Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.999
Peer-review started: August 19, 2016
First decision: September 6, 2016
Revised: September 26, 2016
Accepted: October 30, 2016
Article in press: October 31, 2016
Published online: February 14, 2017
Processing time: 178 Days and 22.4 Hours
AIM
To investigate potential effects of poly I:C on mucosal injury and epithelial barrier disruption in dextran sulfate sodium (DSS)-induced acute colitis.
METHODS
Thirty C57BL/6 mice were given either regular drinking water (control group) or 2% (w/v) DSS drinking water (model and poly I:C groups) ad libitum for 7 d. Poly I:C was administrated subcutaneously (20 μg/mouse) 2 h prior to DSS induction in mice of the poly I:C group. Severity of colitis was evaluated by disease activity index, body weight, colon length, histology and myeloperoxidase (MPO) activity, as well as the production of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 17 (IL-17) and interferon-γ (IFN-γ). Intestinal permeability was analyzed by the fluorescein isothiocyanate labeled-dextran (FITC-D) method. Ultrastructural features of the colon tissue were observed under electron microscopy. Expressions of tight junction (TJ) proteins, including zo-1, occludin and claudin-1, were measured by immunohistochemistry/immunofluorescence, Western blot and real-time quantitative polymerase chain reaction (RT-qPCR).
RESULTS
DSS caused significant damage to the colon tissue in the model group. Administration of poly I:C dramatically protected against DSS-induced colitis, as demonstrated by less body weight loss, lower disease activity index score, longer colon length, colonic MPO activity, and improved macroscopic and histological scores. It also ameliorated DSS-induced ultrastructural changes of the colon epithelium, as observed under scanning electron microscopy, as well as FITC-D permeability. The mRNA and protein expressions of TJ protein, zo-1, occludin and claudin-1 were also found to be significantly enhanced in the poly I:C group, as determined by immunohistochemistry/immunofluorescence, Western blot and RT-qPCR. By contrast, poly I:C pretreatment markedly reversed the DSS-induced up-regulated expressions of the inflammatory cytokines TNF-α, IL-17 and IFN-γ.
CONCLUSION
Our study suggested that poly I:C may protect against DSS-induced colitis through maintaining integrity of the epithelial barrier and regulating innate immune responses, which may shed light on the therapeutic potential of poly I:C in human colitis.
Core tip: Poly I:C, a toll-like receptor 3 agonist, has been previously reported to protect against acute colitis. The potential effects of poly I:C on mucosal injury and epithelial barrier disruption were investigated in mouse models of dextran sulfate sodium (DSS)-induced acute colitis. Poly I:C administration dramatically protected against DSS-induced colitis, with ameliorated ultrastructural changes of colon epithelium, intestinal permeability and tight junction protein expressions. Poly I:C may protect against DSS-induced colitis through maintaining integrity of the epithelial barrier and regulating innate immune responses.
- Citation: Zhao HW, Yue YH, Han H, Chen XL, Lu YG, Zheng JM, Hou HT, Lang XM, He LL, Hu QL, Dun ZQ. Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis. World J Gastroenterol 2017; 23(6): 999-1009
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.999
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are common chronic diseases that are characterized by abnormal mucosal immune response to luminal bacteria[1,2]. The innate and adaptive immune responses have been suggested to engage in the initiation of inflammation and relapse of disease activity, with increased intestinal levels of tumor necrosis factor-α (TNF-α), interleukin 17 (IL-17) and interferon-γ (IFN-γ)[3,4]. The increased intestinal inflammation may, in turn, lead to impaired mucosal barrier function and intestinal permeability, which allow subsequent translocation of microorganisms to the mucosal lymphatic tissue. The down-regulated expressions of junction complex proteins have been demonstrated in the intestinal mucosa of patients with IBD[5]. The impaired gut epithelial barrier function may cause persistent immune activation and, thereby, enhance inflammation of the intestinal mucosa[6].
Epithelial barrier function is determined by intestinal permeability, which is mainly maintained by tight junctions (TJs)[7]. As the most apical intercellular structure of the junctional complex in epithelial cells, TJs serve as the permeability barrier to paracellular transport of solutes[8,9]. Increased intestinal permeability has been reported to associate with the pathogenesis of IBD[10]. Therefore, maintenance of TJ and barrier function may be beneficial for patients with IBD[11-14].
Toll-like receptors (TLRs) are ancient microbial pattern recognition receptors that play an essential role in initiation of immune responses. Subcutaneous administration of poly I:C, a synthetic TLR3 agonist, has been reported to dramatically protect against dextran sulfate sodium (DSS)-induced acute colitis[15]. However, another study also indicated that abnormal activation of TLR3 signaling by poly I:C broke down the mucosal homeostasis and caused mucosal damage in the small intestine[16]. TLR3 signaling may be involved in the process of epithelial destruction and mucosal injury[17]. However, the potential effects of TLR3 activation by poly I:C on mucosal injury and epithelial barrier disruption in DSS-induced acute colitis have not been well investigated up to now. Therefore, the present study aimed to investigate the potential role of poly I:C administration on the intestinal mucosal barrier function and intestinal permeability in the mouse model of DSS-induced acute colitis.
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC, Approval ID: I07-038-3). Male C57BL/6 mice (8 wk, 18-22 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) [License No.: SCXK (Beijing) 2006-0009]. Animals were housed under standard conditions in a barrier facility, according to the protocols of IACUC and Hebei Medical University Vivarium (GB 14925-2001).
Poly I:C was prepared as described previously[18]. Briefly, poly I:C (GE Healthcare, Piscataway, NJ, United States) was dissolved in sterile phosphate-buffered saline (PBS) at a concentration of 2 mg/mL and heated at 50 °C until solubilization. The solution was then slowly cooled down to room temperature for proper annealing. The same lot of poly I:C was employed throughout the study.
Acute colitis was induced by DSS (40000-50000 MW; Sigma, St. Louis, MO, United States) as described in our previous report[19]. Briefly, 30 C57BL/6 mice were randomly assigned to three groups: control group, model group, and poly I:C group (n = 10 per group). Mice in the poly I:C group were administrated poly I:C subcutaneously (20 μg/mouse) in 100 μL PBS at 2 h prior to DSS treatment. Mice in the control and model groups were given normal saline. Animals were then given either regular drinking water (control group) or 2% (w/v) DSS drinking water (model and poly I:C groups) ad libitum for 7 d, and resumed on water for the remainder of the experiments.
Rachmilewitz disease activity index (DAI) was assessed by an investigator blinded to the protocol and according to the well-established scoring system[20]. The data of body weight (BW), stool consistency and occult blood (OB) in the stool were recorded. Loss in BW was scored as: 0: no weight loss, 1: 1%-5% weight loss from baseline, 2: 5%-10%, 3: 10%-20%, and 4: more than 20% weight loss. Stool consistency was scored as: 0: well-formed pellets, 2: pasty and semi-formed stools that do not adhere to the anus, and 4: liquid stools that adhere to the anus. OB was scored as: 0: no blood, 2: positive hemoccult, and 4: gross bleeding. These scores were added together and divided by 3 to result in the DAI ranging from 0 (healthy) to 4 (maximal activity of colitis).
The entire colon (from the cecum to anus) was removed and the lengths were measured as an inflammation marker. The degree of colonic damage was examined macroscopically using a 0-4 scale[19]: 0: normal colon tissue; 1: minimal colon wall thickening without congestion; 2: moderate colon wall thickening with congestion; 3: moderate colon wall thickening, rigidity and congestion; and 4: marked colon wall thickening, rigidity, and congestion. The extent of tissue damage was assessed microscopically using a semi-quantitative scoring system[21]. In detail, the proximal colon was removed, fixed in 10% formalin and embedded in paraffin. The sections of 4 μm thickness were stained with hematoxylin and eosin (HE) and scored using the following parameters: (1) severity of inflammation (0-3: none, slight, moderate, severe); (2) extent of injury (0-3: none, mucosal, mucosal and submucosal, transmural); and (3) crypt damage (0-4: none, basal 1/3 damaged, basal 2/3 damaged, only surface epithelium intact, entire crypt and epithelium lost). The total score was the sum of each parameter multiplying an equivalent reflecting the percentage of tissue involved (× 1: 0%-25%, × 2: 26%-50%, × 3: 51%-75%, × 4: 76%-100%).
Colons (100 mg wet weight) were isolated from each group and homogenized in 1 mL buffer (0.05% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer, pH 6.0). The resulting homogenates were centrifuged at 2000 g and 4 °C. The supernatants were harvested and stored at -80 °C for Myeloperoxidase (MPO) activity assay. The samples (10 μL) were transferred to a 96-well plate and incubated with 3 μL odianisidine hydrochloride (20 mg/mL) in 290 μL 50 mmol/L phosphate buffer and 3 μL H2O2 (20 mmol/L). The reaction was stopped by adding 3 μL sodium azide (30%). Light absorbance at 460 nm was read. MPO activity was determined by the curve obtained from the standard MPO[22].
For immunohistochemistry staining, the sections were boiled 10 min in 10 mmol/L citrate (pH 6.0) for antigen retrieval. The slides were then incubated with mouse monoclonal antibodies against claudin-1, occludin (Santa Cruz Biotechnology, Dallas, TX, United States), TNF-α, and IL-17 (Sigma), and rabbit polyclonal antibodies against zo-1 (1:150; Invitrogen, Carlsbad, CA, United States) and IFN-γ (1:300; Santa Cruz Biotechnology), followed by peroxidase-conjugated secondary antibodies (1:1500). The signals were visualized by a diaminobenzidine peroxidase substrate kit (Vector Laboratories, Burlingame, CA, United States). For immunofluorescence staining, the sections were incubated with mouse monoclonal antibodies against claudin-1 and occludin (1:200; Santa Cruz Biotechnology) and rabbit polyclonal antibody against zo-1 (1:150; Invitrogen), and subsequently with FITC- or Cy3-conjugated secondary antibodies. Images were captured under a Leica DMIRE2 confocal laser scanning microscope.
In vivo permeability assay was performed to assess barrier function by using a FITC-labeled dextran method[23]. Briefly, food and water were withdrawn for 4 h and mice were then gavaged with permeability tracer (FITC-D, 60 mg/100 g body weight, MW 4000; Sigma). Serum was collected, and fluorescence intensity and FITC-dextran concentrations were determined. Permeability was calculated by linear regression of sample fluorescence.
Specimens were fixed with 2.5% glutaraldehyde for 3 h at room temperature. The samples were then washed in acetone, critical point dried, and coated with gold for scanning electron microscopy (SEM).
Total RNA was extracted from colon tissues using Trizol reagent (Gibco-BRL Co., Grand Island, NY, United States) according to the manufacturer's protocol and quantified using a UV spectrophotometer. Total RNA of 5 μg was used for first-strand cDNA synthesis. Real-time polymerase chain reaction (PCR) was performed with Quantitect™ SYBR W Green PCR Mastermix (Qiagen, Hilden, Germany) using 2 μL of cDNA template. The amplification program was 95 °C for 5 min, 45 cycles of 95 °C for 1 min and 60 °C for 10 s. The primers used are summarized in Table 1. The relative quantitative analysis was performed by 2-ΔΔCt method using Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal control.
| Gene | Primers | Length, bp | |
| zo-1 | Forward | 5’-TCATCCCAAATAAGAACAGAGC-3 | 198 |
| Reverse | 5’-GAAGAACAACCCTTTCATAAGC-3’ | ||
| Occludin | Forward | 5’-CTTTGGCTACGGAGGTGGCTAT-3’ | 86 |
| Reverse | 5’-CTTTGGCTGCTCTTGGGTCTG-3’ | ||
| Claudin-1 | Forward | 5’-GCTGGGTTTCATCCTGGCTTCT-3’ | 110 |
| Reverse | 5’-CCTGAGCGGTCACGATGTTGTC-3’ | ||
| IL-17 | Forward | 5’-TATCCCTCTGTGATCTGGGAAG-3’ | 161 |
| Reverse | 5’ATCTTCTCGACCCTGAAAGTGA-3’ | ||
| IFN-γ | Reverse | 5’-ATGAACGCTACACACTGCATCTT-3’ | 139 |
| Forward | 5’-TTTCTTCCACATCTATGCCACTT3’ | ||
| TNF-α | Reverse | 5’-GGTTCTGTCCCTTTCACTCACT-3’ | 169 |
| Forward | 5’-GAGAAGAGGCTGAGACATAGGC-3’ | ||
| GAPDH | Reverse | 5’-GAGACCTTCAACACCCCAGC-3’ | 263 |
| Forward | 5’-ATGTCACGCACGATTTCCC-3’ | ||
Protein concentration was determined by using Coomassie brilliant blue G250. Protein samples (100 μg) were resolved on 8% SDS polyacrylamide gel, electrotransferred to nitrocellulose membrane, and subsequently blocked in 5% skim milk in PBS. The membranes were incubated overnight at 4 °C with rabbit polyclonal antibodies against zo-1 (1:100) and IFN-γ (1:200), mouse monoclonal antibodies against claudin-1 (1:200), occludin (1:200), TNF-α (1:200), and IL-17 (1:200), and rabbit anti-GAPDH monoclonal antibody (1:100). The secondary antibodies used were goat anti-rabbit or -mouse IgG (1:2000) and were incubated with membranes at room temperature for 2 h. Blots were visualized using enhanced chemiluminescence detection reagents (Santa Cruz Biotechnology) and quantified by Bandscan 5.0 software using GAPDH as the internal control.
Data were expressed as mean ± SD and analyzed with SPSS 13.0 software (SPSS, Inc., Chicago, IL, United States). Comparison between the groups was done by Student's t-test. Comparison among the groups was conducted by one-way ANOVA analysis followed by LSD post-hoc test. Statistical significance was considered at P < 0.05.
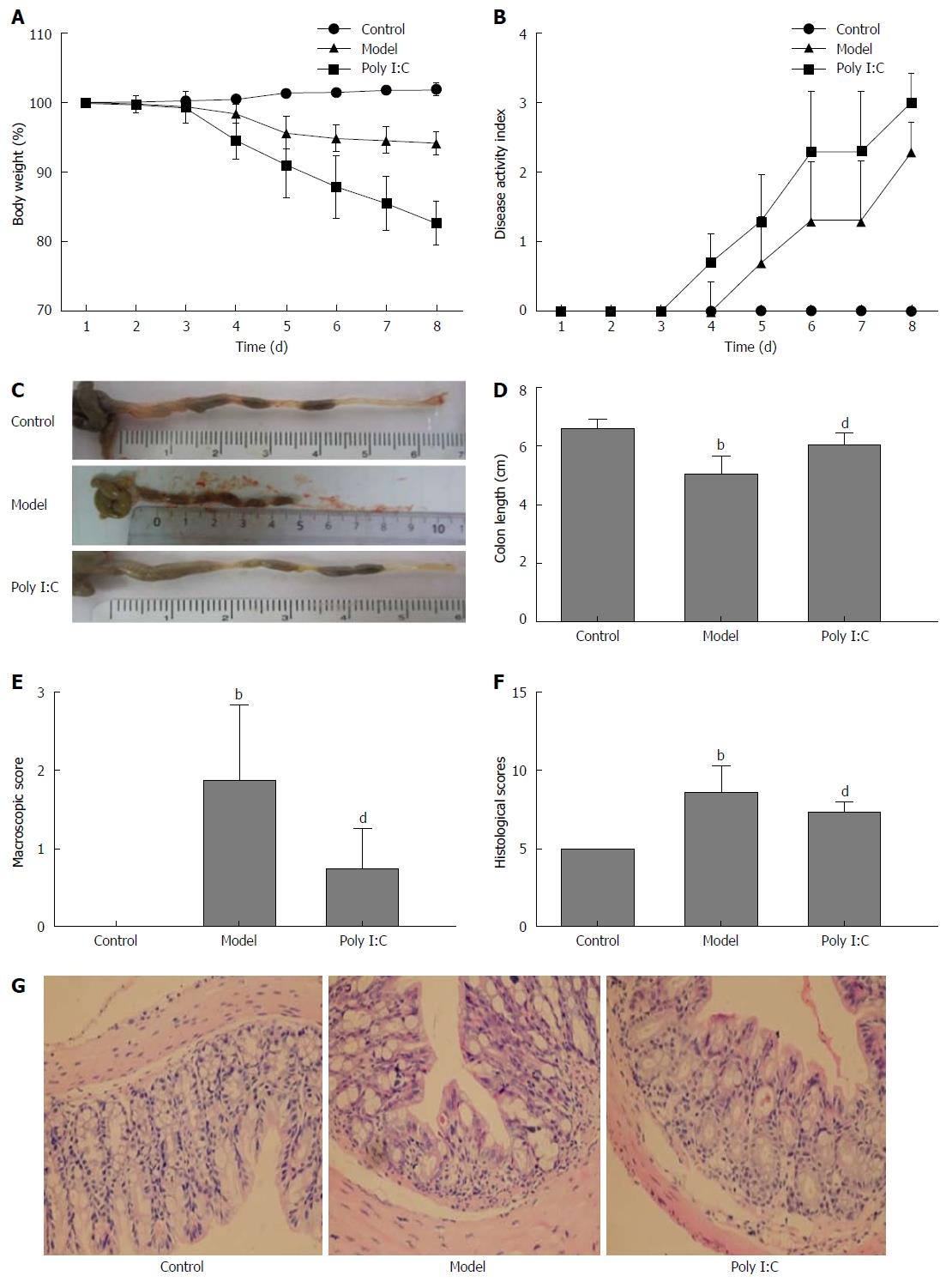
DSS caused significant damage to the colon tissue, with the gut inflammation and weight loss noted in the model group during 1 to 8 d observation. Administration of poly I:C partially reversed the DSS-induced effects, with less BW loss (Figure 1A). The general condition of mice was evaluated using a DAI that scored the extent of BW loss, stool consistency, and OB. As shown in Figure 1B, DAI was significantly enhanced in the DSS-induced model group, and this effect was partially alleviated by poly I:C. The colitic parameters were further quantified after monitoring clinical development of colitis for 8 d. As shown in Figure 1 C and D, the colon length in the model group was significantly shorter than that of the control group (P < 0.01), while pretreatment with poly I:C significantly reduced the DSS-induced colon shortening (P < 0.01). Macroscopically, mice in the model group had significantly higher inflammation score than those in the control and poly I:C groups (P < 0.01; Figure 1E). Histopathological analysis in the model group showed extensive ulceration of the epithelial layer, edema and crypt damage of the bowel wall. The fibrosis of muscularis mucosae and infiltration of granulocytes and mononuclear cells into the mucosa were also observed (Figure 1G). The histology score was significantly higher in the model group than that in the control group (P < 0.01). In contrast, poly I:C pretreatment obviously reversed this DDS-induced effect, with comparatively lower histology score noted in the poly I:C group (Figure 1H).
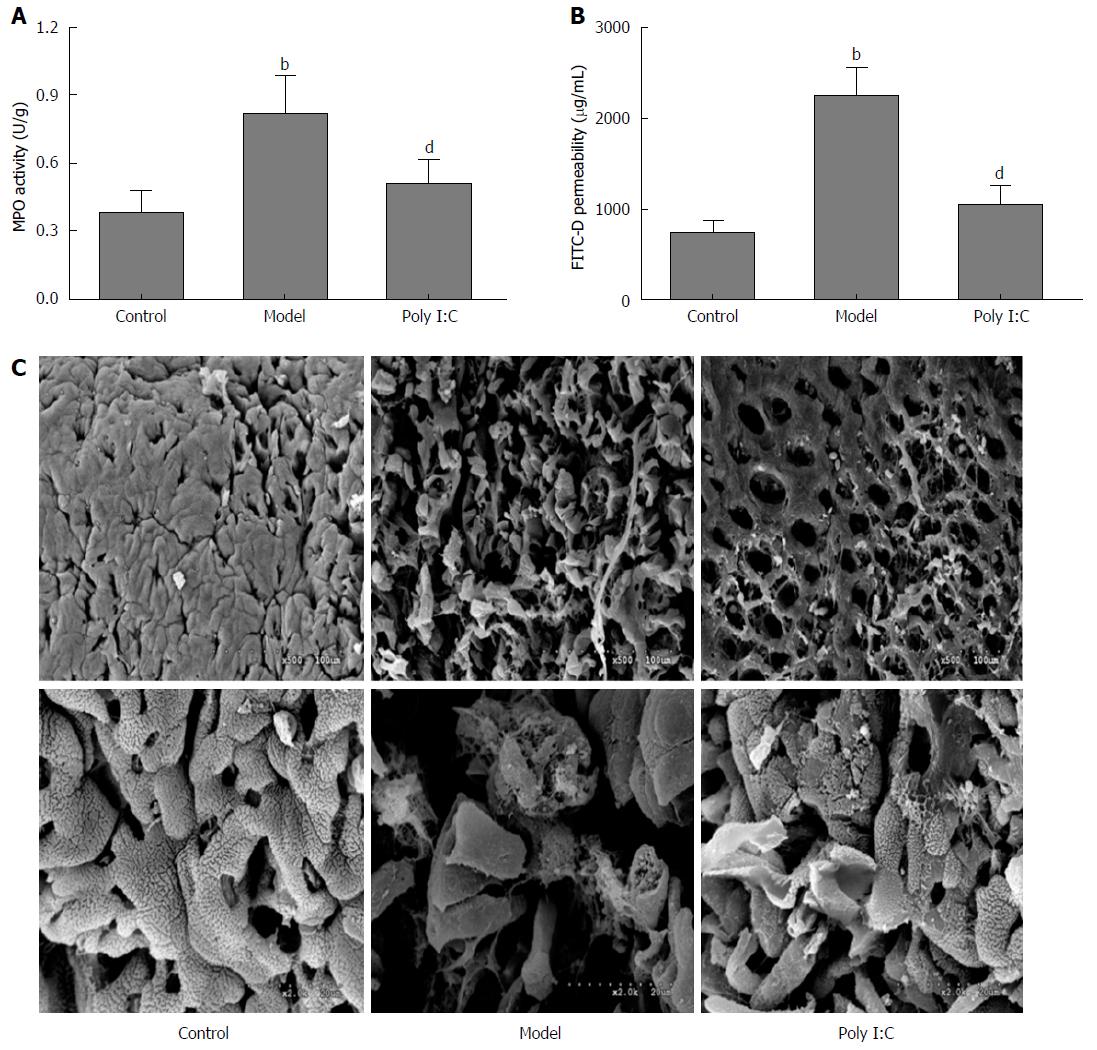
MPO activity is shown in Figure 2A. Mice in the model group showed significantly higher MPO activity than that in the control group (P < 0.01), while the data were significantly lower in mice of the poly I:C group (P < 0.01). To investigate the effect of poly I:C on paracellular permeability, intestinal permeability to FITC-D was determined. The results showed the increased permeability to 4-kDa FITC-D induced by colitis, while mice in the poly I:C group showed a lower intestinal permeability than that of the model group (P < 0.01; Figure 2B). SEM observations of the colonic mucosa showed severe mucosal loss with typical histological inflammation feature in the mice of the model group (Figure 2C). Besides, enterocytes in the mice of the model group showed less glycocalyx and more irregular surface than the control group. By contrast, pretreatment with poly I:C obviously ameliorated the DSS-induced ultrastructural changes, with few histological lesions observed.
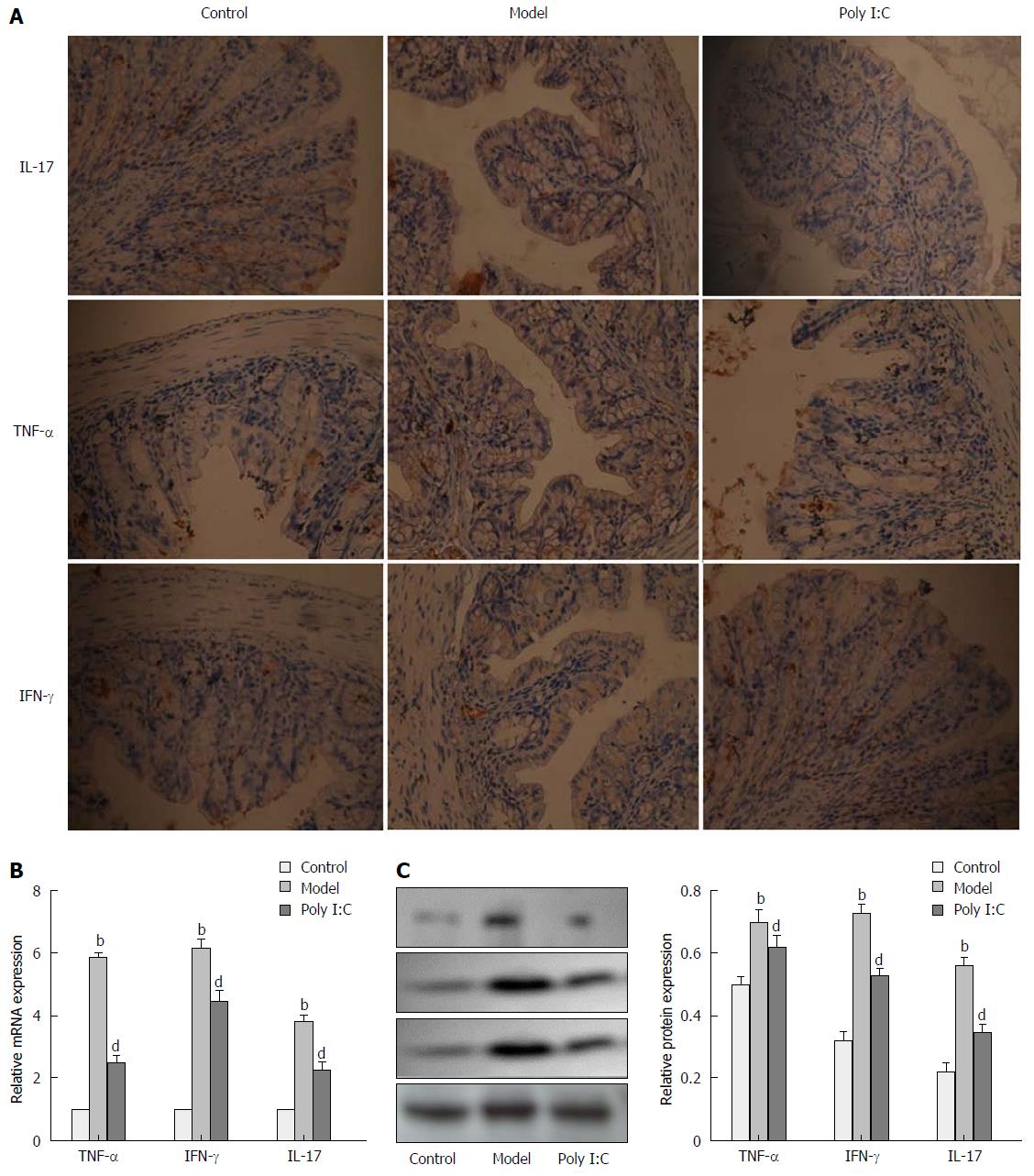
To determine the anti-inflammatory effect of poly I:C on the DSS-induced colitis, expressions of inflammatory markers, including IL-17, TNF-α and IFN-γ, were analyzed by real-time quantitative polymerase chain reaction (RT-qPCR), immunohistochemistry, and Western blot. RT-qPCR analysis showed the up-regulated expressions of IL-17, TNF-α and IFN-γ in the mice of the model group, while pretreatment with poly I:C caused the down-regulated expression of these inflammatory cytokines (P < 0.01; Figure 3A). These results were further confirmed by immunohistochemistry and Western blot analysis, which indicated the enhanced expressions of IL-17, TNF-α and IFN-γ in the model group. By contrast, poly I:C pretreatment markedly reversed the DSS-induced up-regulation of the inflammatory cytokines (P < 0.01; Figure 3B and C).
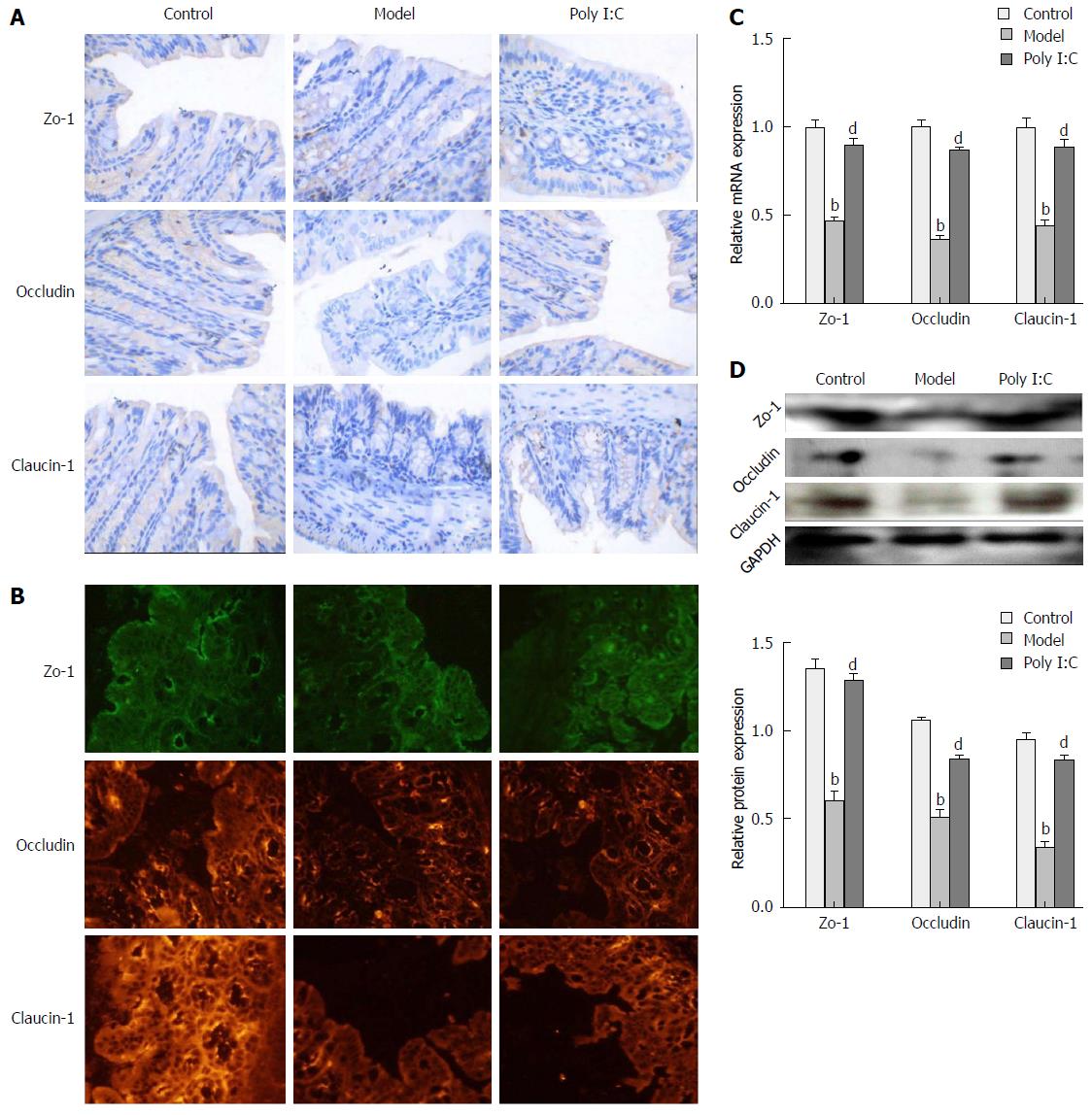
To investigate the protective effect of poly I:C on the DSS-induced disruption of TJ, the expression of TJ markers, including zo-1, occludin and claudin-1, were analyzed by immunohistochemistry, immunofluorescence, RT-qPCR, and Western blot. Immunohistochemistry and immunofluorescence assays showed that, in the control group, TJ proteins were expressed in the cytomembrane of epithelial cells, and most commonly in the spinous and granular layers, whereas expressions of these TJ proteins in the model group were significantly decreased. In the poly I:C group, however, the increased expression of TJ proteins were observed in the both cytomembrane and cytoplasm of spinous and granular layers in the mucosa (Figure 4A and B). Further quantitative analysis of TJ protein expressions by RT-qPCR and Western blot confirmed the down-regulated expression of TJ proteins in the model group when compared with the control group. Meanwhile, this effect was compromised by poly I:C pretreatment, with the comparatively higher expressed TJ markers (P < 0.01; Figure 4C and D).
Poly I:C is a ligand for TLR3 that is involved in the innate immune response to viral infection[24]. Administration of poly I:C in wild-type mice has been reported to dramatically protect against DSS-induced colitis, as demonstrated by parameter analysis of body weight, rectal bleeding, colonic MPO activity, histopathology, and etc[15]. However, another study also indicated that abnormal activation of TLR3 signaling by poly I:C broke down the mucosal homeostasis and caused intestinal mucosal damage[16]. Therefore, the potential role of poly I:C in the mucosal injury and epithelial barrier disruption was further investigated in DSS-induced acute colitis. The findings of our study showed that poly I:C administration obviously ameliorated the clinical symptoms of DSS-induced acute colitis, as demonstrated by less BW loss, lower DAI score, longer colon length, and improved macroscopic and histological scores. These results were consistent with the findings reported by Vijay-Kumar et al[15], which indicated the protective effect of TLR3 activation by poly I:C on DSS-induced acute colitis.
The intestinal barrier has been reported to be defective and associated with paracellular leakiness in inflammatory bowel conditions of UC[25]. TJ protein is the most apical component responsible for restricting paracellular permeability of the intestinal mucosa[26]. Poly I:C administration has been demonstrated to protect against DSS-induced acute colitis. However, abnormal activation of TLR3 signaling by poly I:C has been reported to break down the mucosal homeostasis and cause mucosal damage in the small intestine[16]. TLR3 signaling may be involved in the process of epithelial destruction and mucosal injury[17]. These conflicting results urged us to study the effect of poly I:C on paracellular permeability and epithelial barrier function in DSS-induced acute colitis models.
Corroborating the findings of our previous study[19] and the study of others[15], the present study showed increased paracellular permeability and disrupted TJ induced by DSS, as assessed by FITC-D level, ultrastructure of colon mucosa, and expression and distribution of TJ proteins (zo-1, occludin, and claudin-1). However, unlike the results of our study, another study also indicated that colonic paracellular permeability was not significantly altered by DSS exposure, and DSS mice only showed a trend of increased expressions of claudin-1 and claudin-2[27]. This variation may be partially explained by the different methods used to establish the acute colitis models.
The potential effects of poly I:C administration were then analyzed. Paracellular permeability and TJ were found to be significantly ameliorated when mice were pretreated with poly I:C, as demonstrated by decreased FITC-D permeability, ameliorated ultrastructural changes, and enhanced expression of TJ proteins at the both mRNA and protein levels. The direct effect of poly I:C on intestinal barrier function has been investigated in vitro by Moyano-Porcile et al[28], and indicated that acute exposure of rat ileum and colon tissues to poly I:C reduced colon permeability to macromolecules, while increasing ileum permeability to micromolecules. Intraperitoneal injection of poly I:C has been reported to cause severe mucosal injury of the small intestine, along with the increased levels of IL-15[16].
Activation of TLR-3 seems to cause adverse effects on various epithelial barriers, like the blood-brain barrier and nasal epithelial barriers[29,30]. However, to the best of our knowledge, this study is the first to investigate the effect of poly I:C on intestinal barrier function in DSS-induced acute colitis. Our results suggested that administration of poly I:C in DSS-induced colitis models may help to maintain the integrity of ultrastructure of the epithelial mucosa and junction complex, and therefore decrease paracellular permeability and recover epithelial barrier function.
TJ is known to be the rate-limiting step in transepithelial transport and the principal determinant of intestinal permeability[31]. Increased intestinal permeability and disrupted TJ may cause bowel inflammation[32,33]. TNF-α is a proinflammatory cytokine that has long been established as a key player in the pathogenesis of IBD diseases. Proinflammatory cytokines such as TNF-α and IFN-γ have also been linked to the increased paracellular permeability[34-36]. Ligation of TLR3 by poly I:C has been suggested to be effective in IBD-associated gut inflammation[15]. This may shed light on the barrier protective effect of poly I:C as a likely candidate mechanism that contributes to therapeutic efficacy in IBD diseases. Therefore, the expressions of proinflammatory cytokines (IL-17, TNF-α, and IFN-γ) in colon epithelium were analyzed. Consistent with the result of the above mentioned study, our study showed the increased production of proinflammatory cytokines TNF-α, IL-17 and IFN-γ in mice of the DSS-induced model group. However, mice pretreated with poly I:C showed ameliorated DSS-induced high-expression of these proinflammatory cytokines. These results suggested that poly I:C may ameliorate inflammation in the colitis models.
In conclusion, the findings of our study showed evidence to suggest that poly I:C may protect against DSS-induced acute colitis through maintaining epithelial integrity and regulating the innate immune responses, which may shed light on the therapeutic potential of poly I:C in clinical colitis.
The toll-like receptor 3 (TLR3) agonist poly I:C has been reported to protect against acute colitis.
TLR3 signaling may be involved in the process of epithelial destruction and mucosal injury. However, the potential effects of TLR3 activation by poly I:C on the mucosal injury and epithelial barrier disruption in dextran sulfate sodium (DSS)-induced acute colitis have not been well investigated up to now.
The potential roles of poly I:C administration in intestinal mucosal barrier function and intestinal permeability were investigated in a mouse model of DSS-induced acute colitis. Poly I:C administration dramatically protected against DSS-induced colitis. It also ameliorated DSS-induced ultrastructural changes of the colon epithelium, as well as fluorescein isothiocyanate labeled-dextran permeability and tight junction protein expressions.
Poly I:C may protect against DSS-induced colitis through maintaining integrity of the epithelial barrier and regulating innate immune responses, which may shed light on the therapeutic potential of poly I:C in human colitis.
In general, the present work is well done and the paper describes results of interest for the inflammatory bowel disease research community working with animal models of gut inflammation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Sandvik AK S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Liu WX
| 1. | Song-Zhao GX, Maloy KJ. Experimental mouse models of T cell-dependent inflammatory bowel disease. Methods Mol Biol. 2014;1193:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Geem D, Harusato A, Flannigan K, Denning TL. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Guéry L, Hugues S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed Res Int. 2015;2015:314620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Park JS, Yi TG, Park JM, Han YM, Kim JH, Shin DH, Tak SJ, Lee K, Lee YS, Jeon MS. Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. J Clin Biochem Nutr. 2015;57:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A. Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol. 2015;8:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Luo K, Cao SS. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:328791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Borkowski AW, Kuo IH, Bernard JJ, Yoshida T, Williams MR, Hung NJ, Yu BD, Beck LA, Gallo RL. Toll-like receptor 3 activation is required for normal skin barrier repair following UV damage. J Invest Dermatol. 2015;135:569-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Clark PR, Kim RK, Pober JS, Kluger MS. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases. PLoS One. 2015;10:e0120075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Luissint AC, Bennett A, Nishio H, Hilgarth R, McCall I, Nusrat A, Parkos C. CLMP Expression is Increased in the Intestinal Epithelium Under Inflammatory Conditions and Regulates Intercellular Adhesion, Proliferation and Migration. FASEB j. 2015;29:282-289. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Mielke L, Preaudet A, Belz G, Putoczki T. Confocal laser endomicroscopy to monitor the colonic mucosa of mice. J Immunol Methods. 2015;421:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hummel S, Veltman K, Cichon C, Sonnenborn U, Schmidt MA. Differential targeting of the E-Cadherin/β-Catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl Environ Microbiol. 2012;78:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 2013;8:e59838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (2)] |
| 15. | Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol. 2007;178:4548-4556. [PubMed] [DOI] [Full Text] |
| 17. | Zhou R, Wei H, Sun R, Zhang J, Tian Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc Natl Acad Sci USA. 2007;104:7512-7515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Vijay-Kumar M, Gentsch JR, Kaiser WJ, Borregaard N, Offermann MK, Neish AS, Gewirtz AT. Protein kinase R mediates intestinal epithelial gene remodeling in response to double-stranded RNA and live rotavirus. J Immunol. 2005;174:6322-6331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 21. | Vowinkel T, Mori M, Krieglstein CF, Russell J, Saijo F, Bharwani S, Turnage RH, Davidson WS, Tso P, Granger DN. Apolipoprotein A-IV inhibits experimental colitis. J Clin Invest. 2004;114:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Shimizu T, Suzuki T, Yu HP, Yokoyama Y, Choudhry MA, Bland KI, Chaudry IH. The role of estrogen receptor subtypes on hepatic neutrophil accumulation following trauma-hemorrhage: direct modulation of CINC-1 production by Kupffer cells. Cytokine. 2008;43:88-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Nagy JA, Herzberg KT, Masse EM, Zientara GP, Dvorak HF. Exchange of macromolecules between plasma and peritoneal cavity in ascites tumor-bearing, normal, and serotonin-injected mice. Cancer Res. 1989;49:5448-5458. [PubMed] |
| 24. | Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I: C). Brain Behav Immun. 2007;21:490-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Vodovotz Y, Constantine G, Faeder J, Mi Q, Rubin J, Bartels J, Sarkar J, Squires RH, Okonkwo DO, Gerlach J. Translational systems approaches to the biology of inflammation and healing. Immunopharmacol Immunotoxicol. 2010;32:181-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Wu HL, Gao X, Jiang ZD, Duan ZT, Wang SK, He BS, Zhang ZY, Xie HG. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology. 2013;304:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Carlsson AH, Yakymenko O, Olivier I, Håkansson F, Postma E, Keita AV, Söderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Moyano-Porcile V, Olavarría-Ramírez L, González-Arancibia C, Bravo JA, Julio-Pieper M. Short-term effects of Poly(I: C) on gut permeability. Pharmacol Res. 2015;101:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Ohkuni T, Kojima T, Ogasawara N, Masaki T, Fuchimoto J, Kamekura R, Koizumi J, Ichimiya S, Murata M, Tanaka S. Poly(I: C) reduces expression of JAM-A and induces secretion of IL-8 and TNF-α via distinct NF-κB pathways in human nasal epithelial cells. Toxicol Appl Pharmacol. 2011;250:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 847] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Zhang HS, Fong GH, Xi QL, Wu GH, Bai CG, Ling ZQ, Fan L, Xu YM, Qin YQ. PHD3 Stabilizes the Tight Junction Protein Occludin and Protects Intestinal Epithelial Barrier Function. J Biol Chem. 2015;290:20580-20589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 472] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 33. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2711] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 34. | Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Gilbert S, Zhang R, Denson L, Moriggl R, Steinbrecher K, Shroyer N, Lin J, Han X. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol Med. 2012;4:109-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |