Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1367
Peer-review started: October 6, 2016
First decision: October 20, 2016
Revised: November 6, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 28, 2017
Processing time: 143 Days and 20.2 Hours
To explore the pharmacokinetics and pharmacodynamics of Da-Cheng-Qi decoction (DCQD) in the liver of rats with severe acute pancreatitis (SAP) based on an herbal recipe tissue pharmacology hypothesis.
Healthy male Sprague-Dawley rats were randomly divided into a sham operation group (SOG); a model group (MG); and low-, median- and high-dose treatment groups (LDG, MDG, and HDG, respectively). Different dosages (6, 12 and 24 g/kg for the LDG, MDG, and HDG, respectively) of DCQD were administered to the rats with SAP. The tissue concentrations of aloe-emodin, rhein, emodin, chrysophanol, honokiol, rheo chrysophanol, magnolol, hesperidin, naringenin and naringin in the liver of the treated rats were detected by high-performance liquid chromatography tandem mass spectrometry. Alanine transaminase (ALT) and aspartate transaminase (AST) in serum, inflammatory mediators in the liver and pathological scores were evaluated.
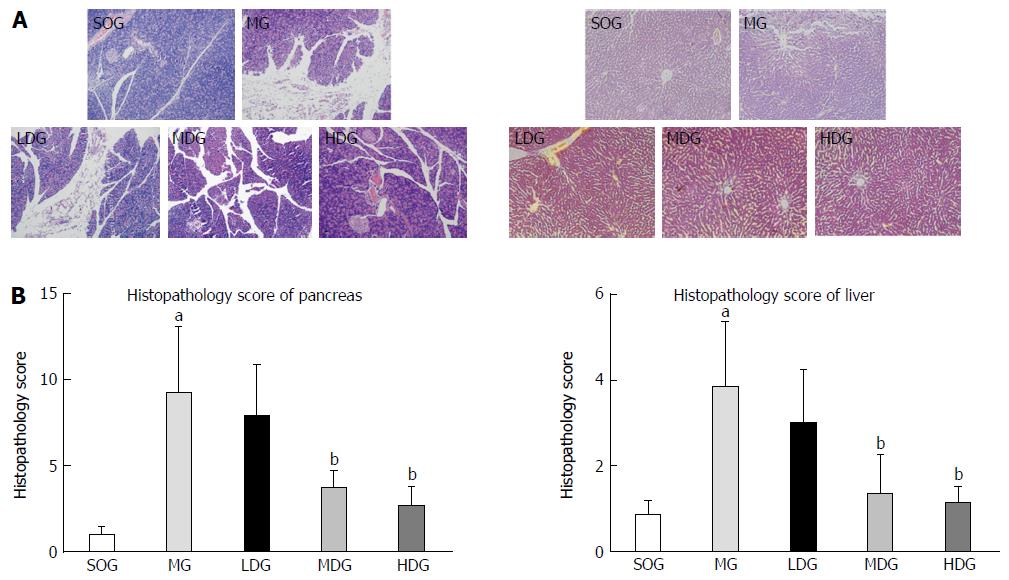
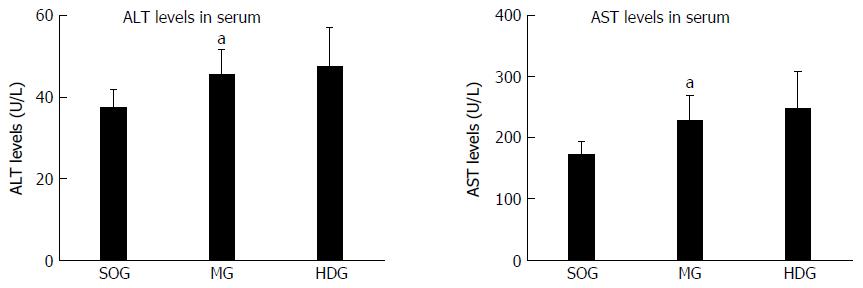
The major components of DCQD were detected in the liver, and their concentrations increased dose-dependently. The high dose of DCQD showed a maximal effect in ameliorating the pathological damages, decreasing the pro-inflammatory mediators tumor necrosis factor-α and interleukin (IL)-6 and increasing anti-inflammatory mediators IL-4 and IL-10 in the liver. The pathological scores in the pancreas for the MG were significantly higher than those for the SOG (P < 0.05). DCQD could reduce the pathological scores in the pancreas and liver of the rats with SAP, especially in the HDG. Compared to the SOG, the ALT and AST levels in serum were higher in the MG (P < 0.05), while there was no statistical difference in the MG and HDG.
DCQD could alleviate liver damage by altering the inflammatory response in rats with SAP based on the liver distribution of its components.
Core tip: Our study group had raised the herbal recipe tissue pharmacology hypothesis, which assumed that the effect of herb formula is related to its target tissue distributions or concentrations of the effective components in target tissues. This study was to investigate the mechanism by which Da-Cheng-Qi decoction (DCQD) ameliorates acute liver injury complicated with severe acute pancreatitis in rats by detecting the tissue distributions of the components from DCQD in the liver and the inflammatory mediators as well as the pathological scores.
- Citation: Zhang YM, Ren HY, Zhao XL, Li J, Li JY, Wu FS, Su H, Tang WF. Pharmacokinetics and pharmacodynamics of Da-Cheng-Qi decoction in the liver of rats with severe acute pancreatitis. World J Gastroenterol 2017; 23(8): 1367-1374
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1367
Acute pancreatitis (AP) is a sudden inflammation of the pancreas and remote tissues. Approximately 20% of AP patients develop a severe form with systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS), which can affect the lung, kidney, intestine, liver and heart[1,2]. One retrospective study showed that hepatic failure associated with a high mortality rate was present in 83% of patients with severe acute pancreatitis (SAP) (among the 178 consecutive patients with SAP admitted to the surgical department from 1994 to 1998, 113 treated in the general intensive care unit were included in the study)[3]. The liver triggering massive inflammatory response during AP was first described in 1996[4]. Inflammatory mediators from the pancreas can directly injure liver cells via the portal vein and can also stimulate Kupffer cells to express tumor necrosis factor-α (TNF-α), interleukin 1 (IL-1), interleukin 6 (IL-6) and other cytokines that are involved in the development of local and systemic inflammatory responses associated with SAP[5]. Additionally, the activation of Kupffer cells mediates lung injury in acute hemorrhagic necrotizing pancreatitis[6,7]. Inhibitors of Kupffer cell activation, such as gadolinium chloride (GdCl3), can decrease serum TNF-α levels and relieve remote organ injury in AP[6]. In the early stage of SAP, the liver stimulates TNF-α generation. However, with the progression of the disease, excessive TNF-α was released into the blood circulation, which led to remote organ damages; this indicated that liver injury plays a central role in the development of MODS in SAP[8]. Therefore, it is important to improve therapies for liver injury to decrease the severity of SAP.
Da-Cheng-Qi decoction (DCQD), a famous traditional Chinese medicine (TCM) prescription, has been widely used for the treatment of AP for over 30 years in China[9]. DCQD is composed of Rheum palmatum L. (Dahuang, dried marshmallow root or rhizome, bitter), Magnolia henryi Dunn (Houpu, tree bark, bitter), Citrus aurantium L. (Zhishi, dried immature fruit, bitter), and Natrii Sulfas (Mangxiao, Na2SO4·10H2O, salty)[9]. It has been reported that DCQD can promote gastrointestinal motility, inhibit cytokine activity and inflammatory response, and relieve acute lung injury in AP[10]. Furthermore, our previous study confirmed that the effects of DCQD on the pancreas, intestine and lung were associated with the tissue distribution of its potential target components[11].
We hypothesized that the tissue pharmacology of the herbal recipe is related to its target tissue distribution or the concentration of its effective components in target tissues[12]. This study aimed to explore the relationship between the effects of DCQD and the distribution/concentration of its absorbed components in the liver of rats with SAP.
Male Sprague-Dawley rats (n = 30) weighing 220 ± 15 g were purchased from the Experimental Animal Center of West China Hospital (Chengdu, China). All of the animals were fed and handled according to the Guide for the Care and Use of Laboratory Animals of Sichuan University (Chengdu, China) and the Animal Ethics Committee Guidelines of the Animal Facility of the West China Hospital (protocol number: 2016001A, Chengdu, China). After one week of acclimation, the rats were fasted for 24 h before the induction of SAP and were kept under food-free conditions throughout the experiment.
The Dahuang, Houpu, Zhishi and Mangxiao spray-dried drug powders were purchased from Chengdu Green Herbal Pharmaceutical Co. Ltd. (Chengdu, China). The crude formula components were extracted twice by refluxing with boiling distilled water (1:12, g/mL) for 1 h, and the obtained solution was concentrated and spray-dried. The dry powder was stored at 4 °C until use. DCQD comes from Shang-Han-Lun, a classic TCM book in China, in which the described dosages are 12 g, 24 g, 12 g and 9 g, respectively, and 57 g of DCQD per person (60 kg body weight) is suggested. According to the Method of Pharmacology, the dosage for rats is adjusted to 1/6-1/20 of the human dosage. We chose 1/6.3 to 1/18.9, meaning that the lowest dosage was 6 g/kg body weight (0.6 g/100 g). As previously described[11], the spray-dried powders were mixed and reconstituted with sterile distilled water according to a standard ratio (12:24:12:9) at different concentrations for the crude drug, giving 0.6 g/mL, 1.2 g/mL and 2.4 g/mL of DCQD.
As previously described[11], the rats were randomly divided into a sham operation group (SOG), a model group (MG), a low-dose treatment group (LDG, 6 g/kg BW), a medium-dose treatment group (MDG, 12 g/kg BW) and a high-dose treatment group (HDG, 24 g/kg BW). After anesthetization with 10% chloral hydrate injected into the abdominal cavity at 3 mL/kg body weight, SAP in rats was induced by retrograde injection of 3.5% sodium taurocholate (Sigma, St. Louis, MO, United States) into the biliopancreatic duct (1 mL/kg body weight) at a rate of 0.2 mL/min with a micro-infusion pump[11]. The SOG received a similar injection procedure but with saline. After the rats recovered from the anesthesia, DCQD was administered intragastrically to rats 2 h after operation at the corresponding dosages. Rats in the SOG and MG were given equal volumes of saline.
At 24 h after operation, the rats were sacrificed and pancreas and liver samples were collected for pathological analysis. These tissue samples were fixed with 10% neutral formalin, embedded in paraffin, cut into sections and stained with hematoxylin and eosin. All of the pathological sections were scored in a blinded fashion by two independent pathologists using a scoring system for the extent and severity of tissue injury (0-4, representing edema, neutrophil infiltration, necrosis, and hemorrhage)[11,13].
The homogenate tissue levels of inflammatory mediators, including TNF-α, IL-6, IL-4 and IL-10, were measured using a Milliplex MAP Rat Cytokine/Chemokine magnetic bead immunoassay kit (Millipore Corporation, Billerica, MA)[11]. The values were read with a MAGPIX Luminex xMAP instrument (Luminex Corp, Austin, TX) and analyzed with the MILLIPLEX Analysis software version 3 (Millipore Corporation, Billerica, MA)[11]. Blood samples (5 mL) were collected for centrifugation at 3000 rpm for 7 min at low temperature, and then, the supernatants were obtained for the alanine transaminase (ALT) and aspartate transaminase (AST) detection with an Automatic Biochemical Analyzer (AU5400, SIEMENS, Munich, Germany).
The concentrations of the 10 major components of DCQD (aloe-emodin, rhein, emodin, chrysophanol, honokiol, rheo chrysophanol, magnolol, hesperidin, naringenin and naringin) in liver tissue homogenates (10%) were measured by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) for the tissue distribution analysis[11]. The HPLC-MS/MS system consists of an SIL-HTc autosampler (Shimadzu, Kyoto, Japan), an LC-10ADvp pump (Shimadzu, Kyoto, Japan), and an API3000 triple-quadrupole LC-MS system (Applied Biosystems, Foster City, CA, United States). The conditions for this system were described previously[11,14]. The mean contents of the components of DCQD, which were detected three times in our previous study, were as follows: rhein, 0.86 mg/g; emodin, 2.48 mg/g; aloe-emodin, 1.73 mg/g; chrysophanol 0.55 mg/g; rheochrysidin, 2.61 mg/g; naringin, 3.83 mg/g; naringenin 4.16 mg/g; hesperidin, 11.06 mg/g; honokiol, 1.26 mg/g; and magnolol, 1.11 mg/g[15].
Our study group had detected the ten components in a previous study by HPLC-MS/MS[14]. Quality control (QC) samples were prepared to obtain the following plasma concentrations for the examined components: (1) 3750, 625, 156.25 and 39.06 ng/mL for rhein; (2) 100, 25 and 6.25 ng/mL for emodin; (3) 600, 100, 25 and 6.25 ng/mL for aloe-emodin, chrysophanol, naringin, naringenin, hesperidin, magnolol and honokiol; and (4) 120, 20, 5 and 1.25 ng/mL for rheochrysidin. The spiked plasma samples (standard and QC samples) were pretreated and detected in each analytical batch along with the unknown samples[14]. The detected DCQD samples were stored in the Public Experiment Platform at West China Hospital (Chengdu, China).
Data collection, peak integration, and calibration were all calculated with the Analyst 1.4.2 software. Calibration curves were plotted according to the peak ratios of the analytes to the internal standards (ibuprofen), and the linear regression between the tissue concentration and the peak area ratio was determined by 1/χ2. The concentrations of QC and unknown samples were measured by interpolation from the calibration curves[14].
All of the data were processed with statistical software PEMS 3.1. All of the values are expressed as the mean ± SD. One-way repeated-measure ANOVA, followed by multiple pair-wise comparisons using the Student-Neuman-Keuls procedure, was used to detect differences among the groups. P < 0.05 was considered significantly different.
The ten major components of DCQD were measured in liver tissues. Chrysophanol, emodin, magnolol, rhein, aloe-emodin and rheo chrysophanol increased dose-dependently with the DCQD dosage. The concentration of naringenin was the highest (Figure 1).
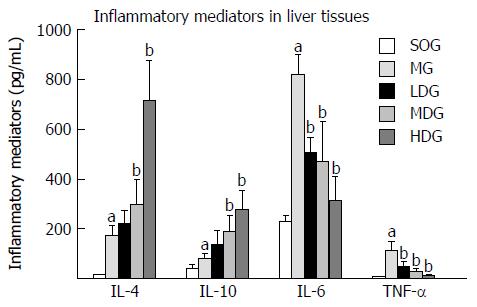
In hepatic tissue, the pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-4 and IL-10) displayed a significant increase in the MG compared with the SOG (P < 0.05). Compared to the MG, the expression of pro-inflammatory cytokines in the liver tissues decreased in the DCQD-treated groups with the lowest levels (P < 0.05), while the levels of anti-inflammatory cytokines were significantly increased and their levels were the highest in the HDG (P < 0.05) (Figure 2).
The pancreas of rats in the SOG was slightly edematous without obvious acinar tissue hemorrhage and necrosis and the liver had no obvious changes. However, the pancreas of rats in the MG showed distinct necrosis, interstitial edema, infiltration of neutrophil and mononuclear cells, and hemorrhage in the tissues. The liver tissues of rats in the MG showed mainly edema and infiltration of neutrophil without necrosis. After treatment with DCQD, we found a significant reduction in inflammatory cell infiltration, hemorrhage, necrosis and interstitial edema in the tissues, and the effects were the best in the HDG. DCQD could reduce the pathological scores in the pancreas and liver of rats with SAP, especially in the HDG (Figure 3).
Based on the highest distribution in the liver tissues of the major components of DCQD in the HDG, the ALT and AST levels were detected in the SOG, MG and HDG. Compared to the SOG, the ALT and AST levels in serum were higher in the MG (P < 0.05), while there was no statistical difference between the MG and HDG (Figure 4).
In our study, we found that the components of DCQD in liver tissues were similar to those in plasma and pancreas or intestine tissues as detected by HPLC-MS/MS[11,14]. As shown above, the concentration of naringenin in the liver was the highest of the components, which was also true in the intestine[11]. However, there were still differences in the distribution of these components in the liver. In the pancreas, the concentration of rhein was the highest in the HDG[11], while naringenin had the highest concentration in the liver, followed by aloe-emodin at 24 h after induction of AP (Figure 1), indicating that naringenin and aloe-emodin might be the major target components of DCQD associated with liver damage in SAP. As described previously, the target tissues of aloe-emodin might be the liver and kidney[16]. In combination with our studies[11,14], the distribution of these DCQD components includes different target tissues, which is consistent with the hypothesis regarding the tissue pharmacology in the herb recipe[12]. For example, the concentration of rhein was highest in the pancreas, the concentration of emodin was highest in the lung, and that of naringenin was highest in the intestine and liver at 24 h after induction of AP[11]. Blood-tissue barriers may contribute to these phenomena[11]. To date, most studies mainly focused on the blood-brain barrier and gut barrier, with research on the blood-pancreas juice barrier (BPJB) and the liver barrier being rare. It has been reported that the BPJB might lead to the selective excretion of some antibiotics, such as chloramphenicol in dogs with chronic pancreatic fistula[17] and anti-tumor drugs including 5-fluorouracil and mitomycin (MMC) during pancreatic adenocarcinoma chemotherapy[18]. We know that the liver barrier consists of a cell-cell barrier, a hepatic cell-blood barrier (the liver sinusoidal cell populations (LSECs) of the reticulo-endothelial system) and a blood-gall barrier. The LSECs of the reticulo-endothelial system could clear the pro-inflammatory molecules derived from the gut, such as lipopolysaccharide (LPS), in the absence of any signs of inflammation[19,20]. Future research could pay more attention to the BPJB and liver barrier, which may play important roles in the treatment of AP with herbal medicine[3].
AP is a potentially lethal acute inflammatory disease mediated by pro- and anti-inflammatory mediators released from the pancreas and other sources[21,22]. During SAP, the pro- and anti-inflammatory responses occurred early and persisted in the systemic circulation for several days[23]. Their main sources are tissue macrophages, and they attract neutrophils and additional macrophages and induce the production of proteases, elastases, and phospholipases, which can cause tissue damage[24]. The pro-inflammatory serum mediator IL-6 increased early and sensitively predicts severity in patients with AP[25]. The release of IL-6 in inflamed pancreatic tissue is associated with the development of remote organ dysfunction[26]. Furthermore, the induction of the hepatic production of acute phase proteins contributes to the pathophysiological roles of IL-6 in the acute phase response[27]. TNF-α is the most important factor involved in inflammatory response and shock, not only inducing apoptosis and necrosis but also entering blood circulation to induce the expression of interleukins, such as IL-6, which can cause inflammatory response waterfall cascades and lead to SIRS[28]. However, the anti-inflammatory cytokines IL-4 and IL-10 can inhibit macrophage function[29]. IL-10 is a marker of Th2 lymphocyte activity[30] and shows a positive effect on the proliferation and differentiation of B lymphocytes, which promote the production of immunoglobulins[22]. IL-10 can reduce the free oxygen radicals affecting macrophages and T-helper lymphocytes[22] and lower necrosis and mortality in AP[31]. IL-10 can also inhibit the secretion of pro-inflammatory cytokines, including TNF-a, IL-1β, IL-6, and IL-8[29]. It appears that balancing pro-inflammatory and anti-inflammatory cytokines is crucial in the progression of inflammation in AP[32].
In this study, we examined the levels of IL-4, IL-6, IL-10 and TNF-α as predictors of inflammatory changes in AP. Our results showed that IL-6 levels were higher than those of other inflammatory mediators, while TNF-α levels were lowest in the liver. The role of pro-inflammatory IL-6 in the prediction of AP severity seems more valuable than TNF-α, which is in line with other studies[11,25]. We found that the pro-inflammatory cytokines IL-6 and TNF-α and the anti-inflammatory cytokines IL-4 and IL-10 displayed a relative balance (Figure 2). DCQD treatment could increase the expression of IL-4 and IL-10 and inhibit the expression of TNF-α and IL-6, which ameliorated SAP-associated liver damages; better effects were observed at higher DCQD dosages (Figure 2). These data provided evidence for the regulatory effect of DCQD on the balance of pro-inflammatory and anti-inflammatory mediators to ameliorate liver damages and reduce AP severity. This effect was similar to the effects of DCQD on lung, pancreas and intestine damages[11]. Similar results can be found in other studies. It has been reported that DCQD could alleviate liver injury by decreasing the levels of TNF-α, IL-6 and NO[33]. DCQD might promote the synthesis of hepatic cell RNA to maintain the structure and function of hepatic cells[34]. Purgative therapy with DCQD improved hepatocyte apoptosis in rats with acute hepatic injury induced by LPS/D-galactosamine by down-regulating the caspase-2 and BAX protein expression, up-regulating BCL-2 expression and adjusting the BCL-2/BAX balance[35]. As mentioned above, naringenin might be the major DCQD target component in the liver of rats with SAP. It has been reported that naringenin increases resistance to oxidative stress and inflammation and protects against multiple organ injury in various animal models[36]. Furthermore, naringenin reduces macrophage infiltration by significantly decreasing hepatic pro-inflammatory mediators and the expression of relevant genes including TNF-α, IL-6, IL-1β, inducible nitric oxide synthase, matrix metalloproteinases (MMP-2 and -9), and EGF-like module-containing mucin-like hormone receptor-like 1[36]. Our study found that aloe-emodin might be another effective target component of DCQD in the liver. Hu et al[37] showed that aloe-emodin conferred anti-inflammatory effects through a likely mechanism involving a decrease in pro-inflammatory cytokine production via inhibition of the nuclear factor κB (NF-κB), MAPK, and PI3K pathways. Of course, other components could also cause anti-inflammatory effects. For example, rhein could inhibit NF-κB activation and sequentially suppress its downstream targets, including inducible nitric oxide synthase, IL-6, and TNF-α, by inhibiting IKKβ in LPS-activated macrophages[38]. Emodin could enhance peritoneal macrophage (pMΦ) phagocytosis and apoptotic cell clearance by altering intercellular adhesion molecule-3 expression in SAP or SIRS[39]. Based on the close relationship between DCQD or its components and inflammatory mediators, future studies should focus on the molecular mechanism of the components and their target tissues.
According to our results, the effect of DCDD on the liver damages was mainly dose-dependently improving the inflammatory injuries[11]. As early as 1998, Zhao et al[40] had determined that the inhibitory effect of DCQD on acute phase protein levels was dose-dependent in MODS. It was recently reported that DCQD improved the pathological scores and decreased serum amylase and lipase dose-dependently in a study showing that DCQD attenuates inflammatory responses by inhibiting the high mobility group box 1 -mediated NF-x03BA, B and P38 MAPK signaling pathways in SAP[41]. Additionally, the concentrations of nine major components of DCQD, all except for naringenin, were increased in a dose-dependent manner (Figure 1). The concentrations of the ten major components of DCQD increased dose-dependently in the intestine[11]. Therefore, a DCQD dose-concentration effect may exist for the treatment of AP, which would support the tissue pharmacology herbal recipe. However, studies substantiating these results remain rare. In this study, DCDD showed no obvious effect in ameliorating the ALT and AST levels. We considered that the action time of the single dose of DCQD might be too short or the dosage of DCQD and the concentrations of the components in the tissues was not enough to reduce the ALT and AST levels. This issue needs to clarify in further studies.
In conclusion, most of the DCQD components could be absorbed into the liver of rats with SAP. Additionally, DCQD may ameliorate histopathological damages and inflammatory responses by increasing the expression of anti-inflammatory cytokines and inhibiting the expression of pro-inflammatory cytokines, which may be associated with the DCQD intake dosages.
Acute lung injury and acute liver injury are the determinants of morbidity and mortality at early stage of sever acute pancreatitis (SAP). The Chinese herbal formula Da-Cheng-Qi decoction (DCQD) may ameliorate acute lung injury complicated with SAP by improving gastrointestinal function and reducing the inflammatory response. However, the mechanism of DCQD in protecting against liver injury during the course of SAP remains unclear.
Authors tested the herbal recipe tissue pharmacology hypothesis, which proposes that the effect of the herbal formula is related to its target tissue distributions or the concentrations of the effective components in the target tissues. This hypothesis may be important for screening the effectively absorbed components of an herbal formula.
Based on the herbal recipe tissue pharmacology hypothesis, this study confirmed that the tissue distributions of emodin, rhein, aloe-emodin, chrysophanol, rheochrysidin, naringin, naringenin, hesperidin, magnolol and honokiol in the liver of rats treated with high dose DCQD showed a maximal effect in ameliorating the pathological damages. DCQD may ameliorate the liver injury complicated with acute pancreatitis (AP) by alleviating the inflammatory response.
This study provides insights for the implementation and further screening for effective components of Chinese herbs in ameliorating acute liver injury complicated with AP.
A high-performance liquid chromatography tandem mass spectrometry method with a long analytical column and negative MRM mode was confirmed as a specific, sensitive, accurate and reproducible method to successfully identify the 10 major components of DCQD in dog plasma after oral administration.
Overall this is a well conducted animal study and the authors did a very good job to investigate the mechanism by which DCQD ameliorates acute liver injury complicated with AP in rats. It is greatly appreciated.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Akamatsu N, Barreto S, Liao KF, Manenti A, Peng SY S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 512] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 2. | Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Multiple organ dysfunction associated with severe acute pancreatitis. Crit Care Med. 2002;30:1274-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Closa D, Bardají M, Hotter G, Prats N, Gelpí E, Fernández-Cruz L, Roselló-Catafau J. Hepatic involvement in pancreatitis-induced lung damage. Am J Physiol. 1996;270:G6-13. [PubMed] |
| 5. | Folch-Puy E. Importance of the liver in systemic complications associated with acute pancreatitis: the role of Kupffer cells. J Pathol. 2007;211:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Liu HB, Cui NQ, Li DH, Chen C. Role of Kupffer cells in acute hemorrhagic necrotizing pancreatitis-associated lung injury of rats. World J Gastroenterol. 2006;12:403-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Folch E, Prats N, Hotter G, López S, Gelpi E, Roselló-Catafau J, Closa D. P-selectin expression and Kupffer cell activation in rat acute pancreatitis. Dig Dis Sci. 2000;45:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Liu W, Liu X, Sheng M, Pei Y, Lei R, Zhang S, Tao R. Role of liver in modulating the release of inflammatory cytokines involved in lung and multiple organ dysfunction in severe acute pancreatitis. Cell Biochem Biophys. 2015;71:765-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Xia Q. The research on the mechanism of’TongLi GongXia’method to treat acute pancreatitis. Zhongguo Puwai Jichu Linchuang Zazhi. 2001;8:131-132. |
| 10. | Wan MH, Li J, Huang W, Mukherjee R, Gong HL, Xia Q, Zhu L, Cheng GL, Tang WF. Modified Da-Cheng-Qi Decoction reduces intra-abdominal hypertension in severe acute pancreatitis: a pilot study. Chin Med J (Engl). 2012;125:1941-1944. [PubMed] |
| 11. | Zhao X, Zhang Y, Li J, Wan M, Zhu S, Guo H, Xiang J, Thrower EC, Tang W. Tissue Pharmacology of Da-Cheng-Qi Decoction in Experimental Acute Pancreatitis in Rats. Evid Based Complement Alternat Med. 2015;2015:283175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Tang W, Wan M, Huang X. Tissue pharmacology of recipe: a new hypothesis. Zhongguo Zhongyao. 2005;36:1-3. |
| 13. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1256] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 14. | Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Tang W, Wan M, Zhu Z, Chen G, Huang X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin Med. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zhu L, Zhao J, Peng X, Wan M, Huang X, Tang W. Pharmacological study on free anthraquinones compounds in rhubarb in rats with experimental acute pancreatitis. Zhongguo Zhongyao. 2014;39:304-308. |
| 17. | Burns GP, Stein TA, Kabnick LS. Blood-pancreatic juice barrier to antibiotic excretion. Am J Surg. 1986;151:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Zhao Y, Liao Q, Xue C. [The effect of blood-pancreatic juice barrier on antitumor drugs excretion]. Zhonghua Waike Zazhi. 1997;35:302-304. [PubMed] |
| 19. | Catala M, Anton A, Portoles MT. Characterization of the simultaneous binding of Escherichia coli endotoxin to Kupffer and endothelial liver cells by flow cytometry. Cytometry. 1999;36:123-130. |
| 20. | van Oosten M, van de Bilt E, van Berkel TJ, Kuiper J. New scavenger receptor-like receptors for the binding of lipopolysaccharide to liver endothelial and Kupffer cells. Infect Immun. 1998;66:5107-5112. [PubMed] |
| 21. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 510] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 22. | Fisic E, Poropat G, Bilic-Zulle L, Licul V, Milic S, Stimac D. The Role of IL-6, 8, and 10, sTNFr, CRP, and Pancreatic Elastase in the Prediction of Systemic Complications in Patients with Acute Pancreatitis. Gastroenterol Res Pract. 2013;2013:282645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Stimac D, Fisić E, Milić S, Bilić-Zulle L, Perić R. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. J Clin Gastroenterol. 2006;40:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Farkas G, Márton J, Nagy Z, Mándi Y, Takács T, Deli MA, Ábrahámd CS. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neurosc Lett. 1998;242:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Yin K, Zhao G, Huang X, Gao G, Sun H, Wei Q, Liu Q, Li M, Xu C, Zhu S. Inhibition of RhoA expression by adenovirus-mediated siRNA combined with TNF-α induced apoptosis of hepatocarcinoma cells. Biomed Mater Eng. 2015;26 Suppl 1:S2055-S2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Oswald IP, Gazzinelli RT, Sher A, James SL. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578-3582. [PubMed] |
| 30. | Geissler K. Current status of clinical development of interleukin-10. Curr Opin Hematol. 1996;3:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L, Vento M, Lopez-Rodas G, Sastre J. Role of redox signaling, protein phosphatases and histone acetylation in the inflammatory cascade in acute pancreatitis. Formerly Current Drug Targets-Inflammation Allergy. 2010;9:97-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Jiang HY, Wang CY. Effects of Da Cheng Qi Decoction on the content of TNF-α, IL-6 and NO in rats with acute liver injury. Jilin Zhongyiyao. 2008;28:8-9. |
| 34. | ZL L. Effects of Da Cheng Qi Decoction on Nucleic Acld Content in the Liver of Experimental Rats. Guangzhou Zhongyiyao Daxue Xuebao. 1988;5:211-213. |
| 35. | Wang FL, Yang HZ, Li YM, Wu WK, Zou ZC. [Prevention and treatment mechanism of qingxia therapy (based on yinchenhao decoction and dachengqi decoction) on hepatocyte apoptosis in rats with acute hepatic injury induced by lipopolysaccharide/D-galactosamine]. Zhong Yao Cai. 2014;37:848-852. [PubMed] |
| 36. | Chtourou Y, Fetoui H, Jemai R, Ben Slima A, Makni M, Gdoura R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur J Pharmacol. 2015;746:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Hu B, Zhang H, Meng X, Wang F, Wang P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol. 2014;153:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Gao Y, Chen X, Fang L, Liu F, Cai R, Peng C, Qi Y. Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic Biol Med. 2014;72:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Ni Q, Zhang W, Sun K, Yin C, An J, Shang D. In vitro effects of emodin on peritoneal macrophage intercellular adhesion molecule-3 in a rat model of severe acute pancreatitis/systemic inflammatory response syndrome. Biomed Rep. 2014;2:63-68. [PubMed] |
| 40. | Zhao Q, Cui N, Li J. Clinical and Experimental Study of Effect on Acute Phase Protein Level of Multiple Organ Dysfunction Syndrome Treated with Dachengqi Decoction. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:453-456. [PubMed] |
| 41. | Chen Z, Chen Y, Pan L, Li H, Tu J, Liu C, Dai X, Zhang X, Sun G, Feng D. Dachengqi Decoction Attenuates Inflammatory Response via Inhibiting HMGB1 Mediated NF-κB and P38 MAPK Signaling Pathways in Severe Acute Pancreatitis. Cell Physiol Biochem. 2015;37:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |