Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1067
Peer-review started: September 12, 2016
First decision: October 20, 2016
Revised: November 16, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 14, 2017
Processing time: 153 Days and 16.6 Hours
AIM
To assess the impact of disease characteristics on the quality of life (QOL) in children with inflammatory bowel diseases (IBD).
METHODS
This was a cross-sectional study conducted at the First Department of Pediatrics of the University of Athens at the “Aghia Sophia” Children’s Hospital. Children diagnosed with Crohn’s disease (CD) or ulcerative colitis (UC), who were followed as outpatients or during a hospitalization, participated, after informed consent was obtained from their legal representative. QOL was assessed by the IMPACT-III questionnaire. Demographic data and disease characteristics were also collected. Statistical analyses included parametric (Student’s t-test and Pearson’s r) and non-parametric (Mann-Whitney test, Fisher’s test and Spearman’s rho) procedures.
RESULTS
Ninety-nine patients (UC: 37, 73.0% females, CD: 62, 51.6% females), aged 12.8 ± 2.6 years were included. Overall, as well as, sub-domain scores did not differ between UC and CD (overall score: 73.9 ± 13.3 vs 77.5 ± 11.2, respectively, P = 0.16). In the entire sample, total score was related to physician’s global assessment (PGA, patients classified as “mild/moderate” active disease had, on average, 14.8 ± 2.7 points lower total scores compared to those “in remission”, P < 0.001) and age at IMPACT completion (Pearson’s r = 0.29, P = 0.05). Disease activity assessed by the indices Pediatric Ulcerative Colitis activity index, Pediatric Crohn’s disease activity index or PGA was significantly associated with all subdomains scores. Presence of extraintestinal manifestations had a negative impact on emotional and social functioning domains.
CONCLUSION
Disease activity is the main correlate of QOL in children with IBD, underlining the importance of achieving and sustaining clinical remission
Core tip: This study demonstrated that disease activity is the main correlate of quality of life (QOL) in children with inflammatory bowel diseases (IBD). Furthermore, several factors, that pose increased risks for impaired QOL for children with IBD, were identified. In brief, children of younger age, the early years after the diagnosis and the presence of extra-intestinal manifestations were inversely related to IMPACT-III scores. Therefore, in children with these specific features, physicians should be more vigilant in order to recognize and address issues related to their QOL promptly.
- Citation: Chouliaras G, Margoni D, Dimakou K, Fessatou S, Panayiotou I, Roma-Giannikou E. Disease impact on the quality of life of children with inflammatory bowel disease. World J Gastroenterol 2017; 23(6): 1067-1075
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1067
Inflammatory bowel diseases (IBD) are immune mediated disorders with a genetic component, characterized by chronic inflammation of the gastrointestinal tract. Although patients suffering from these conditions have a normal life expectancy, the need for long-term medication, frequent hospitalizations, surgeries and the relapsing nature of the disease significantly negatively affect their quality of life (QOL). The term QOL encompasses the patients' subjective perception of their health state, as well as the impact of their disease on their physical, social and emotional wellbeing[1].
There are several studies published on the subject, the majority of which support that patients with IBD, as with other chronic diseases, have an impaired QOL compared to the normal population[1-3]. Most researchers have concluded that in patients with IBD the disease activity is the main predictive factor of QOL; however it is not yet defined how other disease characteristics, such as disease duration, recurrent hospitalizations or different treatment modalities affect their QOL.
In this study we assessed the QOL in Greek pediatric patients with IBD and attempted to identify how it is affected by disease characteristics.
The study was conducted at the First Department of Pediatrics of the University of Athens, Greece after approval by the Ethics Committee of the "Aghia Sophia" Children's Hospital in Athens, Greece. Informed consent was obtained from all legal representatives of the children who participated.
This was a cross-sectional study. Candidates for inclusion were children diagnosed with UC or CD according to the revised Porto criteria[4], who were hospitalized or followed in the outpatient IBD clinic. All patients had undergone at least one full ileocolonoscopy with biopsies, esophago-gastro-duodenoscopy with biopsies and magnetic resonance enterography for small bowel assessment.
At the time of evaluation, a number of parameters were recorded: Demographic data, disease activity, disease duration, current treatment and number of hospitalizations in the previous 3 mo. For the disease activity evaluation the Pediatric Crohn's disease activity index (PCDAI)[5] or the Pediatric Ulcerative Colitis activity index (PUCAI)[6] were used. For PCDAI the score ranges from 0 to 100, whereas for PUCAI the score ranges from 0 to 85 points. Based on the activity indices patients were classified as being in remission (PCDAI ≤ 10 or PUCAI ≤ 10), in relapse with mild activity (10 < PCDAI ≤ 30 or 10 < PUCAI ≤ 34) and in relapse with moderate/severe activity (PCDAI > 30 or PUCAI > 34). Physician's global assessment (PGA) was also recorded at the time of evaluation. PGA is a validated instrument through which the physician is able to evaluate disease activity clinically, on a 4 point scale (inactive, mild, moderate and severe disease).
QOL was assessed by the IMPACT-III questionnaire, which is a 35-item self-report tool that assesses QOL in children and adolescents with IBD. Children indicate on a 5-point Likert scale the extent to which they are bothered by specific aspects of their health condition. It consists of 6 subscales: bowel symptoms, systemic symptoms, emotional functioning, social functioning, body image and treatment/interventions. Scores range from 35 to 175, with higher scores suggesting better QOL. We used the Greek version of the questionnaire, created by one of the authors (Roma-Giannikou E), which has been translated from the original one designed by Otley and the Pediatric Inflammatory Bowel Disease Working Group on Quality of Life in 2002[7]. The patients completed the IMPACT-III questionnaires, in the presence of their parent(s), in approximately 20 min.
Statistical analysis was performed by a medical biostatistician (GC). Summary statistics for continuous variables are presented as mean ± SD and compared by t-test, whereas in cases of small samples (< 30) or skewed distributions, median and interquartile range are presented and the Mann-Whitney test was used. Categorical data are presented as absolute (n) and relative (%) frequencies and compared by Fisher's exact test. Correlations were assessed by Pearson's correlation coefficient (r), or Spearman's rho. Due to the small number of observations in the PGA-category «moderate», categories «mild» and «moderate» were collapsed into one class and compared to patients in «clinical remission». Similarly, as the numbers of subjects in the moderate/severe categories, according to PCDAI/PUCAI classification, were very small, patients were grouped as "in remission" or "in relapse with mild/moderate activity" and a new joined variable was formed including all individuals. After univariate analyses were performed, a stepwise backward regression analysis was performed to assess significant parameters at the multivariate level, grouping all IBD patients. Level of statistical significance, for univariate analyses, was set to 0.05. For the stepwise approach, the level for entering a covariate into the model was 0.051 whereas for removing, it was 0.05. All analyses were performed with Stata 11.0 MP statistical software (Stata Corp, TX, United States).
A total of 99 patients (62 CD, 37 UC) were included in the analysis. Demographic data and disease characteristics are shown in Table 1. Patients with CD were older at the time of diagnosis, as well as at the time of IMPACT completion and were more likely to be treated with an anti-TNFα agent. Table 2 illustrates total and sub-domain IMPACT scores, overall as well as according to diagnosis. In general, patients with CD scored higher in all scales compared to ulcerative colitis group; however the results did not reach statistical significance, not even after adjusting for PGA level (all P values > 0.05). The only exception was emotional functioning domain, where among patients in clinical remission, according to PGA, CD children scored significantly higher compared to UC children (79.3 ± 16.2 vs 70.0 ± 21.1, P = 0.05).
| Overall (n = 99) | CD (n = 62) | UC (n = 37) | P value | |
| Gender, females | 59 (59.6) | 32 (51.6) | 27 (73.0) | 0.063 |
| Age (yr) | 12.8 ± 2.6 | 13.4 ± 2.4 | 11.6 ± 2.5 | 0.0011 |
| Age at diagnosis (yr) | 9.9 ± 3.1 | 10.7 ± 2.9 | 8.6 ± 3.0 | < 0.0011 |
| Number of hospitalizations4 | 2 (1-4) | 2 (1-4) | 3 (1-6) | 0.092 |
| Disease duration at IMPACT completion (yr) | 2.8 ± 2.6 | 2.7 ± 2.5 | 3.0 ± 2.7 | 0.481 |
| Disease activity | NA | 7.2 ± 10.3 | 14.0 ± 20.5 | NA |
| Disease status, in remission | 70 (70.7) | 45 (72.6) | 25 (67.6) | 0.653 |
| Physician global assessment | 0.93 | |||
| Clinical remission | 80 (80.8) | 50 (80.6) | 30 (81.1) | |
| Mild | 14 (14.1) | 9 (14.5) | 5 (13.5) | |
| Moderate | 5 (5.1) | 3 (4.8) | 2 (5.4) | |
| Medications at IMPACT completion | ||||
| Antibiotics | 2 (2.0) | 2 (3.2) | 0 (0.0) | 0.523 |
| Steroids | 39 (39.4) | 24 (38.7) | 15 (40.5) | 0.993 |
| Immunomodulators | 68 (68.7) | 47 (75.8) | 21 (56.8) | 0.113 |
| Biologic agents | 31 (31.3) | 25 (40.3) | 6 (16.2) | 0.033 |
| Enteral nutrition | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Aminosalicylates | 52 (52.5) | 15 (24.2) | 37 (100.0) | < 0.0013 |
| Overall (n = 99) | CD (n = 62) | UC (n = 37) | P value1 | |
| Total score | 76.2 ± 12.1 | 77.5 ± 11.2 | 73.9 ± 13.3 | 0.16 |
| Individual domains | ||||
| Bowel symptoms | 76.6 ± 14.8 | 78.0 ± 14.0 | 74.2 ± 15.5 | 0.22 |
| Systemic symptoms | 80.8 ± 17.4 | 82.0 ± 16.7 | 78.8 ± 18.5 | 0.36 |
| Emotional functioning | 72.4 ± 19.0 | 75.2 ± 18.3 | 67.9 ± 19.6 | 0.06 |
| Social functioning | 79.9 ± 12.4 | 80.6 ± 12.0 | 78.6 ± 13.0 | 0.42 |
| Body image | 71.5 ± 17.9 | 72.6 ± 19.3 | 69.8 ± 15.4 | 0.46 |
| Treatment/interventions | 69.0 ± 19.9 | 70.0 ± 18.4 | 67.3 ± 22.4 | 0.51 |
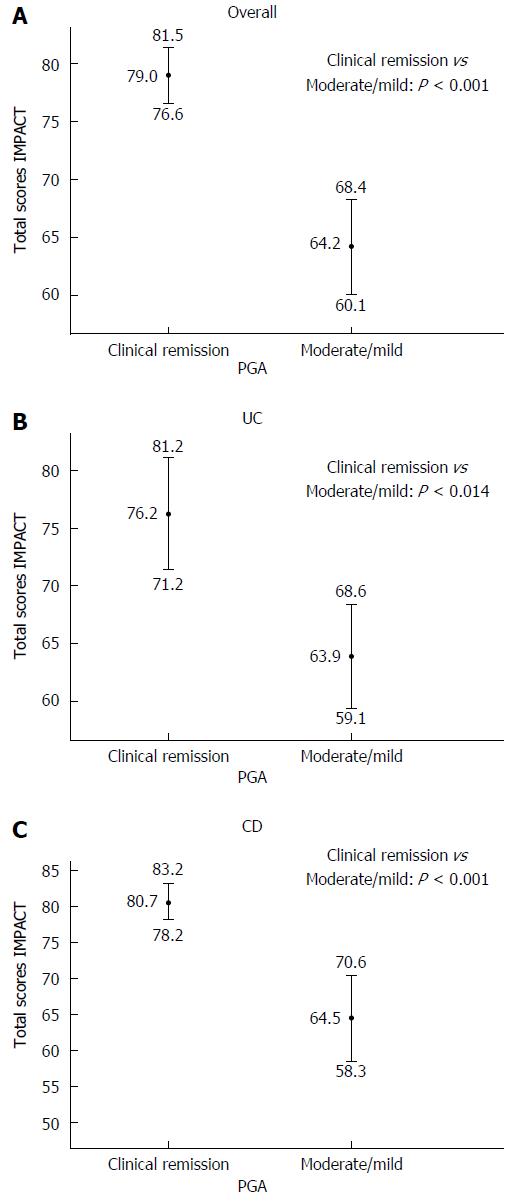
In the entire study sample of IBD patients, the total IMPACT score was positively related to disease duration (Pearson's r = 0.20, P = 0.04), age at IMPACT completion (Pearson's r = 0.19, P =0.05) and inversely related to disease activity assessed by PUCAI and PCDAI ("in remission" vs "mild/moderate": 79.6 ± 11.4 vs 68.0 ± 10.0, respectively, P < 0.001). Sub-analyses confirmed the relation between total IMPACT score and disease activity in both IBD groups (UC: Pearson's r = -0.45, P = 0.005, CD: Pearson's r = -0.42, P < 0.001) and between total IMPACT score and age at IMPACT completion only in the UC group (Pearson's r = 0.29, P = 0.05). Disease duration was not a significant predictor in either group, in stratified analyses. Overall, total scores showed a significant trend across the PGA scale, with lower scores corresponding to worst clinical assessment (Mann-Whitney test, P < 0.001; Figure 1A). The same result occurred in the stratified analyses (Mann-Whitney test, UC: P = 0.014 and CD: P < 0.001; Figure 1B and C respectively).
Gender, number of hospitalizations in the previous 3 mo and type of medication were not correlated to total IMPACT scores neither overall or per group (all P values > 0.10).
Finally a multivariate stepwise regression analysis was performed in the entire study sample. In the final model, PGA and age at IMPACT-III completion remained significant. More specifically, patients classified as "mild/moderate" had, on average, 14.5 ± 2.7 points lower total scores compared to those "in remission" (P < 0.001). In addition, it was estimated that one year increase in age at IMPACT-III completion increases the total score by an average of 0.8 ± 0.4 points, regardless of PGA classification (P = 0.044).
Bowel symptoms: Overall, the bowel symptoms domain score was significantly related to disease duration (Pearson's r = 0.27, P = 0.005), age at IMPACT completion (Pearson's r = 0.23, P = 0.018) and disease activity (in remission vs mild/moderate activity: 81.4 ± 12.7 vs 65.3 ± 10.0, respectively, P < 0.001). In the stratified analysis the direction of the correlations was retained; however in UC only disease activity remained a statistically significant predictor (Pearson's r = -0.56, P < 0.001), whereas in the CD group disease duration (Pearson's r = 0.31, P = 0.013) and disease activity (Pearson's r = -0.54, P < 0.001) reached statistical significance. Patients with CD who were being treated with steroids had significantly lower scores in the bowel symptoms domain compared to steroid-free CD patients (on-steroids vs no-steroids: 73.8 ± 13.8 vs 80.7 ± 13.9, P = 0.047). After stratification according to PGA, significance was marginally not achieved for patients in remission (on-steroids vs no-steroids: 77.8 ± 11.3 vs 83.7 ± 12.1, P = 0.09). No other significant associations between bowel symptoms domain scores and type of medications were observed. As shown in Tables 3, 4 and 5, across the study population, overall as well as in the sub-group analyses, there was a clear trend for bowel symptoms scores resulting in significantly lower scores for patients in the moderate/mild category compared to those classified in remission. The regression analysis showed that PGA, disease activity and disease duration were significant covariates in the final model. Based on PGA, children classified as "mild/moderate" had, on average, 9.6 ± 4.1 points lower total scores compared to those "in remission" (P = 0.022), whereas according to activity indices a similar difference was estimated between the two categories (9.8 ± 3.6, P = 0.008). An interesting point is that these results are adjusted for the level of the other covariate. For example, patients classified "in remission", according to PGA, have significantly different scores depending on their disease status based on activity indices. In the regression analysis it was, also, estimated that one year increase in disease duration increases the total score by an average of 1.0 ± 0.5 points, irrespective of PGA classification or disease activity (P = 0.037).
| Domain | PGA | P value1 | |
| Clinical remission | Mild/moderate | ||
| Bowel symptoms | 80.0 ± 13.3 | 62.4 ± 11.8 | < 0.001 |
| Systemic symptoms | 84.2 ± 16.0 | 66.6 ± 15.9 | < 0.001 |
| Emotional functioning | 75.8 ± 18.6 | 58.3 ± 13.5 | < 0.001 |
| Social functioning | 82.5 ± 11.0 | 69.0 ± 11.9 | < 0.001 |
| Body image | 73.2 ± 17.9 | 64.5 ± 16.4 | 0.032 |
| Treatment/interventions | 70.9 ± 20.1 | 61.0 ± 17.1 | 0.007 |
| Domain | PGA | P value1 | |
| Clinical remission | Mild/moderate | ||
| Bowel symptoms | 77.4 ± 15.1 | 60.7 ± 9.2 | 0.007 |
| Systemic symptoms | 82.8 ± 17.6 | 61.9 ± 11.6 | 0.002 |
| Emotional functioning | 70.0 ± 21.1 | 58.7 ± 4.5 | 0.050 |
| Social functioning | 80.8 ± 12.7 | 69.0 ± 10.0 | 0.032 |
| Body image | 70.3 ± 16.6 | 67.9 ± 8.9 | 0.600 |
| Treatment/interventions | 68.9 ± 24.2 | 60.7 ± 11.5 | 0.300 |
| Domain | PGA | P value1 | |
| Clinical remission | Mild/moderate | ||
| Bowel symptoms | 81.6 ± 12.1 | 63.4 ± 13.4 | < 0.001 |
| Systemic symptoms | 85.0 ± 15.1 | 69.4 ± 17.9 | 0.005 |
| Emotional functioning | 79.3 ± 16.2 | 58.0 ± 16.9 | < 0.001 |
| Social functioning | 83.4 ± 9.9 | 68.9 ± 13.2 | 0.001 |
| Body image | 75.0 ± 18.6 | 62.5 ± 19.6 | 0.036 |
| Treatment/interventions | 72.2 ± 17.5 | 61.1 ± 20.2 | 0.070 |
Systemic symptoms: With respect to the systemic symptoms domain score, disease activity was a significant correlate overall (in remission vs mild/moderate activity: 84.3 ± 16.0 vs 72.4 ± 18.0, respectively, P = 0.001), as well as in the UC and CD groups (Pearson's r = -0.45, P = 0.005 and Pearson's r = -0.30, P = 0.015, respectively). Similarly to the bowel symptoms domain, PGA was significantly and negatively related to systemic symptoms domain (Tables 3, 4 and 5). With the exception of steroids, treatment was not related to systemic symptoms. Patients receiving steroids scored significantly lower in the systemic symptoms domain (on-steroids vs no-steroids: 76.5 ± 18.8 vs 83.6 ± 18.9, P = 0.045). When the analysis was repeated, after stratifying according to PGA class, the effect was retained for those patients in remission (on-steroids vs no-steroids: 79.2 ± 19.8 vs 86.6 ± 18.9, P = 0.039) but not for those with active disease (P = 0.34). In the final model only PGA remained statistically significant ("mild/moderate" had, on average, 17.5 ± 4.1 points lower total scores compared to those "in remission" (P < 0.001)
Emotional functioning: The same pattern was observed for the emotional functioning domain score and disease activity, as well: (overall: in remission vs mild/moderate activity: 75.9 ± 19.4 vs 64.0 ± 15.2, respectively, P = 0.004, UC: Spearman's rho = -0.33, P = 0.04, CD: Spearman's rho = -0.32, P = 0.011). In addition, disease duration appeared to be positively and significantly related to the emotional functioning domain score overall, but not in the sub-group analyses (overall: Pearson's r = 0.20, P = 0.046). An interesting finding was the negative effect of extra-intestinal manifestations on emotional functioning (yes vs no: 55.0 ± 19.8 vs 73.4 ± 18.6, P = 0.046). In relation to PGA, in accordance to previous results, patients classified as "mild/moderate" had significantly worse scores compared to those "in remission" (Tables 3, 4 and 5). Therapeutic modalities did not affect emotional functioning. The multivariate analyses, where all candidate parameters were included and a stepwise process rejected the statistically insignificant, revealed a quite different final model. PGA and disease duration were, again, significant correlates [PGA: "mild/moderate" had, on average, 15.7 ± 4.4 points lower scores compared to those "in remission" (P = 0.001); one year increase in disease duration increases the score by an average of 1.6 ± 0.7 points (0.019)]. Moreover, it was shown that patients with extra-intestinal manifestations had lower scores (average difference: -19.7 ± 0.7, P = 0.017) and the same was demonstrated for gender, with boys having significantly higher scores compared to girls (average difference: 7.4 ± 3.6, P = 0.045).
Social functioning: A positive trend was recorded between age and social functioning scores, overall (Pearson's r = 0.21, P = 0.030), but not in stratified analyses. The previously described inverse relation between domain scores and disease activity was also observed in the social functioning domain score not only in the entire IBD population (overall: in remission vs mild/moderate activity: 83.7 ± 10.7 vs 70.8 ± 11.5, respectively, P < 0.001), but also per IBD group (UC: Pearson's r = -0.52, P < 0.001 and CD: Pearson's r = -0.43, P < 0.001). Disease duration appeared to be positively and significantly related to social functioning domain score in the entire study population (Pearson's r = 0.21, P = 0.034). Patients in the "mild/moderate" group according to PGA classification had impaired social functioning compared to those "in remission" (Tables 3, 4 and 5). Medications were not associated with the social functioning score, overall nor in the stratified analyses. Nevertheless, in the final modeling, PGA was not a significant parameter. Higher disease activity assessed by PCDAI and PUCAI resulted in worse social functioning ("mild/moderate" vs "in remission", average difference -13.2 ± 2.3, P < 0.001) and the presence of extra-intestinal manifestations, also, was related to lower scores compared to no extra-intestinal manifestations (average difference: -12.1 ± 4.9, P = 0.015).
Body image and treatment/interventions: For both, body image and treatment/interventions domains, no statistically significant relationship to the assessed disease characteristics or prescribed medications was found. Regarding PGA, in general, the previously described trends were also observed, although correlations for body image and treatment/intervention domains in UC patients (P = 0.6 and P = 0.3, respectively), and treatment/intervention in CD patients (P = 0.07) did not reach statistical significance (Tables 3, 4 and 5).
The results of the present study indicate that disease activity is the major factor associated with low QOL in children with IBD. The analysis demonstrated a clear inverse relationship between disease activity and IMPACT-III, total and subdomain, scores. The same trend was observed for CD and UC patients, separately. Additionally, it was shown that physician's assessment (through the PGA scale) was a strong correlate of QOL. Interestingly, in the multivariate analysis, PGA absorbed the statistical significance of disease activity, as a correlate of IMPACT-III total score. This could reflect the greater ability of physician's perspective to detect subtle variations in the patient's physical and psychological status. These findings are in accordance with the majority of previous studies, which also found disease activity as the major negative predictor of QOL in IBD patients[8-17].
Published data have been inconsistent regarding comparisons of QOL between patients with CD and UC. Some reports suggest that no such differences exist[18,19], whereas, others describe poorer QOL in patients with CD, due to its worse clinical course, constant need of treatment and higher likelihood for surgery[20]. In our analysis, CD patients reported better QOL scores compared to UC patients, although the difference was not statistically significant. In the subdomain analyses statistical significance was reached for the emotional functioning domain. Notably, CD patients in our study were older and were more likely to be receiving an anti-TNF agent. Both these parameters have been shown to improve QOL, although for the latter no such association was recorded in our sample[21,22].
The assessment of the association between age and QOL in pediatric IBD populations has generated controversial results. We recorded a positive, statistically significant association between age and total IMPACT-III scores, independently of PGA classification. In 2002, Loonen et al[23] concluded that adolescents have impaired QOL scores compared to younger children, whereas Gallo et al[18] found no association. An interesting study published by Deepal et al[24] in 2012, supported that post-colectomy QOL in UC patients was better when the diagnosis was made under the age of twelve. A possible interpretation of our result is that, as children grow into adolescence, they may be able to develop more efficient coping mechanisms and therefore be less vulnerable to the psychological effect of a chronic disease.
An area which has been sparsely investigated is the impact of disease duration and received mediations on QOL. Although some studies failed to find any correlation[19,25], others have indicated improved QOL scores with longer disease duration[9,26,27]. Similarly to the latter, we also showed that disease duration is positively correlated to IMPACT-III total scores. Moreover, in subdomain-analyses, the same positive trend was observed for bowel symptoms, emotional functioning and social functioning. This finding could reflect an adaption process to a new life-style, which, particularly for growing, peri-pubertal children, may be cumbersome and prolonged. In pediatric IBD, bowel symptoms, emotional and social issues are of major concern and their course contribute significantly to QOL[28].
Our findings, also, suggest that, with the exception of steroids, type of medication had no effect on QOL. Nevertheless, patients on steroid therapy scored significantly lower in the systemic symptoms domain and CD patients on steroids recorded impaired bowel symptoms domain subscores. Surprisingly, for systemic symptoms, the effect was retained for the group of patients in remission, after stratifying according to PGA class, whereas for bowel symptoms in CD it was marginally lost. This is probably due to the fact that patients usually receive steroids during and shortly after a flare, when systemic symptoms are present or recent, consequently affecting their QOL. Another interpretation could be that steroid therapy can cause mood impairments, although this should affect the emotional functioning of the patients as well. Furthermore, steroid therapy imposes dietary restrictions and requires supplemental medication (vitamin D). All the above might contribute to the observed effect. Previous studies have, also, demonstrated the negative impact of steroid therapy in the QOL of patients[29,30]. In contrast to recent reports, we did not find any association between the use of biological agents and IMPACT-III, total and subdomain, scores[21,22,31].
An interesting observation, not frequently reported in literature, was the negative association between extra-intestinal manifestations and emotional functioning. This seems to be in accordance with one previous study which concluded that musculoskeletal manifestations had a detrimental effect on QOL[32]. In our population, three patients had sclerosing cholangitis and two patients suffered from type II peripheral arthritis. The latter has a clinical course independent of IBD activity[33]; therefore it could contribute to an impaired QOL even if a patient is in remission. The former is known to have an irreversible, progressive course leading ultimately to liver failure. It would be reasonable to assume that the knowledge of having such a destructive, chronic, untreatable disease, severely affects emotional functioning in these patients.
The main drawback of our study is the cross-sectional nature of the analysis that does not permit detection of causal relations. Furthermore small sub-sample sizes may have prevented some comparisons from reaching statistical significance due to reduced power.
In conclusion, The QOL of patients with IBD is directly and mainly dependent on the activity of their disease and this relationship is optimally assessed by PGA rather than activity indices. Extra-intestinal manifestations and use of steroids should always raise the concern of impaired QOL, even if clinical remission of intestinal disease has been achieved. Disease duration and age have a positive impact on QOL; therefore the first years after the diagnosis, particularly in younger children, is the most sensitive period requiring intensive, supportive interventions.
The inflammatory bowel diseases (IBD) are chronic conditions of the intestines requiring frequent hospitalizations and long-term medications. The risk for surgeries, complications and extra-intestinal manifestations is increased in children suffering from these disorders. All the above result in a significant psychosocial burden which negatively affects their quality of life (QOL).
The spectrum of the clinical evolution of a child suffering from IBD presents many fluctuations and is affected by the age of onset, disease duration, medications and medication-related side effects, and disease-related complications, such as surgeries and extra-intestinal manifestations. The main research question, which is of direct clinical importance, is the identification of factors that predispose in impaired QOL. This would allow treating physicians to, timely, intervene in a timely manner and try to minimize the negative consequences, which is a top priority in growing adolescents.
Several, different, disease-related parameters were found to influence the QOL in pediatric IBD patients, such as disease activity, use of steroids and extra-intestinal manifestations. The significant relation of the physician’s global assessment to the QOL underlines the importance of the clinician’s subjective impression, apart from the standardized activity indices. The time frame of maximum vulnerability appears to be during the early years after the diagnosis, especially in younger children
The observations derived from the present study could serve as a guide for identifying IBD sub-groups at high risk for impaired QOL. Based on these results, physicians treating children with IBD, could implement early strategies in order to manage, or optimally prevent, significant deteriorations. On the other hand, focused research on these high-risk patients could help to clarify the biologic and psychological mechanisms underlying the above described processes.
IBD is a group of chronic intestinal disorders, which may present in early childhood. QOL encompasses information from different aspects of a child’s perception on everyday life. It can be assessed by the IMPACT-III questionnaire which is a self-reporting, validated, structured scale offering quantitative assessment on the QOL, overall, as well as on distinct sub-domains.
This study was well conducted and nicely written, it can be of assistance to the scientific community.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Capasso R, Gazouli M, Hokama A, Lakatos PL S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | Kunz JH, Hommel KA, Greenley RN. Health-related quality of life of youth with inflammatory bowel disease: a comparison with published data using the PedsQL 4.0 generic core scales. Inflamm Bowel Dis. 2010;16:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Timmer A, Preiss JC, Motschall E, Rücker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2011;CD006913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 3. | Kilroy S, Nolan E, Sarma KM. Quality of life and level of anxiety in youths with inflammatory bowel disease in Ireland. J Pediatr Gastroenterol Nutr. 2011;53:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 989] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 5. | Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle JT. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 803] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 813] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 7. | Otley A, Smith C, Nicholas D, Munk M, Avolio J, Sherman PM, Griffiths AM. The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002;35:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Bernklev T, Jahnsen J, Aadland E, Sauar J, Schulz T, Lygren I, Henriksen M, Stray N, Kjellevold O, Vatn M. Health-related quality of life in patients with inflammatory bowel disease five years after the initial diagnosis. Scand J Gastroenterol. 2004;39:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Otley AR, Griffiths AM, Hale S, Kugathasan S, Pfefferkorn M, Mezoff A, Rosh J, Tolia V, Markowitz J, Mack D. Health-related quality of life in the first year after a diagnosis of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Graff LA, Walker JR, Lix L, Clara I, Rawsthorne P, Rogala L, Miller N, Jakul L, McPhail C, Ediger J. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4:1491-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Hill R, Lewindon P, Muir R, Grangé I, Connor F, Ee L, Withers G, Cleghorn G, Davies P. Quality of life in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2010;51:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Hjortswang H, Ström M, Almer S. Health-related quality of life in Swedish patients with ulcerative colitis. Am J Gastroenterol. 1998;93:2203-2211. [PubMed] [DOI] [Full Text] |
| 13. | Perrin JM, Kuhlthau K, Chughtai A, Romm D, Kirschner BS, Ferry GD, Cohen SA, Gold BD, Heyman MB, Baldassano RN. Measuring quality of life in pediatric patients with inflammatory bowel disease: psychometric and clinical characteristics. J Pediatr Gastroenterol Nutr. 2008;46:164-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Engelmann G, Erhard D, Petersen M, Parzer P, Schlarb AA, Resch F, Brunner R, Hoffmann GF, Lenhartz H, Richterich A. Health-related quality of life in adolescents with inflammatory bowel disease depends on disease activity and psychiatric comorbidity. Child Psychiatry Hum Dev. 2015;46:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Gray WN, Denson LA, Baldassano RN, Hommel KA. Disease activity, behavioral dysfunction, and health-related quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Abdovic S, Mocic Pavic A, Milosevic M, Persic M, Senecic-Cala I, Kolacek S. The IMPACT-III (HR) questionnaire: a valid measure of health-related quality of life in Croatian children with inflammatory bowel disease. J Crohns Colitis. 2013;7:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Szigethy E, McLafferty L, Goyal A. Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am. 2010;19:301-318, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Gallo J, Grant A, Otley AR, Orsi M, MacIntyre B, Gauvry S, Lifschitz C. Do parents and children agree? Quality-of-life assessment of children with inflammatory bowel disease and their parents. J Pediatr Gastroenterol Nutr. 2014;58:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kalafateli M, Triantos C, Theocharis G, Giannakopoulou D, Koutroumpakis E, Chronis A, Sapountzis A, Margaritis V, Thomopoulos K, Nikolopoulou V. Health-related quality of life in patients with inflammatory bowel disease: a single-center experience. Ann Gastroenterol. 2013;26:243-248. [PubMed] |
| 20. | Cohen RD. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Szabó D, Kökönyei G, Arató A, Dezsőfi A, Molnár K, Müller KE, Lakatos PL, Papp M, Lovász BD, Golovics PA. Autoregressive cross-lagged models of IMPACT-III and Pediatric Crohn’s Disease Activity Indexes during one year infliximab therapy in pediatric patients with Crohn’s disease. J Crohns Colitis. 2014;8:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Loonen HJ, Grootenhuis MA, Last BF, Koopman HM, Derkx HH. Quality of life in paediatric inflammatory bowel disease measured by a generic and a disease-specific questionnaire. Acta Paediatr. 2002;91:348-354. [PubMed] [DOI] [Full Text] |
| 24. | Dalal DH, Patton D, Wojcicki JM, Clark AL, Garnett EA, Heyman MB. Quality of life in patients postcolectomy for pediatric-onset ulcerative colitis. J Pediatr Gastroenterol Nutr. 2012;55:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Pallis AG, Vlachonikolis IG, Mouzas IA. Assessing health-related quality of life in patients with inflammatory bowel disease, in Crete, Greece. BMC Gastroenterol. 2002;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Jäghult S, Saboonchi F, Johansson UB, Wredling R, Kapraali M. Identifying predictors of low health-related quality of life among patients with inflammatory bowel disease: comparison between Crohn’s disease and ulcerative colitis with disease duration. J Clin Nurs. 2011;20:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Haapamäki J, Turunen U, Roine RP, Färkkilä MA, Arkkila PE. Impact of demographic factors, medication and symptoms on disease-specific quality of life in inflammatory bowel disease. Qual Life Res. 2009;18:961-969. [PubMed] [DOI] [Full Text] |
| 28. | Griffiths AM, Nicholas D, Smith C, Munk M, Stephens D, Durno C, Sherman PM. Development of a quality-of-life index for pediatric inflammatory bowel disease: dealing with differences related to age and IBD type. J Pediatr Gastroenterol Nutr. 1999;28:S46-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Bernklev T, Jahnsen J, Schulz T, Sauar J, Lygren I, Henriksen M, Stray N, Kjellevold Ø, Aadland E, Vatn M, Moum B. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur J Gastroenterol Hepatol. 2005;17:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Romberg-Camps MJ, Bol Y, Dagnelie PC, Hesselink-van de Kruijs MA, Kester AD, Engels LG, van Deursen C, Hameeteman WH, Pierik M, Wolters F. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis. 2010;16:2137-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | DeBoer MD, Barnes BH, Stygles NA, Sutphen JL, Borowitz SM. Changes in inflammation and QoL after a single dose of infliximab during ongoing IBD treatment. J Pediatr Gastroenterol Nutr. 2012;54:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | van der Have M, Brakenhoff LK, van Erp SJ, Kaptein AA, Leenders M, Scharloo M, Veenendaal RA, van der Heijde DM, van der Meulen-de Jong AE, Hommes DW. Back/joint pain, illness perceptions and coping are important predictors of quality of life and work productivity in patients with inflammatory bowel disease: a 12-month longitudinal study. J Crohns Colitis. 2015;9:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Peluso R, Manguso F, Vitiello M, Iervolino S, Di Minno MN. Management of arthropathy in inflammatory bowel diseases. Ther Adv Chronic Dis. 2015;6:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |