Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1250
Peer-review started: November 11, 2016
First decision: December 19, 2016
Revised: December 26, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 21, 2017
Processing time: 102 Days and 15 Hours
To study the morphology of the enteric nervous system and the expression of beta-2 adrenergic (B2A) receptors in primary colorectal cancer.
In this study, we included forty-eight patients with primary colorectal cancer and nine patients for control tissue from the excision of a colonic segment for benign conditions. We determined the clinicopathological features and evaluated the immunohistochemical expression pattern of B2A receptors as well as the morphological changes of the enteric nervous system (ENS). In order to assess statistical differences, we used the student t-test for comparing the means of two groups and one-way analysis of variance with Bonferroni’s post hoc analysis for comparing the means of more than two groups. Correlations were assessed using the Pearson’s correlation coefficient.
B2A receptors were significantly associated with tumor grading, tumor size, tumor invasion, lymph node metastasis (P < 0.05), while there were no statistically significant associations with gender, CRC location and gross appearance (P > 0.05). We observed, on one hand, a decrease of the relative area for both Auerbach and Meissner plexuses with the increase of the tumor grading, and on the other hand, an increase of the relative area of other nervous elements not in the Meissner plexus or in the Auerbach plexus with the tumor grading. For G1 tumors we found that epithelial B2A area showed an inverse correlation with the Auerbach plexus areas [r(14) = -0.531, P < 0.05], while for G2 tumors, epithelial B2A areas showed an indirect variation with both the Auerbach plexus areas [r(14) = -0.453, P < 0.05] and the Meissner areas [r(14) = -0.825, P < 0.01]. For G3 tumors, the inverse dependence increased for both Auerbach [r(14) = -0.587, P < 0.05] and Meissner [r(14) = -0.934, P < 0.05] plexuses.
B2A receptors play an important role in colorectal carcinogenesis and can be utilized as prognostic factors. Furthermore, study of the ENS in colorectal cancer may lead to targeted molecular therapies.
Core tip: In the present study, we provide the first description of beta-2 adrenergic (B2A) receptors found on the nuclear membrane of colon adenocarcinoma cells. B2A receptors have a high level of expression in the neoplastic cells from colorectal adenocarcinoma, and a significant association was found between the expression of B2A receptors and tumor grading. Regarding the enteric nervous system and its associations with colorectal cancer, we observed a decrease of the relative area, both for Auerbach and Meissner plexuses, with the increase of the tumor grading, and an increase of the relative area of other nervous elements with the tumor grading.
- Citation: Ciurea RN, Rogoveanu I, Pirici D, Târtea GC, Streba CT, Florescu C, Cătălin B, Puiu I, Târtea EA, Vere CC. B2 adrenergic receptors and morphological changes of the enteric nervous system in colorectal adenocarcinoma. World J Gastroenterol 2017; 23(7): 1250-1261
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1250
Despite the progress of recent years in the field of antineoplastic therapy, colorectal cancer still remains one of the major public health problems. In the United States, at January 1st 2016, out of 15.5 million patients suffering from cancer, 724690 men had cancer of the colon and rectum; this cancer being the third most prevalent in women after breast and uterine corpus cancer[1]. Regarding the mortality, colon and rectum cancer rank as the fourth cause of mortality in the world with 694000 deaths, representing about 8.5% of the total deaths caused by cancer in 2012[2]. Translational studies that identify new cancer prognostic markers, molecular changes that appear at the initiation of carcinogenesis and during the progression phase, as well as treatment targets such as elements of the nervous system, are thus needed[3].
The idea of establishing connections between cancer and psychological influences originates from the Greek doctor Galen, who in the second century before Christ, showed that women with melancholic temperament develop breast cancer more frequently than other temperamental types[4].
The enteric nervous system (ENS) is the most complex part of the autonomic nervous system[5]. It consists of three major ganglionic plexuses (mucous plexus, submucosal plexus also called Meissner, and myenteric plexus also called Auerbach), together with many aganglionar plexuses[6]. The Auerbach plexus is found starting at the esophagus and extending to the terminal part of the digestive tract, between circular and longitudinal muscular layers[7]. The ENS consists of approximately 500 million neurons, more than in the peripheral autonomic nervous system[8]. The ENS is developed from the neural crest, from the sacral and vagal segments of the neural tube[9].
The ENS is connected with the central nervous system via sympathetic and parasympathetic pathways, a connection known as the cerebroenteral axis[10]. The background of the connections between the two divisions of the autonomic nervous system is represented by neurotransmitters such as acetylcholine for the parasympathetic nervous system and norepinephrine for the sympathetic nervous system[5]. Although norepinephrine is not found in the enteric neurons, but only in the sympathetic afferent fibers that they receive, Li et al[11] showed that norepinephrine transporter, which plays an important role in mediating the effects of norepinephrine, is expressed by enteric neurons. Some of these neurotransmitters act on receptors which are also expressed by colorectal carcinoma cells[4,11]. B2A receptors are part of the family of catecholamine receptors, and their activation can initiate multiple signaling pathways in colorectal tumorigenesis[12]. Moreover, blocking them may slow down or even stop the progression of the neoplastic process, but in this case, the studies are contradictory[12].
Our aim was to compare the morphological changes of the ENS and to evaluate the expression changes of the B2A receptors in primary colorectal cancer versus normal colic mucosa, and also to investigate significant associations between these parameters and the clinicopathological features.
In this study, we analyzed specimens from forty-eight consecutive patients who underwent surgery with potentially curative resection for primary colorectal cancer, performed in the 1st Surgery Clinic of the Emergency County Hospital, Craiova, Romania. Patients were diagnosed with colorectal cancer in the 1st Medical Clinic, Gastroenterology Department of the Emergency County Hospital of Craiova between 2015 and 2016. As controls, we used normal colorectal tissue taken from nine patients who were diagnosed with intestinal obstruction or other pathologies that needed surgery in order to perform the excision of a colonic segment. The study was approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova (registration no. 53/19.05.2016), according to the Declaration of Helsinki. All patients included in the study provided written acceptance and informed consent. In order to obtain clinical information from the patients, we reviewed their medical records for gender, age, tumor size, sites of the primary tumor (right, transverse, left and sigmoid colon and rectum), T and N stages according to the American Joint Committee on Cancer, histological grading according to World Health Organization criteria including well, moderate, or poor differentiated colorectal adenocarcinoma[13].
After we reviewed the slides and confirmed the pathologies and tumor gradings, three μm-thick serial sections were cut from each block, deparaffinized in xylene, rehydrated in graded alcohol series, and subjected to enzymatic immunohistochemistry utilizing a rabbit-anti-human anti-beta-2 adrenergic receptor (NBP2-15564, diluted as 1:200, Novus Biologicals, United Kingdom) and a rabbit anti-human anti-S100 (Z0311, diluted as 1:100, Dako, Glostrup, Denmark) primary antibodies. Briefly, the sections were first processed for antigen retrieval by microwaving in citrate buffer pH 6 for 20 min, incubated in 1% hydrogen peroxide in distilled water for 30 min to block the endogenous peroxidase activity, kept for another 30 min in 3% skimmed milk in PBS, then incubated with the primary antibodies at 4°C for 18 h. Next day, the signal was amplified for 30 minutes utilizing a species-specific peroxidase polymer-based system adsorbed for human immunoglobulins (Nikirei-Bioscience, Tokyo, Japan). The signal was finally detected with 3,3’-diaminobenzidine (DAB) (Dako) and the slides were coverslipped in DPX (Sigma-Aldrich, St. Louis, MO, United States) after a hematoxylin and eosin staining. All slides stained for each of the primary antibodies have been processed at the same time for protocol consistency together with control slides stained either with DAB or with hematoxylin and eosin in order to obtain pure spectral signatures of the respective stains (see further below). Negative controls were obtained by omitting the primary antibodies.
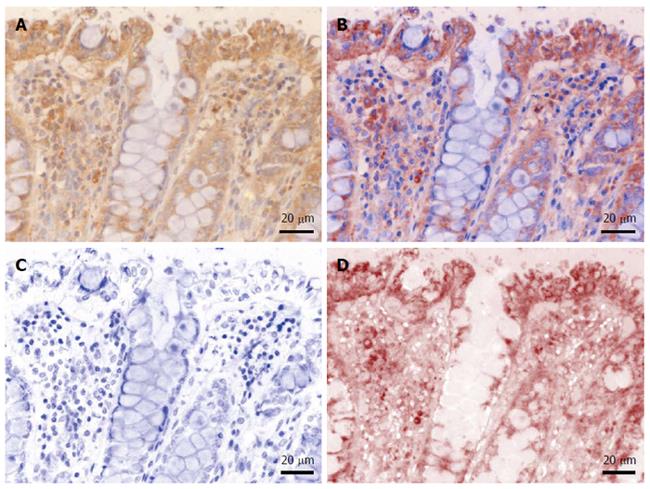
In order to evaluate and quantify the immunohistochemical expression of the targets, light microscopy images were acquired on a Nikon Eclipse 90i motorized microscope (Elta90, Bucharest, Romania) equipped with a Nuance FX multispectral camera and the Nuance analysis software (Perkin Elmer, Hopkinton, MA, United States). After building a spectral library from individual slides stained with either hematoxylin or DAB (as described above), we were able to efficiently unmix and characterize the immunohistochemical expression patterns of interest (Figure 1). Furthermore, unmixed DAB signal was quantified as area and integrated optical density (average intensity/density of each area of interest) on 10 random images captured with a 20 × objective, using the Image-Pro Plus AMS 7 image analysis software (Media Cybernetics, Bethesda, MD, United States); the resulting data were averaged for each patient, and finally averaged and compared for each histopathological grade. Stroma was also considered separately in this analysis, by manually defining regions of interest in the captured images prior to their analysis.
The total identifiable submucosal (Meissner's), myenteric (Auerbach's), and intratumoral nervous plexuses, as well as the multiaxonal bundles of nerves (> 20 μm) were imaged on the slides stained for S100 protein, and areas measured by manually defining the respective regions of interest. For referring densitometry data to total histological areas, whole hematoxylin-eosin slides were scanned on a desktop Benq 5560 scanner together with a microscopic stage micrometer at 4.800 dpi against a white background. Image files were loaded in Image-Pro Plus, and after pixel-size calibration for 1 mm against the microscopic stage micrometer, images were segmented and areas measured based on the same RGB profile created to automatically select the tissue on all the slides.
Data obtained after using the Image-Pro Plus AMS software were exported and plotted in Microsoft Office Excel 2010 (Microsoft Corporation, Redmond, Washington, United States) and were analyzed by using SPSS software (IBM SPSS Statistics, Version 20.0). In order to assess statistical differences we used the student t-test for comparing the means of two groups and one-way ANOVA (ANOVA - analysis of variance) with Bonferroni’s post hoc analysis in order to compare the means of more than two groups. Correlations were assessed using the Pearson’s correlation coefficient. Data were reported as mean ± SD or the standard error of the means (SE). In all cases, P < 0.05 was used to indicate statistical significance.
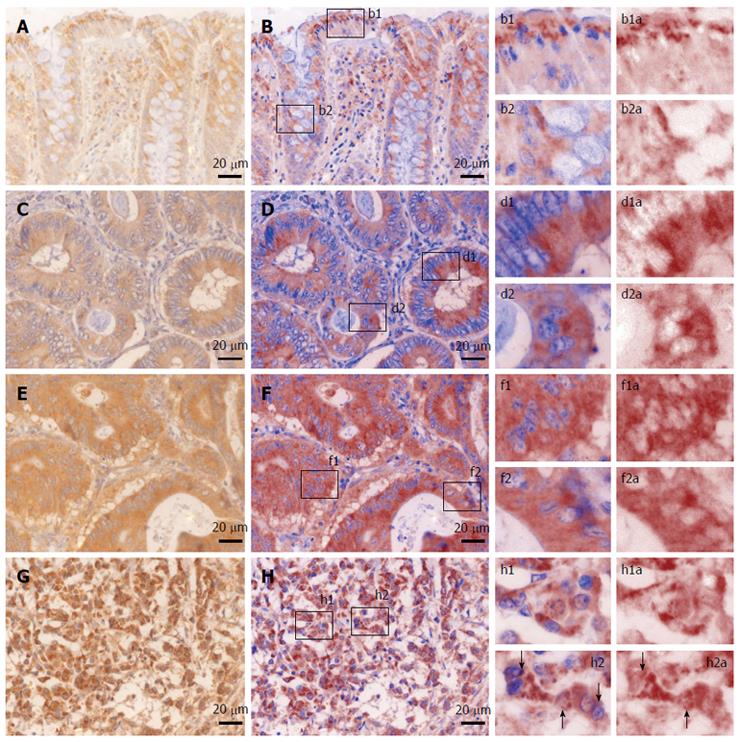
We first sought to characterize the morphological expression of B2A receptor, taking advantage of the multispectral unmixing microscopy (Figure 2). Besides a faint diffuse cytoplasmic reaction on normal colon mucosae, B2A exhibited a granular-like pattern in the cytoplasm of the enterocytes above the nuclei, towards the luminal side of the covering epithelium. In the goblet cells, the signal was localized most frequently below the nucleus. Stromal cells also showed granular staining in their cytoplasmic compartment.
In well differentiated adenocarcinomas, most of the signal was still located towards the luminal side of the tumor cells, although on occasion granules could be clearly identified in the basal pole of the cells.
In moderately differentiated adenocarcinomas, the signal seemed to lose its granular appearance, becoming more diffuse and more intense in the epithelial cells.
In poorly differentiated adenocarcinomas, the intensity of the signal increased in the cytoplasm of the tumor cells, where on a general intense background, one could also identify very intense hotspots surrounding the nuclei with a random-like disposition against them. This time, a clear-cut granular signal could now be observed on occasions in the nuclei or the folds of the nuclear membrane of the tumor cells. A direct counting of the epithelial tumor cells exhibiting this intranuclear/close perinuclear expression pattern of B2A revealed a heterogenous distribution with an average of 12.84% positive- cells [± 12.10% (SD), min = 3%, max = 49%].
On average, there was no signal difference between different tumor stages for the staining of the stromal elements.
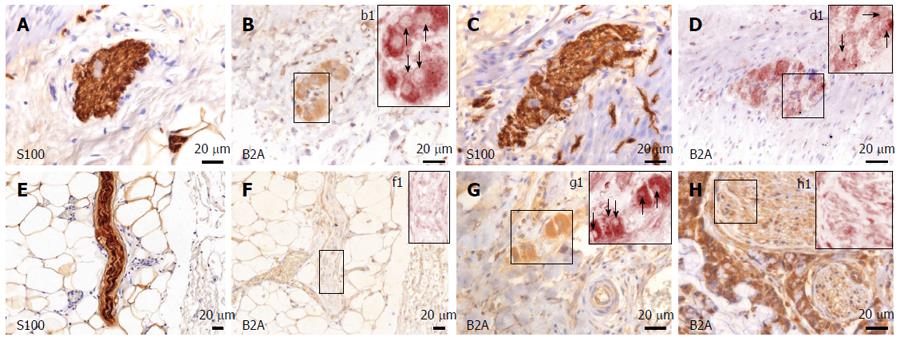
B2A receptors were also present in both stromal cells as well as nerves and nervous ganglia. In both submucosal and myenteric plexuses from the normal colon, the marker exhibited a dense-diffuse pattern in the cytoplasm of the ganglion cells, as well as in the satellite cells (Figure 3). Its expression was much fainter in the Schwann cell cytoplasm within the nerve bundles associated with non-tumor tissue. In the tumor tissue, there was a somewhat denser appearance in the cytoplasm of ganglion cells, and interestingly, larger nerve bundles showed a clear-cut increase in signal density compared to non-tumor tissue. Smaller nerve bundles (deemed thinner than 20 μm for data stratification) were stained very faintly, as in the control tissue.
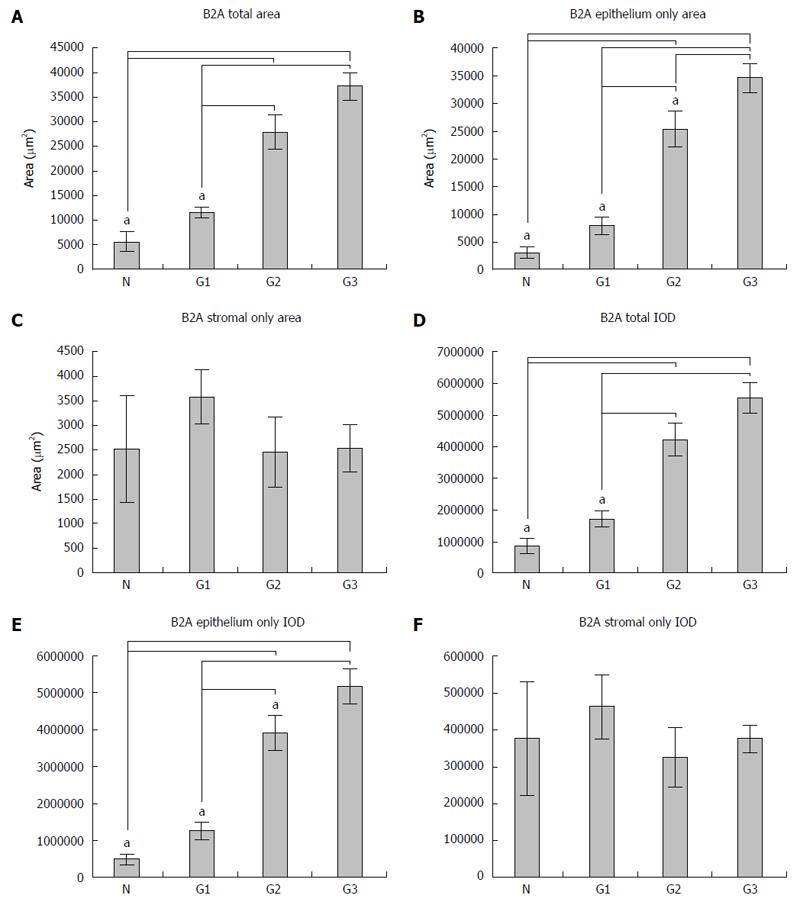
In this study, nine patients as controls and sixteen for each tumor grading were included. The expression of B2A receptors was analyzed by determining both the area and the integrated optical density (IOD) of the signal, both in the total tissue and in the epithelial and stromal tissue for all the cases included in the study, as described in the Materials and Methods section; this was expressed as the mean ± SD. Regarding the expression of B2A receptors in the total tissue belonging to each patient included in the study, we observed that there was a gradual increase of both the area and IOD (Figure 4A and D) from the normal tissue to G1, G2 and G3 differentiated adenocarcinoma (from 5598.4 ± 3393.9 μm2 for area and 862176.0 ± 469798.7 for IOD in normal tissue to 11583.3 ± 5521.3 μm2 for area and 1717361.9 ± 886266.2 for IOD in G1, 27891.1 ± 12118.5 μm2 for area and 4240529.2 ± 1795221.7 for IOD in G2 and 37218.5 ± 9738.5 μm2 for area and 5560460.5 ± 1720879.6 for IOD in G3). In this instance, we observed a statistical significance on one-way analysis of variance with post hoc comparisons using the Bonferroni’s test between normal tissue and G2, G3 (P = 0.000) and also between G1 and G2, G3 (P = 0.000) both for the area of B2A and for the IOD.
Regarding the expression of B2A receptors only in the epithelium areas, this had a similar growth to its expression in the total tissue (Figure 4B and E); from normal tissue to G1, G2 and G3 differentiated adenocarcinoma (from 3076.5 ± 1895.5 μm2 for area and 485440.8 ± 299641.9 for IOD in normal tissue to 7997.6 ± 5344.5 μm2 for area and 1255032.6 ± 834235.5 for IOD in G1, 25423.0 ± 11100.6511 μm2 for area and 3914461.91 ± 1673240.4 for IOD in G2 and 34670.1 ± 9146.9 μm2 for area and 5184455.0 ± 1670704.2 for IOD in G3). Moreover, in this situation, we also observed a statistically significant difference between the normal tissue and G2, G3, similar to the expression of B2A receptors in the total tissue (P = 0.000) and G1, G2 and G3 (P = 0.000), but in addition, we also noticed a statistically significant difference between G2 and G3 (P = 0.047) only for the area, while for IOD there was no significant difference (P = 0.141).
No significant differences between the means of B2A receptor expression and IOD in the stroma of the normal colonic mucosa and different gradings of the colorectal adenocarcinoma could be observed (Figure 4C and F). These data show an increase of the expression of B2A receptors with the tumor grading with regard to the epithelium and not the stroma.
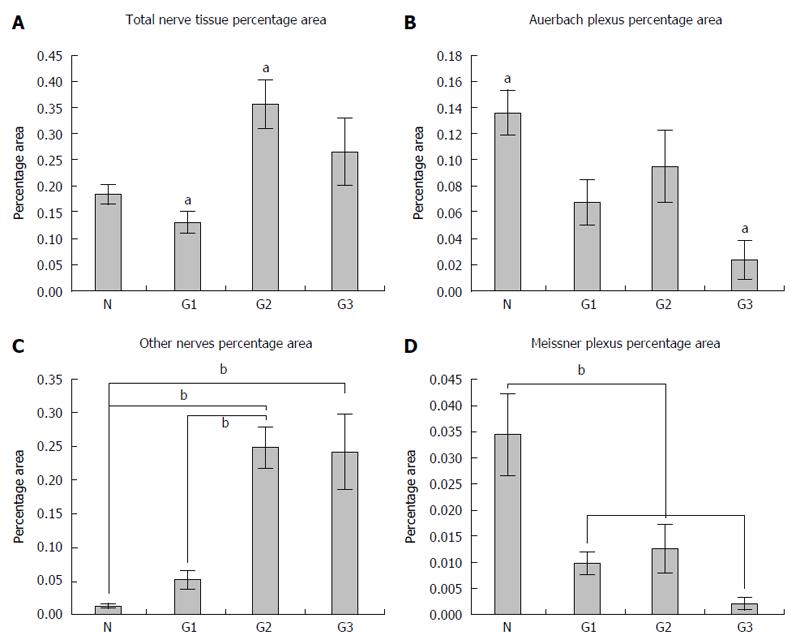
We analyzed the submucosal (Meissner’s), myenteric (Auerbach’s) and intratumoral nervous plexuses, as well as those of multiaxonal bundles of nerves (larger than 20 μm) that could not be included either in the Auerbach or in the Meissner plexus, by calculating the percentage average of the area of total nervous tissue separately for both the normal colonic tissue and in different tumor gradings.
The density of the total nervous tissue expressed by percentage area recorded the smallest values in G1 graded tumors (0.129% ± 0.052%), followed by normal colonic tissue (0.184% ± 0.041%), G2 graded tumors (0.355% ± 0.131%) and G3 graded tumors (0.264% ± 0.172%) (Figure 5A). Here, statistical differences between the percentage area from the normal colonic tissue and percentage area from G2 tumors (P = 0.013) were observed.
The density of the Auerbach plexus relative areas revealed a net decrease from the normal tissue (0.136% ± 0.039%) to G1 (0.067% ± 0.043%), G2 (0.094% ± 0.078%) with a minimum for G3 gradings (0.023% ± 0.040%) (Figure 5B). As far as this plexus’ density was concerned, significant differences were recorded between the percentage area from the normal colonic tissue and the percentage area from G3 (P = 0.013) tumors.
On the other hand, larger nervous bundles showed about the same pattern for the total analyzed areas (Figure 5C), but with a growth of the percentage nervous area in the normal colonic mucosa (0.013% ± 0.006%), in G1 (0.052% ± 0.033%), G2 (0.248% ± 0.087%) and in G3 (0.241% ± 0.146%) tumors. In this case, significant differences between the percentage nervous area in the normal colonic tissue and the area in G2 (P = 0.001) and G3 tumors (P = 0.002), and also between the percentage nervous area in G1 and the percentage nervous area in G2 (P = 0.004) and G3 tumors (P = 0.008), were recorded.
Furthermore, differences between the relative area of Meissner plexuses revealed the same pattern as for the Auerbach plexuses (Figure 5D), with a maximum in the normal colonic tissue (0.034% ± 0.017%), followed by G1 (0.009% ± 0.005%), G2 (0.012% ± 0.013%) and with a minimum in G3 tumors (0.002% ± 0.003%).
These data suggest, on one hand, a decrease of the relative area of both Auerbach and Meissner plexuses with increasing tumor grading, and on the other hand, an increase of the relative area of other nervous elements that could not be included either in the Meissner plexus or in the Auerbach plexus, again with the tumor grading.
Both B2A receptor expression area and IOD were significantly linked with tumor size, tumor invasion and lymph node metastasis, while there were no statistically significant connections with gender, CRC location and gross appearance. Regarding the involvement of age, there was no significant difference between B2A area in patients under 60 years old and patients over this age, while between B2A IOD and patients included in these age groups we found a significant difference (P = 0.018). These data are summarized in Table 1.
| Clinicopathological features | n | B2A only area (μm2) mean ± SD | P value1 | B2A only IOD mean ± SD | P value1 |
| Gender | |||||
| Male | 38 | 25332.4 ± 15753.5 | 0.126 | 2861294.1 ± 1735364.6 | 0.109 |
| Female | 19 | 19450.2 ± 11703.5 | 3833335.5 ± 2431953.4 | ||
| Age group | |||||
| < 60 | 15 | 28796.9 ± 13781.3 | 0.085 | 4742202.6 ± 2372372.902 | 0.018a |
| ≥ 60 | 42 | 21584.2 ± 14860.9 | 3086368.8 ± 2092543.9 | ||
| Tumor size | |||||
| < 5 cm | 23 | 15778.7 ± 13661.5 | 0.003a | 2463149.731 ± 2229187.1 | 0.008a |
| ≥ 5 cm | 25 | 28241.1 ± 13584.4 | 4188867.1 ± 2074271.4 | ||
| CRC location | |||||
| Right and transvers colon | 9 | 24817.5 ± 19874.7 | 0.412 | 3818617.6 ± 3131069.5 | 0.375 |
| Left colon, sigmoid and rectum | 39 | 23347.2 ± 14047.3 | 3492859.3 ± 2140467.2 | ||
| Gross appearance | |||||
| Exophytic | 20 | 23166.8 ± 14796.3 | 0.429 | 3260358.1 ± 2057001.7 | 0.208 |
| Infiltrative, ulcero-infiltrative | 28 | 24010.8 ± 15114.6 | 3852705.4 ± 2501409.4 | ||
| Tumor invasion | |||||
| T1-2 | 17 | 11110.3 ± 6784.4 | 0.000a | 1650300.5 ± 1004078.8 | 0.000a |
| T3-4 | 31 | 36025.2 ± 8632.1 | 5433145.7 ± 1423440.9 | ||
| Lymph node metastasis | |||||
| N0-1 | 21 | 19264.3 ± 12628.1 | 0.010a | 2904065.0 ± 1922977.6 | 0.013a |
| N≥ 2 | 27 | 30022.8 ± 15745.7 | 4498210.2 ± 2467186.4 |
Moreover, for G1 tumors we found that epithelial B2A area showed an inverse correlation with the Auerbach plexus areas [r(14) = -0.531, P < 0.05], while for G2 tumors, epithelial B2A areas showed an indirect variation with both the Auerbach plexus areas [r(14) = -0.453, P < 0.05] and the Meissner areas [r(14) = -0.825, P < 0.01]. For G3 tumors, the inverse dependence increased for both Auerbach [r(14) = -0.587, P < 0.05] and Meissner [r(14) = -0.934, P < 0.05] plexuses. In control tissue, we also found a strong indirect correlation between the B2A signal area and the Auerbach relative areas [r(7) = -0.897, P < 0.01], but not with the Meissner plexuses.
Both tumor microenvironment and its constituents have an extremely important role in carcinogenesis and in tumor progression. The interaction of neoplastic and of endothelial cells and the extracellular matrix with the cells of the immune system and also with other elements have been carefully analyzed for their contribution as putative molecular targeted (antiangiogenic) therapies[14]. However, the mechanisms that drive the pathogenesis and evolution of neoplasms are far from being understood. In colorectal neoplasm, nervous elements from the tumor microenvironment seem to play an important role. Regarding, for example, perineural invasion, Liebl et al[15], in a study that included 673 patients diagnosed with colorectal cancer, concluded that nerve invasion influences in a negative way the survival of the patients with colorectal cancer. However, it is not known if the nervous elements intervene first in the neuro-neoplastic chain, or if this role belongs to neoplastic cells both at the colorectal level and in other localizations of the neoplastic process.
In our study, we approached the neuro-neoplastic interrelationships in colorectal adenocarcinoma, both as a structural characterization (by showing the changes of the ENS) and as an indirect functional characterization (by analyzing the expression of B2A receptors).
Regarding the morphology of the ENS, we found a decrease of the relative area, both for Auerbach and Meissner plexuses, with the increase of the tumor grading and on the other hand, an increase of the relative area of other nervous elements that could not be stratified in the Meissner plexus or in the Auerbach plexus with the tumor grading. A study from 2004 showed that degenerative changes of the intrinsic neurons of the digestive tract cause either direct proportional variation between the size of nervous ganglions and tumor invasion and also an inverse proportional variation with the expression of synaptophysin in the enteric neurons of the colon[16]. However, Godlewski showed that enteric nervous elements were not found in solid colorectal tumors, a finding which in fact supports some symptoms of patients with colorectal carcinoma[17]. Kozlowska et al[18] recently discovered that atrophy of the myenteric plexus takes place in the immediate vicinity of the colorectal neoplasm, leading to a decrease both in the size of the nervous ganglia and also in the number of neurons per plexus, in comparison with the nervous plexuses located at a distance from the tumor advancing edge, but this atrophy did not occur in the submucosal plexus. The atrophy of the myenteric plexus is most probably not caused by apoptosis, as both the extrinsic pathway of apoptosis activation via caspase 8 (CASP8) and the intrinsic pathway via activation of the caspase 3 (CASP3) are not different between the myenteric plexuses situated in the immediate vicinity of the colorectal neoplasm and those which are located at a distance from this process[18]. Many of the symptoms specific to colorectal cancer, such as change in bowel habit, flatulence, bloating and mucus discharge, constipation due to the alteration of the peristalsis, abdominal pain and others[19] can be explained by the structural alterations of the ENS, highlighted both in our study as well as in the studies mentioned above.
Regarding the neuro-neoplastic interrelationships at the colorectal level, we chose to study the expression of B2A receptors, because many intracellular signaling pathways have been discovered via this type of receptors up till now; these are involved in the initiation, progression and cancer metastasis. In a previous study of a group of three patients, we have recently reported that the expression of B2A receptors increases with the tumor grading, but a large cohort study is still necessary in order to confirm this observation[20]. Neurotransmitters which act on this type of receptor are mainly adrenaline and noradrenaline. The role of noradrenaline in colorectal cancer development was highlighted by an increase in the locomotor activity of SW 480 colon carcinoma cells and by blocking its effects on this type of cells after using the beta1/2 blocker propranolol[21]. Moreover, the norepinephrine transporter, which plays an important role in mediating the effects of norepinephrine, is expressed by the enteric neurons[11]. Also, adrenaline acts on this type of receptor, being the most important agonist of B2A receptors[22]. Several studies have shown the effects of adrenaline in colorectal adenocarcinoma[23-26]. It is well known that B2A receptors for these neurotransmitters are G protein-coupled receptors[27], and their activation may trigger complex intracellular signaling involved in the carcinogenesis process, such as the activation of the arachidonic acid cascade and also other pathways via the second messenger cyclic adenosine monophosphate (cAMP)[14,28]. Other intracellular signaling pathways are: proto-oncogene tyrosine-protein kinase Src (c-Src), involved in cancer progression and also in promoting other signals; mitogen-activated protein kinase (MAPK); protein kinase B(Akt); extracellular signal-regulated kinases (ERK1/2); cAMP response elements (CREB), which are linked to certain DNA sequences that decrease or increase the transcription of variate genes downstream[12,29,30]. This type of receptor was also studied in other types of cancer including for example pancreas cancer[28], lung cancer[31], breast cancer[32] and prostate cancer[33]. Moreover, our analysis revealed a possible intranuclear/tight perinuclear localization of B2A receptors in poorly differentiated adenocarcinoma, suggesting a connection between its arresting on the downstream of the signaling pathways and more drastic cellular dedifferentiation and loss of function/gain of function signaling misbalances. Although more data are not yet available to clearly show the connection between B2A receptor levels and tumor recurrence, corresponding to the clinical outcomes of these patients, we are following up these patients for future reference. To date, functional B2A receptors have been reportedly found on the nuclear membranes of ventricular myocardiocytes[34], but to our knowledge this is the first description for colon adenocarcinoma cells. Further functional and electron microscopy studies will still be needed to clarify the implications of this observation.
In our study, it was not possible to know for sure if the nervous structures that were deemed as other nerves were actually modified ganglia from the two plexuses or whether they represent sympathetic or parasympathetic afferents or efferents. They could not be catalogued either as ganglia belonging to the Meissner plexus or as ganglia belonging to the Auerbach plexus due to the structural changes caused by the neoplastic process. Neuro-neoplastic functional studies are required in order to find out if these nervous elements found in advanced gradings of colorectal adenocarcinoma secrete adrenaline and noradrenaline, and in this case, the regulation of B2A receptors examined in our study would be paracrine; however, the source of catecholamines could only be represented by the medulla, which seems to be least plausible if we take into account the short half-life of the catecholamines that activate B2A receptors[22].
We showed here that B2A area of expression in the tumor epithelium shows a moderate to strong inverse correlation with the areas of the Auerbach and Meissner plexuses. Although these are components of the stroma, there was no significant correlation between stromal or total B2A area and the number of nervous plexuses. There are too few elements to judge a functional association between these factors, but this is to our knowledge the first report of such a correlation for colon cancer. However, there are studies on breast tumors showing that stress-induced neuroendocrine activation leads to a massive increase of metastasis, and treatment of stressed animals with the β-antagonist propranolol reversed the stress-induced inflammatory infiltrate and inhibited tumor metastasis[32]; all these suggesting that the sympathetic nervous system might act as a regulator for tumor spreading and metastasis.
In conclusion, B2A receptors have a high expression level in the neoplastic cells from colorectal adenocarcinoma, and we found a significant association between the expression of B2A receptors and tumor grading. This shows that B2A receptors may play an important role in antineoplastic therapy or that they can be utilized as a prognostic factor. On the other hand, besides the functional changes caused by B2A receptors, structural changes of the ENS caused by the neoplastic process and the upward trend of the relative area of the nerves in colorectal adenocarcinoma might suggest that studies to develop targeted molecular therapies could be aimed not only at angiogenesis but also at neurogenesis in colorectal cancer; this might represent an important treatment pathway.
Colorectal cancer still remains one of the major health problems worldwide. In this article, the authors wanted to compare the morphological changes of the enteric nervous system (ENS) and to evaluate the changes in expression of the beta-2 adrenergic (B2A) receptors in primary colorectal cancer compared with normal colic mucosa, and also to find significant associations between these parameters and clinicopathological features.
The mechanisms that drive the pathogenesis and the evolution of neoplasms are far from being understood. Translational studies that identify new colorectal cancer prognostic markers, molecular changes that appear at the initiation of carcinogenesis and during cancer’s progression, as well as treatment targets such as nervous elements, are thus needed.
The authors provide the first description of B2A receptors found in/on the nuclear membrane of colon adenocarcinoma cells. B2A receptors have a high level of expression in the neoplastic cells from colorectal adenocarcinoma, and a significant association was found between the expression of B2A receptors and tumor grading. Until now, many intracellular signaling pathways have been discovered via this type of receptor, which are involved in the initiation, progression and cancer metastasis. On the other hand, in colorectal neoplasm, nervous system elements from the tumor microenvironment seem to play an important role.
The present study looks into B2A receptors and morphological changes of the ENS in primary colorectal cancer. B2A receptors may play an important role in antineoplastic therapy, and they can be utilized as a critical prognostic factor. On the other hand, the structural changes of the ENS in colorectal adenocarcinoma may lead to targeted molecular therapies not only regarding angiogenesis, but also concerning neurogenesis in colorectal cancer.
B2A receptors are G protein-coupled receptors and their activation may trigger complex intracellular signaling involved in the carcinogenesis process. Neurotransmitters which act on this type of receptor are mainly adrenaline and noradrenaline.
The authors investigated the neuro-neoplastic interrelationship in colon cancer. Authors assessed the expression pattern of B2A receptors and morphological changes of the enteric nervous system in 48 primary colorectal cancers and 9 control non-colon cancer specimens. They concluded that B2A receptors could serve as a prognostic factor in colorectal cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Steel JC S- Editor: Ma YJ L- Editor: Logan S E- Editor: Wang CH
| 1. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3538] [Article Influence: 393.1] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20499] [Article Influence: 2049.9] [Reference Citation Analysis (20)] |
| 3. | Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008;29:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Fife A, Beasley PJ, Fertig DL. Psychoneuroimmunology and cancer: historical perspectives and current research. Adv Neuroimmunol. 1996;6:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Sayegh AI, Washington MC. Back to Basics: Regulation of the Gastrointestinal Functions. J Gastroint Dig Syst. 2012;2:118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1065] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 7. | Hansen MB. The enteric nervous system I: organisation and classification. Pharmacol Toxicol. 2003;92:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Furness JB. Integrated Neural and Endocrine Control of Gastrointestinal Function. Adv Exp Med Biol. 2016;891:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1-G24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 10. | Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Li Z, Caron MG, Blakely RD, Margolis KG, Gershon MD. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J Neurosci. 2010;30:16730-16740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, Cheng CC, Kuo YH, Shi CS. Selective β2-AR Blockage Suppresses Colorectal Cancer Growth Through Regulation of EGFR-Akt/ERK1/2 Signaling, G1-Phase Arrest, and Apoptosis. J Cell Physiol. 2016;231:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Hamilton SR, Bosman FT, Boffetta P. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press. 2010;8:134-146. |
| 14. | Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015;75:1777-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Liebl F, Demir IE, Rosenberg R, Boldis A, Yildiz E, Kujundzic K, Kehl T, Dischl D, Schuster T, Maak M. The severity of neural invasion is associated with shortened survival in colon cancer. Clin Cancer Res. 2013;19:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Sobaniec-Lotowska ME, Ciołkiewicz M, Pogumirski J, Sulkowski S, Sobczak A. Morphometry of synaptophysin immunoreactive ganglion cells in Auerbach plexus in patients with colorectal cancer. Is this a new prognostic factor? Folia Neuropathol. 2004;42:167-170. [PubMed] |
| 17. | Godlewski J. Morphological changes in the enteric nervous system caused by carcinoma of the human large intestine. Folia Histochem Cytobiol. 2010;48:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kozlowska A, Kwiatkowski P, Oponowicz A, Majewski M, Kmiec Z, Godlewski J. Myenteric plexuses atrophy in the vicinity of colorectal cancer tissue is not caused by apoptosis or necrosis. Folia Histochem Cytobiol. 2016;54:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Walter FM, Emery JD, Mendonca S, Hall N, Morris HC, Mills K, Dobson C, Bankhead C, Johnson M, Abel GA. Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br J Cancer. 2016;115:533-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Florescu C, Istrătoaie O, Târtea GC, Pirici D, Streba CT, Cătălin B, Puiu I, Târtea EA, Caragea DC, Ghiluşi MC. Neuro-neoplastic interrelationships in colorectal level - immunohistochemical aspect in three cases and review of the literature. Rom J Morphol Embryol. 2016;57:639-650. [PubMed] |
| 21. | Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866-2869. [PubMed] |
| 22. | Westfall TC, Westfall DP. Adrenergic agonists and antagonists. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th edition. New York: McGraw-Hill 2011; 277-334. |
| 23. | Yao H, Duan Z, Wang M, Awonuga AO, Rappolee D, Xie Y. Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet Cytogenet. 2009;190:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Wong HP, Ho JW, Koo MW, Yu L, Wu WK, Lam EK, Tai EK, Ko JK, Shin VY, Chu KM. Effects of adrenaline in human colon adenocarcinoma HT-29 cells. Life Sci. 2011;88:1108-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Pu J, Bai D, Yang X, Lu X, Xu L, Lu J. Adrenaline promotes cell proliferation and increases chemoresistance in colon cancer HT29 cells through induction of miR-155. Biochem Biophys Res Commun. 2012;428:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep. 2015;33:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Irina G, Katrin A, Terence EH. Organizational Complexity of b-adrenergic Receptor Signaling Systems. In: Wang Q, Benos DJ, Balaban R, Simon SA, editors. Advances in Adrenergic Receptor Biology. Elsevier 2011; 19-49. |
| 28. | Zhang D, Ma QY, Hu HT, Zhang M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 2010;10:19-29. [PubMed] |
| 29. | Tang J, Li Z, Lu L, Cho CH. β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 31. | Al-Wadei HA, Plummer HK, Ullah MF, Unger B, Brody JR, Schuller HM. Social stress promotes and γ-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila). 2012;5:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042-7052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 652] [Cited by in RCA: 634] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 33. | Ventura S, Evans BA. Does the autonomic nervous system contribute to the initiation and progression of prostate cancer? Asian J Androl. 2013;15:715-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hébert TE. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69-78. [PubMed] |