Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.926
Peer-review started: August 24, 2016
First decision: September 28, 2016
Revised: October 6, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: February 7, 2017
Processing time: 151 Days and 18.3 Hours
Guillain-Barre syndrome (GBS)-associated achalasia is a very rare disease of uncertain cause. We report the case of a patient diagnosed with GBS-associated type I achalasia who was successfully treated with peroral endoscopic myotomy (POEM). A 30-year-old man who was diagnosed with GBS 3 mo before was referred to our department with dysphagia and meal-related regurgitation. The results of esophagography, endoscopy, and high-resolution manometry (HRM) revealed type I achalasia. POEM that utilized a submucosal tunneling technique was performed to treat the GBS-associated type I achalasia. After POEM, smooth passage of a contrast agent into the stomach was shown in follow-up esophagography, and follow-up HRM revealed a decrease in the mean integrated relaxation pressure 22.9 mmHg to 9.6 mmHg. The patient remained without dysphagia for 7 mo, even though the patient’s neurological problems were not fully resolved. POEM may be a safe and effective treatment for GBS-associated type I achalasia.
Core tip: Guillain-Barre’ syndrome (GBS)-associated achalasia is a rare disease of uncertain cause. Previously, a case of GBS-associated achalasia that was treated with pneumatic dilation was reported. However, pneumatic dilatation has about a 25%-50% chance that the patient will require another procedure within five years. Here, we present a case of a patient diagnosed with GBS-associated type I achalasia who was successfully treated with peroral endoscopic myotomy.
- Citation: Shin SK, Kim KO, Kim EJ, Kim SY, Kim JH, Kim YJ, Chung JW, Kwon KA, Park DK. Peroral endoscopic myotomy for treatment of Guillain-Barre syndrome-associated achalasia: A rare case. World J Gastroenterol 2017; 23(5): 926-930
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.926
Guillain-Barré syndrome (GBS) is an immune-mediated polyradiculoneuropathy characterized by rapidly progressive, symmetrical, ascending weakness and sensory loss. It is usually triggered by an infection, such as upper respiratory tract infection or gastroenteritis[1]. GBS-associated achalasia is a very rare condition of uncertain cause. Previously, a case of GBS-associated achalasia that was treated with pneumatic dilation was reported[2]. However, pneumatic dilatation has about a 25%-50% chance that the patient will require another procedure within five years[3,4]. Recently, peroral endoscopic myotomy (POEM) has emerged as a safe and effective technique that can adequately treat achalasia. Here, we present a case in which a patient diagnosed with GBS-associated type I achalasia was successfully treated with POEM.
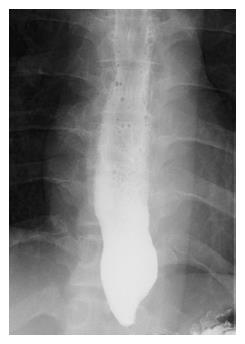
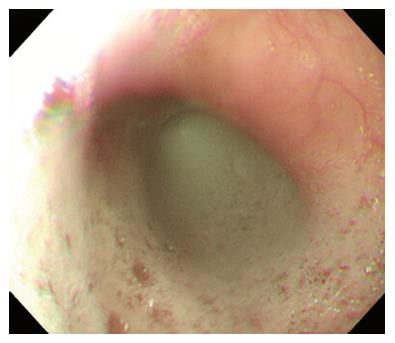
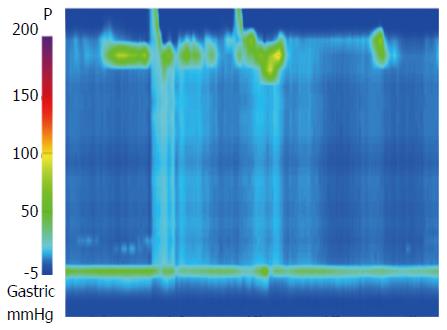
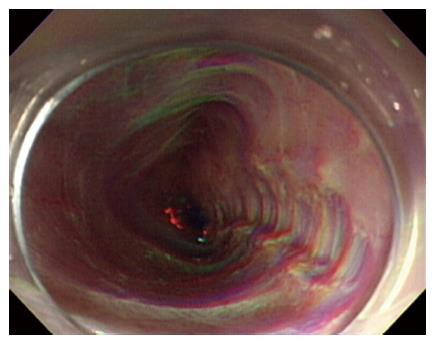
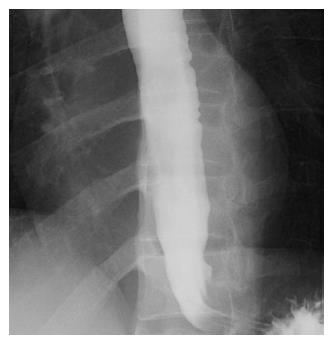
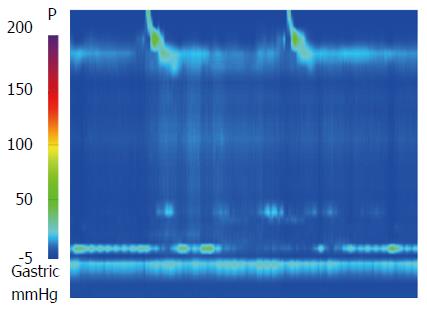
A 30-year-old man was referred to our department with dysphagia and meal-related regurgitation, with a baseline Eckardt symptom score of 11. Three months before, he had suffered from progressive quadriparesis, dysarthria, and facial palsy following gastrointestinal illness that he had about 10 d prior. With the characteristic protein-cell count differentiation of albuminocytologic dissociation detected via cerebrospinal fluid analysis (protein 73.4 mg/dL, WBC 8 /mm3) and high titer of the serum IgM antibody to GD1b, in addition to nerve conduction studies, a diagnosis of GBS was made. Despite receiving five days of intravenous immunoglobulin therapy immediately after the diagnosis of GBS, the patient’s neurological problems had not recovered, and swallowing difficulty was worsening. His family history was unremarkable. Abdominal examinations showed normal bowel sounds, no palpable masses, and no organomegaly. Laboratory tests revealed normocytic normochromic anemia, with a hemoglobin level of 11.1 g/dL. The results of liver and renal function tests were within normal limits. A videofluoroscopic swallowing study (VFSS) on day 30 showed dysfunctional oropharyngeal transfer of the barium paste, subglottic aspiration of the liquid barium, and decreased esophageal peristaltic movement. Oromotor facilitation and electrical stimulation therapy to the pharyngeal muscles were performed for the swallowing difficulty. A follow-up VFSS on day 71 showed slight improvement of the oropharyngeal transfer of barium paste. However, decreased esophageal peristaltic movement was still present, and the dysphagia was aggravated. On day 93, subtle dilated distal esophageal lumen with acute tapering at the lower esophageal sphincter (LES) and narrowing at the esophagogastric junction was shown through esophagography (Figure 1), and dilation of the esophageal lumen and retention of food remnants in the esophagus were identified during endoscopy (Figure 2). A mean integrated relaxation pressure (IRP) of 22.9 mmHg over test swallows with absent peristalsis was shown in high-resolution manometry (HRM) (Figure 3). POEM was performed in the operating room under general anesthesia to treat GBS-associated type I achalasia (Figure 4). To summarize, it consisted of four steps: longitudinal mucosal incision, creation of a submucosal tunnel extending towards the gastroesophageal junction, selective myotomy of the circular muscle fibers, and mucosal closure by clip. After the treatment, smooth passage of a contrast agent into the stomach was shown in follow-up esophagography (Figure 5), and follow-up HRM showed that the mean IRP decreased to 9.6 mmHg (Figure 6). The endoscope could be passed without resistance at 2 mo after the procedure. The patient’s Eckardt symptom score decreased to 2. Although the patient’s neurological problems were not fully recovered at 10 mo after diagnosis of GBS, he could eat a soft diet without problems for 7 mo after POEM.
GBS is an acute, immune-mediated polyneuropathy that often leads to severe weakness[5]. Required criteria for diagnosis include progressive weakness of more than two limbs, areflexia, and progression for no more than four weeks. Other causes of acute neuropathy, such as lead poisoning, vasculitis, botulism, and porphyria, require exclusion. Supportive criteria include relatively mild sensory signs, raised protein levels in the cerebrospinal fluid while maintaining a relatively normal cell count, and neurophysiological evidence of conduction block. Weakness is frequently proximal and distal, unlike dying-back axonopathies, and respiratory involvement occurs in about a quarter of cases[6]. Cranial nerve involvement can be observed in 45%-75% of patients with GBS[7]. In cases of cranial nerve involvement, facial and oropharyngeal weakness can appear, and dysphagia can develop[8].
A definite mechanism of GBS has not been established, but it has been found that the antibodies generated by the activation of immune responses after infections makes a cross-bridge via molecular mimicry with antigens in the peripheral nerves, which destroys them[9,10]. This hypothesis is supported by the fact that anti-anglioside antibodies have been found in GBS patients’ serum. In our case, high titer of the serum IgM antibody to GD1b was found.
On the other hand, GBS-associated achalasia is a very rare disease of uncertain cause. Achalasia is an esophageal motor disorder characterized by the absence of peristalsis and a defective relaxation of the LES which results in impaired bolus transport and food stasis in the esophagus[11,12]. In 1994, Firouzi et al[2] presented a case of GBS with achalasia. An autoimmune response triggered by an infection such as gastroenteritis or respiratory tract infection is discussed as one possible cause of the loss of inhibitory ganglion cells within the myenteric plexus in patients with GBS-associated achalasia[13]. Previously, it was reported that GD1b antibodies preferentially stained the large dorsal root ganglion neurones in animal models[14], and degenerative changes in the ganglion cells of the dorsal motor nucleus of the vagus have been reported in some patients with achalasia[15]. In our case, changes in the ganglion cells of the dorsal motor nucleus of the vagus by GD1b antibodies may have induced GBS-associated achalasia.
Although intravenous immunoglobulin and plasma exchange have proved effective, many patients with GBS still develop severe weakness and have a long disease course, often with incomplete recovery, pain, and fatigue[16]. In our case, despite receiving five days of intravenous immunoglobulin therapy immediately, the patient’s neurological problems had not recovered and swallowing difficulty was worsening.
Treatment of achalasia is currently aimed at decreasing the resting pressure in the LES. In a previous report, GBS-associated achalasia was subsequently diagnosed by manometry and was successfully treated with pneumatic dilation[2]. However, the pneumatic dilatation has about a 25%-50% chance that the patient will require another procedure within five years[4]. An alternative to dilation in patients with achalasia is endoscopic injection of the botulinum toxin. The symptomatic response to this treatment is often short lived, with a greater than 50% recurrence rate within 6 mo[17].
Recently, POEM has been developed as an alternative endoscopic treatment that is effective and less invasive[18]. A major benefit of POEM may be the ability to avoid the risk of unexpected and uncontrolled perforation that may occur during balloon dilation. The clinical effects of the POEM treatment were immediate, and the later HRM showed improvement in the LES pressure and pressurization disappeared[19].
In the present case, we performed POEM for treatment of GBS-associated type I achalasia. After POEM without any complications, mean IRP decreased from 22.9 mmHg to 9.6 mmHg and Eckardt symptom score was reduced from 11 to 2 points. Our initial experience suggests that POEM is a safe and effective treatment for GBS-associated type I achalasia.
A 30-year-old man was referred to our department with dysphagia and meal-related regurgitation, with a baseline Eckardt symptom score of 11.
After the diagnosis of Guillain-Barré syndrome (GBS), a mean integrated relaxation pressure of 22.9 mmHg over test swallows with absent peristalsis was shown in high-resolution manometry, which refers to GBS associated type I achalasia.
Cranial nerve involvement in GBS.
Laboratory tests revealed normocytic normochromic anemia, with a hemoglobin level of 11.1 g/dL, and other laboratory findings were within normal limits.
Subtle dilated distal esophageal lumen with acute tapering at the lower esophageal sphincter and narrowing at the esophagogastric junction was shown through esophagography.
Pathologic confirmation was not performed in our case.
Peroral endoscopic myotomy (POEM) was performed to treat GBS-associated type I achalasia.
Previously, a case of in GBS-associated achalasia that was treated with pneumatic dilation was reported.
GBS is an immune-mediated polyradiculoneuropathy characterized by rapidly progressive, symmetrical, ascending weakness and sensory loss.
The authors’ initial experience suggests that POEM is a safe and effective treatment for GBS -associated type I achalasia.
It is an interesting and well-written case report.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pavlidis TE S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31:491-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Firouzi M, Keshavarzian A. Guillain-Barre syndrome and achalasia: two manifestations of a viral disease or coincidental association? Am J Gastroenterol. 1994;89:1585-1587. [PubMed] |
| 3. | Vanuytsel T, Lerut T, Coosemans W, Vanbeckevoort D, Blondeau K, Boeckxstaens G, Tack J. Conservative management of esophageal perforations during pneumatic dilation for idiopathic esophageal achalasia. Clin Gastroenterol Hepatol. 2012;10:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 459] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 5. | van Doorn PA, Kuitwaard K, Walgaard C, van Koningsveld R, Ruts L, Jacobs BC. IVIG treatment and prognosis in Guillain-Barré syndrome. J Clin Immunol. 2010;30 Suppl 1:S74-S78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Winer JB. Guillain Barré syndrome. Mol Pathol. 2001;54:381-385. [PubMed] |
| 7. | Nanda SK, Jayalakshmi S, Ruikar D, Surath M. Twelfth cranial nerve involvement in Guillian Barre syndrome. J Neurosci Rural Pract. 2013;4:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Yu JY, Jung HY, Kim CH, Kim HS, Kim MO. Multiple cranial neuropathies without limb involvements: guillain-barre syndrome variant? Ann Rehabil Med. 2013;37:740-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barré syndrome. J Neuroimmunol. 1999;100:74-97. [PubMed] |
| 10. | Mogale KD, Antony JH, Ryan MM. The pharyngeal-cervical-brachial form of Guillain-Barré syndrome in childhood. Pediatr Neurol. 2005;33:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Boeckxstaens GE. The lower oesophageal sphincter. Neurogastroenterol Motil. 2005;17 Suppl 1:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Torresan F, Ioannou A, Azzaroli F, Bazzoli F. Treatment of achalasia in the era of high-resolution manometry. Ann Gastroenterol. 2015;28:301-308. [PubMed] |
| 13. | Müller M, Eckardt V, Schrank B, Graap H. [Achalasia and Guillain-Barré syndrome]. Z Gastroenterol. 2009;47:1149-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Gong Y, Tagawa Y, Lunn MP, Laroy W, Heffer-Lauc M, Li CY, Griffin JW, Schnaar RL, Sheikh KA. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain. 2002;125:2491-2506. [PubMed] |
| 15. | Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87:848-856. [PubMed] |
| 16. | Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 17. | Pasha SF, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest Endosc. 2014;79:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1235] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 19. | Talukdar R, Inoue H, Nageshwar Reddy D. Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis. Surg Endosc. 2015;29:3030-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |