of hydrogen medicine: Toward therapeutic and preventive applications of molecular hydrogen against a variety of diseases]. Seikagaku. 2015;87:82-90. [PubMed] |
| 13. | Abe T, Li XK, Yazawa K, Hatayama N, Xie L, Sato B, Kakuta Y, Tsutahara K, Okumi M, Tsuda H. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation. 2012;94:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | He J, Xiong S, Zhang J, Wang J, Sun A, Mei X, Sun X, Zhang C, Wang Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J Surg Res. 2013;185:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Medani M, Collins D, Docherty NG, Baird AW, O’Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Zhang JY, Song SD, Pang Q, Zhang RY, Wan Y, Yuan DW, Wu QF, Liu C. Hydrogen-rich water protects against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol. 2015;21:4195-4209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Zhang JY, Wu QF, Wan Y, Song SD, Xu J, Xu XS, Chang HL, Tai MH, Dong YF, Liu C. Protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. World J Gastroenterol. 2014;20:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zhang J, Wu Q, Song S, Wan Y, Zhang R, Tai M, Liu C. Effect of hydrogen-rich water on acute peritonitis of rat models. Int Immunopharmacol. 2014;21:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Simeoli R, Mattace Raso G, Lama A, Pirozzi C, Santoro A, Di Guida F, Sanges M, Aksoy E, Calignano A, D’Arienzo A. Preventive and therapeutic effects of Lactobacillus paracasei B21060-based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. J Nutr. 2015;145:1202-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Fichna J, Dicay M, Lewellyn K, Janecka A, Zjawiony JK, MacNaughton WK, Storr MA. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm Bowel Dis. 2012;18:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Sałaga M, Polepally PR, Zakrzewski PK, Cygankiewicz A, Sobczak M, Kordek R, Zjawiony JK, Krajewska WM, Fichna J. Novel orally available salvinorin A analog PR-38 protects against experimental colitis and reduces abdominal pain in mice by interaction with opioid and cannabinoid receptors. Biochem Pharmacol. 2014;92:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Qu K, Xu X, Liu C, Wu Q, Wei J, Meng F, Zhou L, Wang Z, Lei L, Liu P. Negative regulation of transcription factor FoxM1 by p53 enhances oxaliplatin-induced senescence in hepatocellular carcinoma. Cancer Lett. 2013;331:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Heinzelmann SM, Villanueva L, Sinke-Schoen D, Sinninghe Damsté JS, Schouten S, van der Meer MT. Impact of metabolism and growth phase on the hydrogen isotopic composition of microbial fatty acids. Front Microbiol. 2015;6:408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhao Y, Tang Y, Suo C, Liu D, Li S, Li H. [Effects of hydrogen-rich saline on endoplasmic reticulum stress during myocardial ischemia-reperfusion in rats]. Zhonghua Yixue Zazhi. 2014;94:3024-3028. [PubMed] |
| 25. | Tanabe H, Sasaki Y, Yamamoto T, Kiriyama S, Nishimura N. Suppressive Effect of High Hydrogen Generating High Amylose Cornstarch on Subacute Hepatic Ischemia-reperfusion Injury in Rats. Biosci Microbiota Food Health. 2012;31:103-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Qu J, Lü X. Hydrogen: a promising novel treatment for hepatic encephalopathy? Free Radic Biol Med. 2013;63:457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Zanello G, Kevans D, Goethel A, Silverberg M, Tyler A, Croitoru K. Genetics and innate and adaptive immunity in IBD. Nestle Nutr Inst Workshop Ser. 2014;79:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol. 2013;108:1835-1842, quiz 1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Lee JW, Im JP, Cheon JH, Kim YS, Kim JS, Han DS. Inflammatory Bowel Disease Cohort Studies in Korea: Present and Future. Intest Res. 2015;13:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Liang J, Sha SM, Wu KC. Role of the intestinal microbiota and fecal transplantation in inflammatory bowel diseases. J Dig Dis. 2014;15:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Thomson A, Hemphill D, Jeejeebhoy KN. Oxidative stress and antioxidants in intestinal disease. Dig Dis. 1998;16:152-158. [PubMed] |
| 32. | Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 33. | Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LS. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci. 2015;6:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 375] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 34. | Scharl M, Vavricka SR, Rogler G. Review: new anti-cytokines for IBD: what is in the pipeline? Curr Drug Targets. 2013;14:1405-1420. [PubMed] |
| 35. | Vermeire S, Van Assche G, Rutgeerts P. Serum sickness, encephalitis and other complications of anti-cytokine therapy. Best Pract Res Clin Gastroenterol. 2009;23:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Blotière PO, Rudant J, Barré A, Racine A, Weill A, Peyrin-Biroulet L, Carbonnel F, Alla F. Conditions of prescription of anti-TNF agents in newly treated patients with inflammatory bowel disease in France (2011-2013). Dig Liver Dis. 2016;48:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Komaki Y, Komaki F, Sakuraba A, Cohen R. Approach to Optimize Anti-TNF-α Therapy in Patients With IBD. Curr Treat Options Gastroenterol. 2016;14:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1539] [Article Influence: 128.3] [Reference Citation Analysis (1)] |
| 39. | Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 40. | Khan I, Tang E, Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep. 2015;5:10581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 41. | Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Molecular Pathways: Immunosuppressive Roles of IRE1α-XBP1 Signaling in Dendritic Cells of the Tumor Microenvironment. Clin Cancer Res. 2016;22:2121-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Liu D, Liu X, Zhou T, Yao W, Zhao J, Zheng Z, Jiang W, Wang F, Aikhionbare FO, Hill DL. IRE1-RACK1 axis orchestrates ER stress preconditioning-elicited cytoprotection from ischemia/reperfusion injury in liver. J Mol Cell Biol. 2016;8:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Choi SK, Lim M, Yeon SI, Lee YH. Inhibition of endoplasmic reticulum stress improves coronary artery function in type 2 diabetic mice. Exp Physiol. 2016;101:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Liu Z, Zhao N, Zhu H, Zhu S, Pan S, Xu J, Zhang X, Zhang Y, Wang J. Circulating interleukin-1β promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovasc Diabetol. 2015;14:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Cao SS. Epithelial ER Stress in Crohn’s Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Lee D, Park S, Bae S, Jeong D, Park M, Kang C, Yoo W, Samad MA, Ke Q, Khang G. Hydrogen peroxide-activatable antioxidant prodrug as a targeted therapeutic agent for ischemia-reperfusion injury. Sci Rep. 2015;5:16592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Yang R, Jia Q, Guo X, Liu X, Ma S, Gao Q, Guan S. [Protective effects of hydrogen sulfide on diaphragmatic muscle of Type 1 diabetic rats and its anti-apoptotic mechanisms]. Zhongnan Daxue Xuebao Yixueban. 2015;40:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Araujo JA, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 49. | Durante W. Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front Biosci (Landmark Ed). 2011;16:2372-2388. [PubMed] |
| 50. | Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X, Fu J, Zou X, Zhang J, Chen X. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS One. 2016;11:e0149032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 51. | Cheng HT, Yen CJ, Chang CC, Huang KT, Chen KH, Zhang RY, Lee PY, Miaw SC, Huang JW, Chiang CK. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta. 2015;1850:2506-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Schumacher A, Wafula PO, Teles A, El-Mousleh T, Linzke N, Zenclussen ML, Langwisch S, Heinze K, Wollenberg I, Casalis PA. Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One. 2012;7:e42301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |