Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1010
Peer-review started: September 30, 2016
First decision: October 20, 2016
Revised: November 4, 2016
Accepted: November 23, 2016
Article in press: November 28, 2016
Published online: February 14, 2017
Processing time: 140 Days and 3.9 Hours
AIM
To explore the induction effects and mechanism of Solanum lyratum Thumb (ST) on human hepatocellular carcinoma SMMC-7721 cells through the mitochondrial pathway.
METHODS
The experiments were conducted on three groups: an experimental group (with ST ethanol extracts’ concentration being 2.5, 5 and 10 mg/L), a negative control group (with only nutrient solution, 0 mg/L ST ethanol extracts), and a positive control group (2.5 mg/L DDP). The inhibition rate of cell proliferation was checked by using the methyl thiazolyl tetrazolium method, and cell apoptosis was tested by TUNEL method. Furthermore, RT-PCR was used to examine mRNA expression of Fas, FasL, caspase-8, caspase-3, p53 and Bcl-2 genes.
RESULTS
Compared with the negative control group, the inhibition and apoptosis rates of the experimental group with different concentrations of ST extracts on human hepatocellular carcinoma SMMC-7721 cells significantly increased (P < 0.05). Besides, the mRNA expression of FasL and Bcl-2 significantly decreased (P < 0.05) while the mRNA expression of Fas, caspase-8, caspase-3 and p53 increased significantly. When compared with the positive control group, the experimental groups with 5 mg/L ST ethanol extracts showed effects similar to the positive control group.
CONCLUSION
ST ethanol extracts induced the apoptosis of hepatocellular carcinoma SMMC-7721 cells through up-regulated Fas, caspase-8, caspse-3 and p53, and down-regulated FasL and Bcl-2 in the mitochondrial pathway.
Core tip: Chinese herbal medicine has a very good effect on the tumor. Solanum lyratum Thumb (ST) belonging to Solanaceae, is generally used to treat tumors, so it is a commonly used anticancer drug. However, the effects and mechanism of ST on tumor cells are unclear. This experiment verified that ST can induce the apoptosis of hepatocellular carcinoma SMMC-7721 cells; moreover, the apoptosis mechanism was related to the expression of Fas, FasL, caspase-8, caspase-3, p53 and Bcl-2 in the mitochondrial pathway. This result provides powerful evidence of the improved apoptosis effects of ST on hepatocellular carcinoma cells.
- Citation: Mo XQ, Wei HY, Huang GR, Xu LY, Chen YL, Qi J, Xian W, Qin YC, Wei LD, Zhao LJ, Huang YQ, Xing W, Pu HQ, Wei PY, Li CG, Liang QC. Molecular mechanisms of apoptosis in hepatocellular carcinoma cells induced by ethanol extracts of Solanum lyratum Thumb through the mitochondrial pathway. World J Gastroenterol 2017; 23(6): 1010-1017
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1010.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1010
Solanum lyratum Thumb (ST), belonging to Solanaceae, is generally used to treat tumors[1-3], including liver, gastric, esophageal and bladder cancers, with exact curative effects, and it is a commonly used anticancer drug. However, the effects on tumor cells are unclear. The occurrence of tumors is closely related to the abnormality of cell differentiation and is a disordered cell apoptosis. Cell apoptosis is strictly controlled by multiple genes and factors. With the development of the technologies used in molecular biology and proteomics, cell apoptosis is gradually being understood, and some new regulatory genes have been found, with the result that the pathway of cell apoptosis is now better recognized. The mitochondrial pathway is currently recognized as one of the important methods of signal transmission in the process of cell apoptosis. Genes including Fas, FasL, caspase-8, caspase-3, p53 and Bcl-2 are involved in regulation of this pathway. Furthermore, the coordinated network regulation system formed by these genes promotes or inhibits cell apoptosis[4-7].
To date, there is no report on whether ST extracts can induce the apoptosis of hepatocellular carcinoma cells through the mitochondrial pathway or by what mechanism such apoptosis occurs. This research aimed to fill this gap in the current knowledge.
Tumor cells: Human hepatocarcinoma SMMC-7721 cells were purchased from the Shanghai Institute of Cell Biology of Chinese Academy of Science, China.
Main reagents: ST was purchased from the biological medicine chain in Baise, Guangxi Province, China. RPMI 1640 cultural medium and fetal bovine serum were purchased from Gibco Company, United States. The detection kit for in situ cell apoptosis was sourced from Beijing Zhongshan Jinqiao Biotech Company, China. Methyl thiazolyl tetrazolium (MTT) was produced by Shanghai Pufei Biotech Co., Ltd, China. The polymerase chain reaction (PCR) primer was bought from Sangon Biotech Shanghai Co., Ltd, China. In addition, the Trizol Reagent Kit and the 2 × SYBRGreen qPCR Mix were purchased from Shanghai Invitrogen Company, China and Beijing Zhuangmeng Co., Ltd, China respectively. The RevertAid First Strand cDNA Synthesis Kit and DNase I were obtained from Fermentas, United States.
Main instruments: The main instruments used included a carbon dioxide incubator (MCO-18AIC), a biosafety cabinet (BHC-1300 II A/B33), an automatic microplate spectrophotometer (Multiskan MK3), an inverted microscope (Cioc), and a BX51 microscope (Olympus). Furthermore, a table-top, high-speed freezing centrifuge (1-15PK), a microcentrifuge (Uni Force 6K) and a RT-PCR instrument (IQ5) were also used in this study.
Ethanol extracts of ST: After being smashed, ST of 50 g was immersed for 3 h at 40 °C in 75% ethanol and filtered. The immersion and filtration were conducted three times. Afterwards, the filter liquors were mixed and dried by using a rotary evaporator, thus obtaining ST extractum.
Setting ST of different concentrations: The ST extractum was dissolved using dimethylsulphoxide (DMSO) to prepare the drug solution with a concentration of 10 mg/mL, which was then diluted by RPMI 1640 cell culture solution to obtain ST extract solutions with concentrations of 0, 2.5, 5 and 10 mg/L.
Medicine intervention for tumor cells: Hepatoma carcinoma SMMC-7721 cells were cultured using the culture solution for 24 h and then the culture solution was discarded. Then, the prepared drug solution containing ST extracts was added to the cells. Moreover, a negative control group and a positive control group were set by adding the drug solution without ST extracts (0 mg/L) and 2.5 mg/L DDP, respectively. The cells were subjected to the effects of the drugs for 48 h.
MTT method: Hepatocellular carcinoma SMMC-7721 cell suspension of 200 μL was put into a culture plate with 96 holes at density of 1 × 104 ml-1 to be cultured for 24 h. Afterwards, the culture solution was removed and discarded, and 200 μl of the prepared ST solutions with different concentrations were added to the culture plate. Four holes were established for each concentration in the experimental group. The negative control group with culture solution (0 mg/L ST extracts) and the DDP positive control group were placed into the incubator to be cultured for 44 h, and then dosed with 20 μl MTT (5 mg/ml), followed by 4 h of continuous culture. After the supernatants were poured out and 150 μl DMSO was added, the solutions were shocked for 10 min. Finally, the wavelength of the enzyme labelling instrument was adjusted to 492 nm to detect the light absorption value (OD value) of the solutions (three replicates), thus allowing for calculation of the average inhibition rate. The formula for calculating the inhibition rate was (1 - OD value of the experimental group/OD value of the control groups) × 100%.
TUNEL method: The experiment was conducted according to the specification of the purchased detection kit for in situ cell apoptosis. Based on the analysis results under a visible light microscope, the apoptosis rate was calculated as the percentage of positive cells counted in randomly 10 high-power fields.
RT-PCR test: (1) Primer design: Primers were designed by using Primer Premier 5.0 software and checked in GenBank. The primers for Fas, FasL, caspase-8, caspase-3, p53, Bcl-2 and β-actin are shown in Table 1; (2) RNA extraction: after medical intervention for tumor cells using ST, total RNA was extracted in accordance with the specification of the purchased Trizol Reagent Kit; (3) reverse transcription: RNA of 5 μl, along with 1 μl random primer and 5 μl RNase-free ddH2O was added into PCR tubes for warm-bath conditioning for 5 min at 70 °C and ice-bath treatment for 10 s, followed by centrifugation. Then, after being dosed with 4 μl buffer, 2.0 μl dNTP mix, 1.0 μL RNase inhibitor and 2.0 μl AMV reverse transcriptase, the tubes underwent warm-bath conditioning at 37 °C for 5 min, 42 °C for 60 min and 70 °C for 10 min, before termination of the reaction; (4) fluorescence quantitative PCR (qPCR) detection: cDNA samples were diluted 6-fold: the 20 μl reaction system contained 10 μl SYBRGreen qPCR master mix, 1 μl upstream primer (10 μmol/L), 1 μl downstream primer R (10 μmol/L), 7 μl ddH2O and 1 μl template (cDNA). The heat cycle was conducted by pre-denaturation for 2 min at 95 °C, denaturation for 10 s at 95 °C, and annealing for 40 s at 60 °C, for a total of 40 cycles; and (5) relative quantitative analysis method: The 2£ΔΔCT method was used for relative quantitative analysis of data. The calculation formulae were expressed as: ΔCT = CT target gene - CT reference gene, and ΔΔCT = ΔCT target gene - the average value of target gene ΔCT in the control groups. 2£ΔΔCT represents the relative expression of target genes.
| Gene name | Primer sequence | Product length, bp |
| Fas | forward: 5′-GAATGCAAGGGACTGATAGC-3′ | 414 |
| Reverse: 5′-TGGTTCGTGTGCAAGGCTC-3′ | ||
| FasL | forward: 5′-GGAATGGGAAGACACATATGGAACTGC-3′ | 239 |
| Reverse: 5′-CATATCTGGCCAGTAGTGCAGTAATT-3′ | ||
| Caspase-3 | forward: 5′-GCTATTGTGAGGCGGTTGT-3′ | 270 |
| Reverse: 5′-CGTTTGGAGTCCCTTTGT-3′ | ||
| Caspase-8 | forward: 5′-TCTGGAGCATCTGCTGTCTG-3′ | 427 |
| Reverse: 5′-TTGACGTCTGTGGTCCGTCC-3′ | ||
| p53 | forward: 5′-CTTTCAACTCTGTCTCCTTC-3′ | 180 |
| Reverse: 5′-TGGGCAACCAGCCCTGTCGT-3′ | ||
| Bcl-2 | forward: 5′-CAGCTGCACCTGACGCCCTT-3′ | 231 |
| Reverse: 5′-GCCTCCGTTATCCTGGATCC-3′ | ||
| β-actin | forward: 5′-ACACTGTGCCCATCTACGAGG-3′ | 621 |
| Reverse: 5′-AGGGGCCGGACTCGTCATACT-3′ |
Inhibition and apoptosis rate were analyzed by using SPSS17 software and data were represented by mean ± standard variance (±s). A homogeneity test of variance and one-way ANOVA were used for comparison among groups. As for further pairwise comparison, if the variance was homogeneous, the Student-Newman-Keuls method was used, while Games-Howell was used when the variance was inhomogeneous. Moreover, χ2 was used for the comparison of the rates. If P < 0.05, the difference was deemed to have been statistically significant.
The inhibition rate of hepatocellular carcinoma SMMC-7721 cells in the experimental group of each ST concentration was significantly higher than that of the negative control group (P < 0.05), and the higher the ST concentration, the higher the inhibition rate. Furthermore, the inhibition rate of the group with 5 mg/L ST was equal to that of the positive control group (Table 2).
| Group | Concentration, mg/L | n | OD value | Inhibition rate, % |
| Three ST groups with different concentrations | 2.5 | 12 | 1.516 ± 0.205 | 18.22 ± 2.32 |
| 5 | 12 | 1.242 ± 0.236 | 36.56 ± 2.51 | |
| 10 | 12 | 0.815 ± 0.276 | 55.98 ± 3.12 | |
| Negative control group | 0 | 12 | 1.842 ± 0.183 | - |
| Positive control group | 2.5 | 12 | 1.135 ± 0.172 | 38.60 ± 1.78 |
The apoptosis rate of hepatocellular carcinoma SMMC-7721 cells in each ST group with different concentrations was significantly higher than that of the positive control group (P < 0.05). The higher the ST concentration, the higher the cell apoptosis rate. Moreover, the apoptosis rate of the group with 5 mg/L ST was identical to that of the positive control group (Table 3).
| Group | Concentration, C/mg•L-1 | Apoptosis rate of SMMC-7721 cells, % |
| Three ST groups with different concentrations | 2.5 | 10.75 ± 2.51 |
| 5 | 17.31 ± 2.33 | |
| 10 | 30.21 ± 3.62 | |
| Negative control group | 0 | 3.75 ± 1.23 |
| Positive control group | 2.5 | 17.36 ± 1.62 |
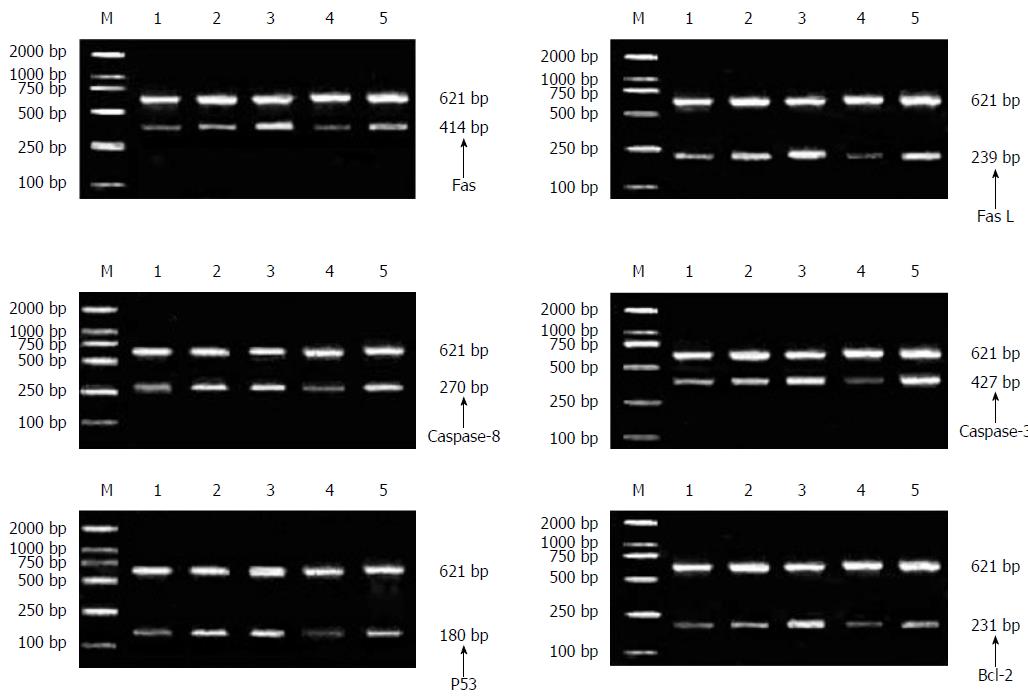
After intervention of ST extracts in each concentration on the hepatocellular carcinoma SMMC-7721 cells, the mRNA expressions of FasL and Bcl-2 decreased markedly (P < 0.05) and the reductions were negatively correlated with the ST concentration. However, the mRNA expressions of Fas, caspase-8, caspase-3 and p53 increased and the increase showed positive correlation with the ST concentration. When the concentration was greater than, or equal to, 5 mg/L, the mRNA expressions increased (P < 0.05). Moreover, the mRNA expressions of these genes in 5 mg/L ST group were equivalent to those of the positive control group, as demonstrated in Figure 1 and Tables 4 and 5.
| Group | Concentration, C/mg•L-1 | mRNA expression of genes | ||||||
| Fas | FasL | Caspase-8 | Caspase-3 | p53 | Bcl-2 | β-actin | ||
| Three ST groups with different concentrations | 2.5 | 52.47 ± 0.10 | 58.25 ± 0.51 | 30.57 ± 0.10 | 22.15 ± 0.02 | 34.17 ± 0.01 | 36.28 ± 0.14 | 17.53 ± 0.14 |
| 5 | 52.74 ± 0.08 | 56.81 ± 0.30 | 30.76 ± 0.03 | 22.33 ± 0.09 | 34.45 ± 0.20 | 35.40 ± 0.51 | 18.15 ± 0.41 | |
| 10 | 53.41 ± 0.64 | 55.94 ± 0.80 | 31.30 ± 0.24 | 23.01 ± 0.47 | 35.11 ± 0.69 | 33.54 ± 0.51 | 19.10 ± 0.91 | |
| Negative control group | 0 | 52.32 ± 0.04 | 59.13 ± 0.07 | 30.44 ± 0.06 | 22.05 ± 0.03 | 34.11 ± 0.02 | 37.59 ± 0.33 | 17.30 ± 0.17 |
| Positive control group | 2.5 | 52.79 ± 0.11 | 56.69 ± 0.34 | 30.77 ± 0.03 | 22.35 ± 0.12 | 34.43 ± 0.20 | 35.53 ± 0.35 | 18.18 ± 0.41 |
| Group | Concentration, C/mg•L-1 | 2-ΔΔCT | |||||
| Fas | FasL | Caspase-8 | Caspase-3 | p53 | Bcl-2 | ||
| Negative control group | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Three ST groups with different concentrations | 2.5 | 1.06 ± 0.06 | 2.30 ± 0.93a | 1.08 ± 0.04 | 1.10 ± 0.12 | 1.13 ± 0.11 | 3.09 ± 0.59a |
| 5 | 1.34 ± 0.25a | 9.02 ± 0.32b | 1.47 ± 0.31a | 1.50 ± 0.27a | 1.43 ± 0.16a | 9.46 ± 0.58b | |
| 10 | 1.63 ± 0.14a | 31.34 ± 0.13b | 2.02 ± 0.71a | 1.82 ± 0.40a | 1.75 ± 0.25a | 47.29 ± 0.68b | |
| Positive control group | 5 | 1.33 ± 0.17a | 10.02 ± 0.57b | 1.49 ± 0.30a | 1.50 ± 0.19a | 1.47 ± 0.11a | 8.92 ± 0.09b |
ST has functions including cooling and dehumidifying, detoxifying, detumescence and anticancer effects, and it can cure cold and fever, icteric hepatitis, gallstone disease, cholecystitis, nephritis and uterine erosion. Clinically, it has certain effects on the treatment of various types of cancers, especially for lung, liver, stomach and cervical cancers[8-10]. The experimental results show that the inhibition and apoptosis rates of hepatocellular carcinoma SMMC-7721 cells in ST groups with different concentrations were significantly higher than those of the negative control group and showed positive correlation with the ST concentration, confirming that ST can inhibit the growth of hepatocellular carcinoma SMMC-7721 cells. However, the molecular mechanism of this anti-hepatoma behavior is not clear and requires further clarification.
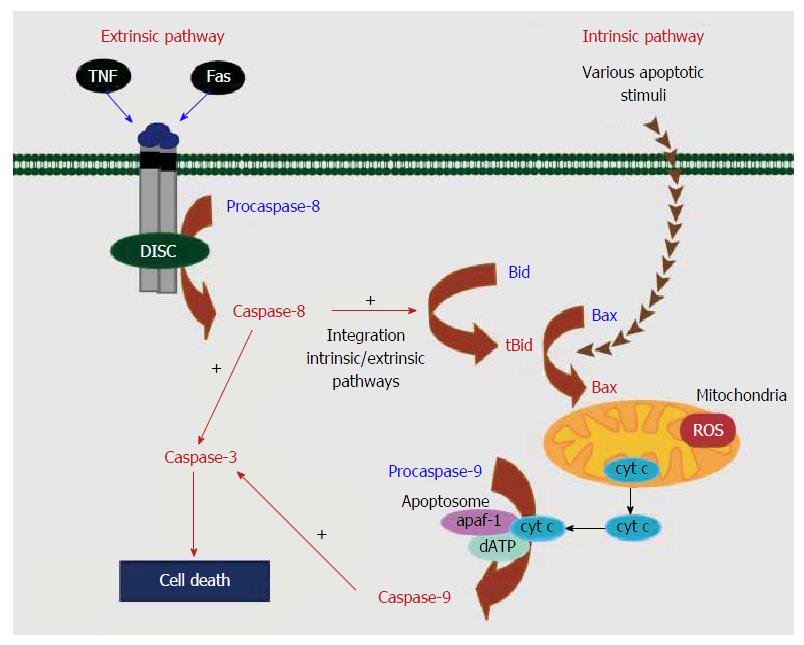
At present, cell apoptosis has been studied and found to be a normal metabolic procedure; however, if the apoptosis process is disordered, a lot of diseases, tumors for instance, can appear. Cell apoptosis is a multi-factor and multi-pathway process regulated by a network, involving a series of proteins, such as caspase family proteins, Bcl-2 family proteins, p53 protein and survivin. Therefore, the exact mechanism of apoptosis is unclear, while the intrinsic and extrinsic pathways of apoptosis have been clarified[11]. As shown in Figure 2, the extrinsic pathway is triggered by death receptors such as Fas and tumor necrosis factor receptor (TNF-R) on the surface of cells[12,13]. While the intrinsic or mitochondrial pathway is induced by many stress conditions, chemical treatment reagents, and medicines.
Under physiological or pathological conditions, no matter whether the apoptosis is induced by DNA damage or other receptors relating to organelles, various receptors affect the intrinsic pathway of apoptosis[14,15]. In the intrinsic pathway of cell apoptosis, the mitochondria control cell activities and are important in regulating cell apoptosis. There are two main changes in mitochondria in the early stage of apoptosis. One is the decrease of mitochondrial internal transmembrane potential, and the other is the increase of mitochondrial external transmembrane permeability.
For the change in interaction of these two aspects, if the difference in the internal and external potentials of the mitochondrial membrane decreases, the mitochondrial transmembrane potential decreases and then the mitochondrial membrane permeability increases, resulting in the release of caspase activated cytochrome C and the activation of caspase protein. The activated caspase affects other protein substrates in the cells, leading to cascade reaction of apoptosis and finally cell apoptosis[16-18].
Caspase, as a cysteine-aspartic specific protease, can excise fragments containing aspartic acids. So far, at least 14 sub-types of caspase with similar molecular structure and high homology have been found. According to their functions, the sub-types can be divided into two categories, namely, initiator and effector caspases. Moreover, caspase plays an essential role in apoptosis. The initiator caspase acts on the inactive effector caspase, thereby activating the effector caspase.
Caspase-8, belonging to the initiator caspase sub-type, is the initiator of the cascade reaction of cell apoptosis. It can self-activate and transmit apoptotic signals in the participation of other proteins and activate downstream effector caspase, thus forming a cascade amplification system to induce cell apoptosis. Caspase-3 (being subjected to the effector caspase) is the most important final excision enzyme and is also an important part of the killing mechanism of cytotoxic T lymphocyte (CTL) cells and can be activated by a variety of factors. As to the killing effects on CTL cells, caspase-3 can be activated by the Fas/FasL pathway and the B pathway of granzymes. When caspase-8, upstream of cells, is activated, the activated caspase-3 excises poly (ADP-ribose) polymerase (PARP) into two fragments, separating two zinc fingers binding with DNA in PARP from the C-terminal catalytic region to influence its normal function. As a result, the activity of Ca/Mg-dependent endonuclease is adversely affected by PARP increases, so that the DNA between nucleosomes is cracked, leading to cell apoptosis[19-21].
p53, as a tumor suppressor gene, slows down or monitors cell division and the integrity of the genome when checking DNA damage loci in the G1 phase. If there is damage, the p53 protein prevents DNA replication and provides sufficient time to repair the damaged DNA[22].
Bcl-2, as a kind of negative regulatory gene of cell apoptosis, can control the membrane potential by changing the redox state of mitochondrial thiols. Furthermore, it can also regulate the permeability of the mitochondrial membrane for some apoptosis protein precursors and locate apoptosis protein precursor Apaf-1 on the mitochondrial membrane to stop apoptosis[23].
Fas and FasL are membrane surface molecules, which regulate the apoptosis induced by toxicity in T-cell development[24]. FasL, with its high-level expression on cell surfaces, identifies Fas on the target cell surface after CTL cells recognize target cells. Then, by triggering the apoptosis process in the interior of target cells using Fas, the programmed cell death of target cells occurs[24,25].
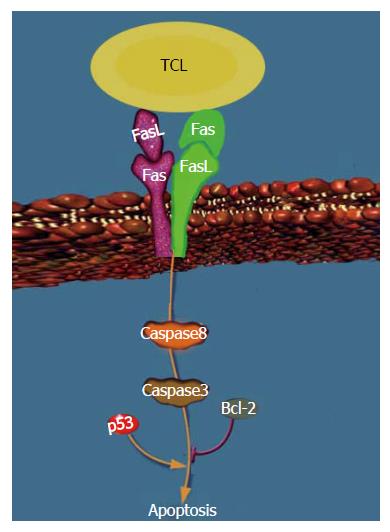
It can be found from this experiment that, after the intervention of ST extracts with different concentrations on hepatocellular carcinoma SMMC-7721 cells, the inhibition and apoptosis rates of the cells increased significantly (P < 0.05). This indicated that ST inhibited hepatocellular carcinoma cells. The mRNA expressions of FasL and Bcl-2 were markedly reduced (P < 0.05), which showed that the negative feedback regulation protected CTL cells to certain extent. In addition, the mRNA expressions of Fas, caspase-8, caspase-3 and p53 increased. Compared with the positive control group, the effects of 5 mg/L ST extracts were equivalent to those of the positive control group, which suggested that these genes were positive regulatory genes for apoptosis of hepatocellular carcinoma cells induced by ST through the mitochondrial pathway. Therefore, ST regulated hepatocellular carcinoma SMMC-7721 cells by the combination of Fas/FasL on the surface of the cells with that on the surface of T cells. The mitochondrial pathway was then stimulated to transmit apoptosis signals, which activated the upstream caspase-8 and further the downstream caspase-3. In this way, a cascade amplification system was formed to induce apoptosis. In addition, the apoptosis process was affected by the synergistic action of tumor suppressor gene p53 and negative feedback gene Bcl-2 for apoptosis, jointly forming the regulation network of ST inducing apoptosis of hepatocellular carcinoma SMMC-7721 cells, as shown in Figure 3.
Although this experiment verified that ST can induce the apoptosis of hepatocellular carcinoma SMMC-7721 cells, the apoptosis mechanism was related to the expression of Fas, FasL, caspase-8, caspase-3, p53 and Bcl-2 in the mitochondrial pathway. This result provides powerful evidence of the improved apoptosis effects of ST on hepatocellular carcinoma cells; however, due to only a few genes being involved in apoptosis, the regulatory effects of other genes were unable to be determined here. Moreover, the apoptosis may be related to other proteins in the caspase family, so the molecular mechanism of the apoptosis induced by ST is not completely explained and needs to be further verified by other studies.
Solanum lyratum Thumb (ST) belonging to Solanaceae is generally used to treat tumors, and it is a commonly used anticancer drug. However, the effects and mechanism of ST on tumor cells are unclear.
Precision treatment of Chinese herbal medicine is the focus of the current research and hot spots of investigations. This study verified the mechanism of Chinese herbal medicine by using molecular biological technology, providing powerful evidence of the improved apoptosis effects of Solanum lyratum Thumb on hepatocellular carcinoma cells.
The experiment verified that ST ethanol extracts up-regulated Fas and down-regulated FasL in the mitochondrial pathway, inducing the up-regulation for the expression of caspase-8 and caspse-3. In addition, ST ethanol extracts induced the apoptosis of hepatocellular carcinoma SMMC-7721 cells through feedback regulation by up-regulating the p53 gene that inhibits cancers and down-regulating the Bcl-2 gene.
These findings provide powerful evidence of the improved apoptosis effects of St on hepatocellular carcinoma cells.
this study is well designed and the results are interesting. The authors try to explore the induction effects and mechanism of ST on human hepatocellular carcinoma SMMC-7721 cells through the mitochondrial pathway.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abdollahi M, Tokunaga Y S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Liu WX
| 1. | Liu SH, Shen XH, Wei XF, Mao XH, Huang T. Immunomodulatory activity of butanol extract from Solanum lyratum in tumor-bearing mice. Immunopharmacol Immunotoxicol. 2011;33:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Guan Y, Zhao H, Yan X, Meng J, Wang W. A study on anti-tumour effect of Solanum lyratum Thunb. extract in S₁₈₀ tumour-bearing mice. Afr J Tradit Complement Altern Med. 2013;10:345-351. [PubMed] |
| 3. | Shanghai: Shanghai Science and Technology Press 2006; Nanjing University of Chinese Traditional Medicine. Dictionary of Chinese Medicine. |
| 4. | Elkholi R, Renault TT, Serasinghe MN, Chipuk JE. Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab. 2014;2:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Loison F, Zhu H, Karatepe K, Kasorn A, Liu P, Ye K, Zhou J, Cao S, Gong H, Jenne DE. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J Clin Invest. 2014;124:4445-4458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Rahman MA, Bishayee K, Huh SO. Angelica polymorpha Maxim Induces Apoptosis of Human SH-SY5Y Neuroblastoma Cells by Regulating an Intrinsic Caspase Pathway. Mol Cells. 2016;39:119-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Neophytou CM, Constantinou C, Papageorgis P, Constantinou AI. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem Pharmacol. 2014;89:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Wei X, Li Zg, Nong S, Huang Xm. The influence of Solanum lyratum Thunb extract on apoptosis and the expression of fas/fasL genes in Hela cells. Zhongyaocai. 2006;29:1203-1206. [PubMed] |
| 9. | Tu S, Wan Fs, Wei X, Nong S, Zhu Wf, Liu Zq. Solamum lyratum Thunbery alkaloid induces human lung adenoearcinoma A549 cells apoptosis by activating FAS-pathway. shizhen Guoyi Guoyao. 2013;24:66-68. |
| 10. | Jia YR, Tian XL, Liu K, Chen C, Wang XL, Zhang CC, Sun LX. Simultaneous determination of four alkaloids in Solanum lyratum Thunb by UPLC-MS/MS method. Pharmazie. 2012;67:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Marí M, Morales A, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. Mitochondrial glutathione: features, regulation and role in disease. Biochim Biophys Acta. 2013;1830:3317-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Marí M, Morales A, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. Mitochondrial glutathione: features, regulation and role in disease. Biochim Biophys Acta. 2013;1830:3317-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Saralamma VV, Nagappan A, Hong GE, Lee HJ, Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH. Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand. Int J Mol Sci. 2015;16:22676-22691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Davidson MT, Deitch EA, Lu Q, Haskó G, Abungu B, Németh ZH, Zaets SB, Gaspers LD, Thomas AP, Xu DZ. Trauma-hemorrhagic shock mesenteric lymph induces endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms. Ann Surg. 2004;240:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | He YC, Zhou FL, Shen Y, Liao DF, Cao D. Apoptotic death of cancer stem cells for cancer therapy. Int J Mol Sci. 2014;15:8335-8351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Castedo M, Ferri K, Roumier T, Métivier D, Zamzami N, Kroemer G. Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods. 2002;265:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Tian H, Zhang DF, Zhang BF, Li HZ, Zhang Q, Li LT, Pei DS, Zheng JN. Melanoma differentiation associated gene-7/interleukin-24 induces caspase-3 denitrosylation to facilitate the activation of cancer cell apoptosis. J Interferon Cytokine Res. 2015;35:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Saito-Hakoda A, Uruno A, Yokoyama A, Shimizu K, Parvin R, Kudo M, Saito-Ito T, Sato I, Kogure N, Suzuki D. Effects of RXR Agonists on Cell Proliferation/Apoptosis and ACTH Secretion/Pomc Expression. PLoS One. 2015;10:e0141960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ram DR, Ilyukha V, Volkova T, Buzdin A, Tai A, Smirnova I, Poltorak A. Balance between short and long isoforms of cFLIP regulates Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA. 2016;113:1606-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Kwon YH, Bishayee K, Rahman A, Hong JS, Lim SS, Huh SO. Morus alba Accumulates Reactive Oxygen Species to Initiate Apoptosis via FOXO-Caspase 3-Dependent Pathway in Neuroblastoma Cells. Mol Cells. 2015;38:630-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Hsiao PC, Lee WJ, Yang SF, Tan P, Chen HY, Lee LM, Chang JL, Lai GM, Chow JM, Chien MH. Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells. Tumour Biol. 2014;35:11903-11911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Lai YJ, Lin CI, Wang CL, Chao JI. Expression of survivin and p53 modulates honokiol-induced apoptosis in colorectal cancer cells. J Cell Biochem. 2014;115:1888-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Hyun HB, Lee WS, Go SI, Nagappan A, Park C, Han MH, Hong SH, Kim G, Kim GY, Cheong J. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol. 2015;46:2670-2678. [PubMed] [DOI] [Full Text] |
| 24. | Chen YF, Yang JS, Chang WS, Tsai SC, Peng SF, Zhou YR. Houttuynia cordata Thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J Biomed Sci. 2013;20:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Choi HS, Seo HS, Kim JH, Um JY, Shin YC, Ko SG. Ethanol extract of paeonia suffruticosa Andrews (PSE) induced AGS human gastric cancer cell apoptosis via fas-dependent apoptosis and MDM2-p53 pathways. J Biomed Sci. 2012;19:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |