Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.876
Peer-review started: October 14, 2016
First decision: December 1, 2016
Revised: December 14, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: February 7, 2017
Processing time: 102 Days and 0.2 Hours
To explore factors associated with persistent hepatitis B virus (HBV) infection in a cohort of hepatocellular carcinoma (HCC)-affected families and then investigate factors that correlate with individual viral load among hepatitis B surface antigen (HBsAg)-positive relatives.
We evaluated non-genetic factors associated with HBV replication in relatives of patients with HCC. Relatives of 355 HCC cases were interviewed using a structured questionnaire. Demographics, relationship to index case, HBsAg status of mothers and index cases were evaluated for association with the HBV persistent infection or viral load by generalized estimating equation analysis.
Among 729 relatives enrolled, parent generation (P = 0.0076), index generation (P = 0.0044), mothers positive for HBsAg (P = 0.0007), and HBsAg-positive index cases (P = 5.98 × 10-8) were associated with persistent HBV infection. Factors associated with HBV viral load were evaluated among 303 HBsAg-positive relatives. Parent generation (P = 0.0359) and sex (P = 0.0007) were independent factors associated with HBV viral load. The intra-family HBV viral load was evaluated in families clustered with HBsAg-positive siblings. An intra-family trend of similar HBV viral load was found for 27 of 46 (58.7%) families. Male offspring of HBsAg-positive mothers (P = 0.024) and older siblings were associated with high viral load.
Sex and generation play important roles on HBV viral load. Maternal birth age and nutritional changes could be the reasons of viral load difference between generations.
Core tip: Familial clustering of chronic hepatitis B infection is identified in this study. Most of the hepatitis B surface antigen (HBsAg) carriers in this cohort are in families of an HBsAg-positive index case. A high prevalence of HBsAg is found in the siblings’ generation and in offspring of an HBsAg-positive mother. The HBsAg status of index cases and HBsAg status of the mother are important factors for determining the persistence of hepatitis B virus (HBV) infection in hepatocellular carcinoma families. Sex and generation are factors associated with HBV replication. Perinatal infection has a great influence on male offspring’s HBV replication.
- Citation: Hsieh AR, Fann CS, Yeh CT, Lin HC, Wan SY, Chen YC, Hsu CL, Tai J, Lin SM, Tai DI. Effects of sex and generation on hepatitis B viral load in families with hepatocellular carcinoma. World J Gastroenterol 2017; 23(5): 876-884
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/876.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.876
In the families of hepatitis B virus (HBV)-infected individuals, clustering of chronic hepatitis B surface antigen (HBsAg) carriers and hepatocellular carcinoma (HCC) are common[1-6]. HBV is highly infectious[7,8], and a substantial number of individuals who are exposed to HBV early in life become chronic HBsAg carriers[4,9-11]. Furthermore, intra-familial transmission of HBV could underlie the high incidence of HCC among family members[3,4].
In addition to sex-related behavioral factors[12,13], genome-wide association studies in Japan indicated that the human leukocyte antigen subunits DP and DQ are associated with HBsAg persistence[14,15]. However, the genes identified as being responsible for clinical progression among chronic HBsAg carriers differ among several genome-wide association studies carried out in China and Taiwan[16-20]. Hence, it is possible that non-genetic factors may play a non-negligible role in determining HBV replication. For example, an increased risk of liver cancer among first-degree relatives of HCC patients was shown to be associated with a prolonged HBV replication phase[1,2]. Therefore, before evaluating genetic factors associated with HBV replication, non-genetic factors that may be associated with HBV viral load should be clarified[2-4,6,9-11,21].
Given the familial clustering of chronic HBsAg carriers in HCC families[2,5,6,9,21] with maternal status, those relatives having a similar genetic background may be instrumental in helping clinicians determine any non-genetic factors that may be associated with persistent HBV infection and viral replication. In this respect, we explored factors associated with persistent HBV infection in a cohort of HCC-affected families and then investigated factors that correlated with individual viral load among HBsAg-positive relatives.
Patients with HCC who were diagnosed at Chang Gung Memorial Hospital, Lin-Kou Medical Center were included as index cases. From 2003 to 2007, relatives of these patients were prospectively invited to complete a survey concerning liver diseases. Spouses of index cases or spouses of their relatives were excluded.
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan (IRB: 91-124), and written informed consent was obtained from all participants before the study. All experiments and data comparisons were carried out in compliance with relevant laws and guidelines and in accordance with the ethical standards of the Declaration of Helsinki.
At entry, basic information that included national citizen identification number, sex, race, alcohol and smoking habits, profession, location of residency at birth, level of education, and family history were obtained through questionnaires and structured interviews.
Each relative that was enrolled in the study underwent liver biochemistry tests for α-fetoprotein and viral markers, as well as a liver ultrasound. Serum HBsAg and hepatitis C virus antibody (anti-HCV) were measured by enzyme-linked immunosorbent assay (Abbott Diagnostics, Chicago, IL, United States). Maternal HBsAg was assayed at enrollment or obtained by reviewing our hospital records.
A quantitative HBV DNA assay was carried out initially with the Digene Hybridization System (Digene Diagnostics, Inc., Beltsville, MD, United States; lower limit of detection, 1.4 × 105 cps/mL). Those with HBV DNA lower than the detectable limit were further assayed using the COBAS Amplicor HBV Monitor Test (Roche Diagnostics, Branchburg, NJ, United States; lower limit of detection, 200 cps/mL). Our previous long-term follow-up study revealed that nearly 40% of HBsAg carriers with persistent normal alanine aminotransferase levels have a level of HBV DNA of > 1.0 × 104 cps/mL[22]. Therefore, relatives with HBV DNA levels of ≥ 1.0 × 105 cps/mL were considered as having high HBV replication, and those with levels < 1.0 × 105 cps/mL were considered as having low HBV replication.
HBV genotype was initially determined with the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method[23], but we later changed to a more sensitive SMITEST HBV Genotyping kit (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan) for all subjects. For those subjects with low HBV DNA level, the S region of the genome was amplified by nested PCR followed by direct sequencing (CEQ 8000 Genetic Analysis System; Beckman Coulter, Brea, CA, United States).
Thomas et al[24] reported that body height at adulthood may predict the nutritional status of a population in a particular birth year. Hence, we estimated the nutritional status of Taiwan based on body height data according to birth year for subjects who received a general checkup between year 2000 and 2004 at Chang Gung Memorial Hospital[9] and in the cohort of HCC families.
The analysis of cohort data was divided into two stages. In the first stage, we searched for factors associated with chronic HBsAg carriers. In the second stage, we examined factors associated with HBV viral load in HBsAg-positive relatives only.
The relatives included in the study were individuals from the same household. Because both individual and familial responses from the same household should be evaluated, we used the generalized estimating equation (GEE) method to determine correlations between the data and each binary response (e.g., for HBsAg status or HBV DNA level) using the exchangeable working correlation structure[25,26] in our first and second stages of the analyses. Univariate and multivariate analyses in the two stages were assessed using the GEE method with the PROC GENMOD procedure in SAS 9.3 (SAS Institute Inc., Cary, NC, United States).
The role of sex hormones in the development and progression of HBV-associated HCC has been reported[12,13]. Therefore, we added a new familial view on HBV replication status in this cohort. We examined intra-familial HBV replication among HBsAg-positive siblings of the same sex in each family. A sex difference with respect to HBV viral load in families clustered with HBsAg-positive siblings. We used logistic regression to explore the sex effect for families in which the mother was positive for HBsAg as well as in all families.
A total of 355 families participated in this study. Of the 330 index cases with data on HBV, 203 (61.5%) were seropositive for HBsAg, 29 (8.8%) were seropositive for both HBsAg and anti-HCV, 75 (22.7%) were seropositive for anti-HCV, and 23 (7.0%) were seronegative for both HBsAg and anti-HCV. The diagnosis of HCC was based on cytology or histology for 180 (50.7%) patients. The others were diagnosed clinically based on a serum α-fetoprotein level and/or imaging studies[27].
There were 806 relatives and 205 spouses in the study. Twenty-five relatives were diagnosed with liver cirrhosis by ultrasound at screening. None of the study relatives had HCC detected on initial screening. Three siblings and three children of the indexed HCC patients developed HCC during the subsequent follow-up study.
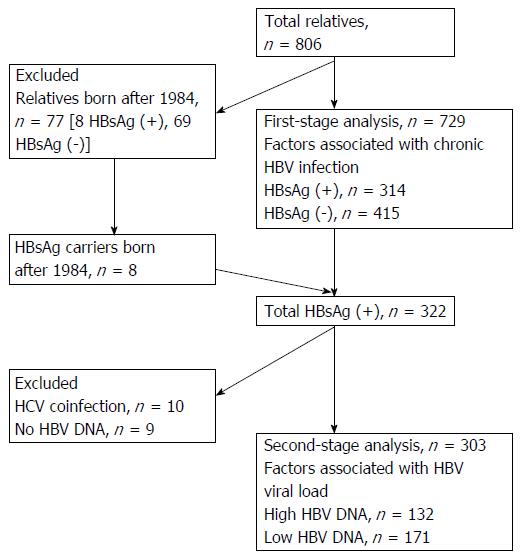
Of the 806 relatives who participated in this study, 77 were born after 1984 when the nationwide vaccination program against HBV started in Taiwan; these 77 subjects were excluded from the first-stage analysis (Figure 1). The dataset used for the first-stage analysis thus contained 729 individuals.
The risk factor of chronically expressing HBsAg was examined in the first stage. The following factors were evaluated: sex, index case sex, age, relation to the index case, HBsAg status of the mother (maternal HBsAg), and HBsAg status of the index case (index HBsAg). Index HBsAg, maternal HBsAg, and index generation were significantly associated with persistent HBV infection (P < 0.0001; Table 1). After controlling for sex, these associations remained statistically significant (P < 0.0001; Table 1).
| Category | HBsAg | OR (95%CI) | Adjusted OR (95%CI)1 | |
| Positive | Negative | |||
| Total family members | 314 | 415 | ||
| Sex | ||||
| Male | 171 (54.46) | 196 (47.23) | 1.25 (0.97-1.61) | |
| Female | 143 (45.54) | 219 (52.77) | ||
| Index sex | ||||
| Male | 229 (72.93) | 302 (72.77) | 1.07 (0.70-1.62) | 1.25 (0.97-1.60) |
| Female | 85 (27.07) | 113 (27.23) | ||
| Age, mean ± SD | 40.49 ± 10.89 | 37.87 ± 11.69 | 1.01 (1.00-1.03) | 1.28 (1.00-1.64) |
| Relation to index | ||||
| Parent | 10 (3.18) | 20 (4.82) | 0.78 (0.37-1.64) | 0.81 (0.38-1.71) |
| Index generation | 86 (27.39) | 36 (8.67) | 3.89 (2.32-6.51)a | 3.97 (2.38-6.63)a |
| Child | 206 (65.61) | 347 (83.61) | ||
| Grandchild | 12 (3.82) | 12 (2.89) | 1.43 (0.66-3.13) | 1.39 (0.65-3.00) |
| Maternal HBsAg | ||||
| Negative | 86 (27.38) | 244 (58.80) | ||
| Positive | 129 (41.08) | 53 (12.77) | 5.03 (3.16-8.01)a | 5.00 (3.13-7.97)a |
| Unknown | 99 (31.53) | 118 (28.43) | 2.01 (1.30-3.38)a | 2.04 (1.33-3.13)a |
| Index HBsAg2 | ||||
| Negative | 48 (15.43) | 203 (49.03) | ||
| Positive | 263 (84.57) | 211 (50.97) | 5.57 (3.56-8.71)a | 5.51 (3.53-8.61)a |
In the multivariate GEE analysis, persistent HBV infection was lower for parents of index cases (OR = 0.24, P = 0.0076; Table 2). The risk was higher for subjects in the index generation (OR = 2.25, P = 0.0044; Table 2), those who had an HBsAg-positive mother (OR = 2.65, P = 0.0007; Table 2), those related to an HBsAg-positive index case (OR = 4.19, P = 5.98 × 10-8), and those of older age (OR = 1.03, P = 0.0037; Table 2).
| Factor | Item | OR (95%CI) | P value |
| Sex | Male | 1.26 (0.94-1.70) | |
| Index sex | Male | 1.28 (0.78-2.10) | |
| Age | 1.03 (1.01-1.05) | 0.0037 | |
| Relation to index | Parent | 0.24 (0.09-0.69) | 0.0076 |
| Index generation | 2.25 (1.29-3.94) | 0.0044 | |
| Grandchild | 2.06 (0.78-5.45) | ||
| Maternal HBsAg | Positive | 2.65 (1.51-4.67) | 0.0007 |
| Unknown | 1.21 (0.72-2.03) | ||
| Index HBsAg | Positive | 4.19 (2.50-7.04) | 5.98 × 10-8 |
Among the 314 HBsAg-positive relatives born before 1984 and 8 relatives born after 1984, for this second-stage analysis we excluded 10 relatives with dual HBV and HCV infections and 9 relatives who did not have an HBV DNA assay (Figure 1). A total of 303 individuals were thus included in the HBV viral load association analysis.
The associations between HBV DNA level and sex, index sex, age, relation to index case, maternal HBsAg, index HBsAg, and HBV genotype were examined. A positive association was found between high HBV DNA level and male sex (OR = 2.12, P = 0.0013; Table 3). A significant association with HBV viral load was noted between parents of index cases and child plus grandchild generations (OR = 4.77, P = 0.0348; Table 3). Index HBsAg status was significantly associated with HBV DNA level (OR = 2.32, P = 0.0221; Table 3). A significant association with HBV viral load was also noted between HBV genotype C and HBV genotype B (OR = 1.71, P = 0.008; Table 3); after controlling for sex, however, the association was of marginal statistical significance (P = 0.064; Table 3).
| Factor | HBV DNA | OR (95%CI) | P value | Adjusted OR (95% CI)1 | P value | |
| ≥ 100000 cps/mL | < 100000 cps/mL | |||||
| Total family members | 132 | 171 | ||||
| Sex | ||||||
| Male | 84 (63.64) | 79 (46.20) | 2.12 (1.34-3.39) | 0.0013 | ||
| Female | 48 (36.36) | 92 (53.80) | ||||
| Index sex | ||||||
| Male | 99 (75) | 121 (70.76) | 1.83 (0.69-2.04) | 1.17 (0.68-2.01) | ||
| Female | 33 (25) | 50 (29.24) | ||||
| Age, mean ± SD | 40.51 ± 12.18 | 39.15 ± 10.55 | 1.01 (0.99-1.03) | 1.02 (0.99-1.04) | ||
| Relation to index | ||||||
| Child and grandchild | 83 (62.88) | 128 (74.85) | ||||
| Parent | 7 (5.30) | 2 (1.17) | 4.77 (1.12-20.31) | 0.0348 | 4.57 (1.15-18.14) | 0.0307 |
| Index generation | 42 (31.82) | 41 (23.98) | 1.51 (0.87-2.62) | 0.64 (0.36-1.14) | ||
| Maternal HBsAg | ||||||
| Negative | 33 (25) | 51 (29.82) | ||||
| Positive | 61 (46.21) | 64 (37.43) | 1.55 (0.84-2.87) | 1.57 (0.84-2.92) | ||
| Unknown | 38 (28.79) | 56 (32.75) | 1.08 (0.57-2.06) | 1.20 (0.62-2.33) | ||
| Index HBsAg | ||||||
| Negative | 12 (9.16) | 32 (18.93) | ||||
| Positive | 119 (90.84) | 137 (81.07) | 2.32 (1.13-4.76) | 0.0221 | 2.47 (1.19-5.15) | 0.0158 |
| HBV genotype2 | 0.0017 | |||||
| N3 | 2 (1.53) | 21 (12.88) | 0.11 (0.03-0.44) | 0.09 (0.02-0.39) | 0.0011 | |
| B | 97 (74.62) | 120 (73.62) | ||||
| C | 31 (23.85) | 22 (13.50) | 1.71(0.94-3.14) | 0.008 | 1.80 (0.97-3.36) | 0.0640 |
In the multivariate GEE analysis, HBV viral load was independently associated with sex (OR = 2.65, P = 0.0007; Table 4) and being the parent of an index case (OR = 6.49, P = 0.0359; Table 4).
| Factor | Item | OR (95%CI) | P value |
| Sex | Male | 2.65 (1.51-4.64) | 0.0007 |
| Index sex | Male | 1.47 (0.73-2.95) | |
| Age | 1.01 (0.98-1.03) | ||
| Relation to index | Parent | 6.49 (1.13-37.27) | 0.0359 |
| Index generation | 1.19 (0.60-2.37) | ||
| Maternal HBsAg | Positive | 1.50 (0.71-3.17) | |
| Unknown | 1.02 (0.49-2.15) | ||
| Index HBsAg | Positive | 1.51 (0.68-3.38) | |
| HBV genotype | N | 0.12 (0.03-0.56) | 0.0066 |
| C | 1.22 (0.59-2.51) |
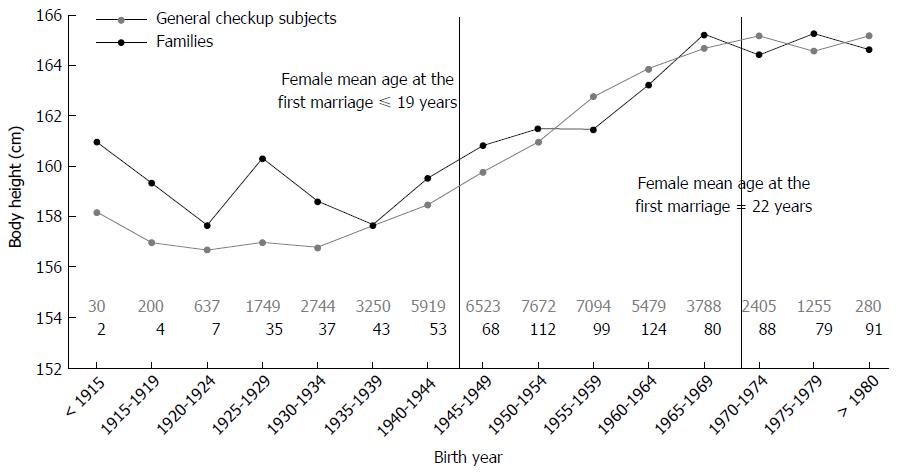
Figure 2 presents data for body height change according to birth year in general checkup subjects and HCC families. The body height of the general checkup subjects and of HCC families increased similarly according to birth year.
Forty-six families were found to have at least two HBsAg-positive siblings of the same sex. Among them, 28 were male sibling families and 18 were female sibling families (Table 5). All siblings had a high HBV viral load in 13 (28.26%) families, and all siblings had a low HBV viral load in 14 (30.43%) families. These two groups (58.69%) revealed a familial trend of HBV replication status; among those siblings, male sibling families generally had a high HBV viral load, whereas female sibling families had a low HBV viral load (OR = 29.96, P = 0.007; Table 5). Maternal HBsAg positivity had a large influence on male offspring in that most of male offspring were in the high HBV viral load group; on the other hand, female offspring were generally in the low HBV viral load group (OR = 21, P = 0.024; Table 5).
| HBV DNA level1 | Maternal HBsAg | Total | ||
| Positive | Unknown | Negative | ||
| Total male siblings | 12 | 9 | 7 | 28 |
| All high level | 7 (58.33)2 | 2 (22.22) | 2 (28.57) | 11 (39.3)3 |
| All low level | 1 (8.33)2 | 2 (22.22) | 1 (14.29) | 4 (14.3)3 |
| Older > younger | 3 (25.00) | 3 (33.33) | 3 (42.86) | 9 (32.1) |
| Younger > older | 1 (8.33) | 1 (11.11) | 1 (14.29) | 3 (10.7) |
| Other | 0 (0.00) | 1 (11.11) | 0 (0.00) | 1 (3.6) |
| Total female siblings | 11 | 3 | 4 | 18 |
| All high level | 2 (18.18)2 | 0 (0.00) | 0 (0.00) | 2 (11.1)3 |
| All low level | 6 (54.55)2 | 1 (33.33) | 3 (75.00) | 10 (55.6)3 |
| Older > younger | 1 (9.09) | 1 (33.33) | 0 (0.00) | 2 (11.1) |
| Younger > older | 1 (9.09) | 0 (0.00) | 1 (25.00) | 2 (11.1) |
| Other | 1 (9.09) | 1 (33.33) | 0 (0.00) | 2 (11.1) |
For 11 families (23.91%), older siblings had a higher level of HBV DNA than their younger siblings; this trend was opposite for only 5 families (10.87%). Older siblings tended to have a higher HBV DNA level than their younger siblings, but the difference was not statistically significant owing to the small number of cases. Because all siblings were generally infected at an early stage of life[4,9-11], this phenomenon contradicts the general trend that HBV replication declines with increasing age[28,29].
This study reveals a familial clustering of chronic HBV infection. As shown in Table 1, most of the chronically HBV-infected carriers (84.57%) in this cohort were families of an HBsAg-positive index case. A high prevalence of HBsAg was apparent for the siblings’ generation (86/122 or 70.49%, P < 0.0001) and for offspring of an HBsAg-positive mother (129/182 or 70.88%, P < 0.0001). These findings remained significant in the multivariate analysis. Notably, the majority of index cases were male (72.93%), indicating that both vertical and horizontal infections were present in HCC families.
HBV replication phase or viral load plays roles in determining the prognosis of chronic persistent HBV infection[2,30]. In our study, we found that sex and generation played independent roles in determining HBV DNA level (Tables 3 and 4). HBV viral load was higher for subjects with HBV genotype C than genotype B in the univariate analysis (P = 0.008; Table 3), but this difference was not statistically significant in the multivariate analysis (Table 4).
Sex is a well-known factor associated with chronic HBV infection[9]. We therefore added a new family view on HBV replication status in this cohort, and we identified a sex difference with respect to HBV viral load in families that had HBsAg-positive siblings (Table 5). HBV viral load was generally higher in male than female siblings (OR = 29.96, P = 0.007). In addition, male siblings in families of an HBsAg-positive mother tended to be in the high HBV DNA group, whereas female siblings were generally in the low HBV DNA group (OR = 21, P = 0.024). Male offspring are more vulnerable to the influence of maternal HBsAg status, whereas female offspring may overcome the maternal influence of persistent HBV replication.
Relatively high HBV replication in older generations has not been well documented in the literature. A study of pregnant women between 1990 and 1995 revealed a progressively decreasing prevalence of hepatitis B e antigen (HBeAg) among chronically HBV-infected carriers[31]. This finding was confirmed in a longer study spanning 1985 to 2000[32], in which the prevalence of HBsAg remained nearly the same, but the prevalence of HBeAg declined progressively from 40% in 1986 to 18% in 2000. This difference between HBsAg and HBeAg prevalence remained apparent even when the ages of the pregnant women were considered[32].
In our previous study of HCC families, we found that older siblings frequently cleared HBeAg later than did their younger siblings[21], and an HBV phylogenetic study yielded similar findings[33]. Among 13 families with an HBsAg-positive mother, the 11 oldest siblings were HBeAg positive whereas only 3 of the youngest siblings were HBeAg positive. These observations provided a clue that maternal age at birth might influence HBV replication in offspring.
The mean age of women entering their first marriage in Taiwan was 18 years before 1917 and remained at about 19 years between 1918 and 1945 (Figure 2)[34]. In the 1970s, however, this mean age had risen to 22 years (http://nccur.lib.nccu.edu.tw/handle/140.119/34632) and increased rather rapidly to 29.2 years by 2010 (http://www.moi.gov.tw/stat/news_content.aspx?sn=5261). Thus, mothers in younger generations of this period between 1918 and 2010 may be 3-5 years older than mothers of the older generations.
A 2014 review article by Bertoletti et al[35] presented an interesting viewpoint that immune responses change during the life of an individual, based on the observed higher mortality of influenza infection at age 30 than at age 20. This implies that a more vigorous immune response produces a more fulminant disease by age 30, whereas a weaker immune response produces a self-limited infection at age 20. A similar situation can be found for chronic HBV infection in that such patients usually enter the immune clearance phase by age 30. We suspect that generational differences might be associated with differences in maternal immunity at the time of an offspring’s birth[36]. Further study will be needed.
Better nutrition is another potential reason for reduced HBV replication in younger generations, and long-term follow-up studies revealed that hepatic steatosis is a good prognostic indicator for chronic HBsAg carriers[28,29]. Hepatic steatosis correlated with a lower risk of HCC, lower mortality rate, and higher chance of spontaneous HBsAg clearance. A recent PNPLA3 polymorphism study on non-alcoholic fatty liver disease found that those SNP genotypes favoring hepatic steatosis development were associated with lower HBV DNA level[37].
During the time frame of our study, we did not have data on the nutritional habits of individuals, but for most participants we obtained body height data, which may reflect long-term nutritional status during the major growth period of humans[24,38]. In our cohort, the mean body height remained < 159 cm for individuals born before 1945. From about 1955 to 1965, however, mean body height increase rapidly to > 164 cm (Figure 2). These findings indicate a significant change in socioeconomic status of the Taiwanese population after the Second World War. Hence, increased food consumption and decreased physical activity may have contributed to the observed increase in the prevalence of hepatic steatosis[39]. Therefore, lifestyle and nutritional habits are factors that may have contributed to our observed shortened HBV replication phase in the younger generation.
We conclude that the generation of the family member, index HBsAg, and maternal HBsAg are important factors for predicting HBV persistence in HCC families. Sex and generation are factors associated with HBV replication. Perinatal infection substantially influences the duration of HBV replication in male offspring.
We are grateful to the National Science Council and the Institute of Biomedical Sciences and Academia Sinica of Taiwan for their support.
Hepatitis B virus (HBV) replication is critical for disease progression. Multiple inconsistent genetic factors have been identified to be involved in the disease progression. Therefore, the non-genetic factors concerning persistent HBV replication should be clarified.
Among 729 relatives enrolled, parent generation, index generation, maternal hepatitis B surface antigen (HBsAg), and index cases HBsAg status were factors associated with persistent HBV infection. Factors associated with HBV viral load were evaluated among 303 HBsAg-positive relatives. Generation and sex were independent factors associated with HBV viral load. The intra-familial HBV viral load was evaluated in families clustered with HBsAg-positive siblings. An intra-family trend of similar HBV viral load was found for 27 of 46 (58.7%) families. Male offspring of HBsAg-positive mothers and older siblings were associated with high viral load.
Based on the finding that older generation and older siblings have higher viral load, the authors suspect that maternal age at birth and nutritional status might be related to generational differences on viral load. HBsAg-positive mothers usually associated with high viral load on male offspring, but not on female offspring.
Sex, generation, maternal age at birth and maternal HBsAg status are factors that should be taken into consideration when genetic factors associated with HBV-related outcome are evaluated.
The manuscript from Hsieh et al reported the sex and generation associated with HBV load in hepatocellular carcinoma family. And perinatal infection is a major effect factor for male offspring’s HBV replication. The entire sets of data are nicely presented, and highly supportive of the conclusion.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu XL S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Yu MW, Chang HC, Liaw YF, Lin SM, Lee SD, Liu CJ, Chen PJ, Hsiao TJ, Lee PH, Chen CJ. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst. 2000;92:1159-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Tai DI, Changchien CS, Hung CS, Chen CJ. Replication of hepatitis B virus in first-degree relatives of patients with hepatocellular carcinoma. Am J Trop Med Hyg. 1999;61:716-719. [PubMed] |
| 3. | Sung JL, Chen DS. Geographical distribution of the subtype of hepatitis B surface antigen in Chinese. Gastroenterol Jpn. 1977;12:58-63. [PubMed] |
| 4. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Tai DI, Chen CH, Chang TT, Chen SC, Liao LY, Kuo CH, Chen YY, Chen GH, Yang SS, Tang HS. Eight-year nationwide survival analysis in relatives of patients with hepatocellular carcinoma: role of viral infection. J Gastroenterol Hepatol. 2002;17:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Chen CH, Chen YY, Chen GH, Yang SS, Tang HS, Lin HH, Lin DY, Lo SK, Du JM, Chang TT. Hepatitis B virus transmission and hepatocarcinogenesis: a 9 year retrospective cohort of 13676 relatives with hepatocellular carcinoma. J Hepatol. 2004;40:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kingsley LA, Rinaldo CR, Lyter DW, Valdiserri RO, Belle SH, Ho M. Sexual transmission efficiency of hepatitis B virus and human immunodeficiency virus among homosexual men. JAMA. 1990;264:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77:801-807. [PubMed] |
| 9. | Tsay PK, Tai DI, Chen YM, Yu CP, Wan SY, Shen YJ, Lin DY. Impact of gender, viral transmission and aging in the prevalence of hepatitis B surface antigen. Chang Gung Med J. 2009;32:155-164. [PubMed] |
| 10. | Beasley RP, Hwang LY, Lin CC, Leu ML, Stevens CE, Szmuness W, Chen KP. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis. 1982;146:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 218] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Beasley RP, Hwang LY, Lin CC, Ko YC, Twu SJ. Incidence of hepatitis among students at a university in Taiwan. Am J Epidemiol. 1983;117:213-222. [PubMed] |
| 12. | Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, Lin SM, Lee SD, Chen PJ. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:2023-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, Chen CJ. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 15. | Mbarek H, Ochi H, Urabe Y, Kumar V, Kubo M, Hosono N, Takahashi A, Kamatani Y, Miki D, Abe H. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20:3884-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Jiang DK, Sun J, Cao G, Liu Y, Lin D, Gao YZ, Ren WH, Long XD, Zhang H, Ma XP. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet. 2013;45:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 17. | Li S, Qian J, Yang Y, Zhao W, Dai J, Bei JX, Foo JN, McLaren PJ, Li Z, Yang J. GWAS identifies novel susceptibility loci on 6p21.32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genet. 2012;8:e1002791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Chan KY, Wong CM, Kwan JS, Lee JM, Cheung KW, Yuen MF, Lai CL, Poon RT, Sham PC, Ng IO. Genome-wide association study of hepatocellular carcinoma in Southern Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, Huang W, Yu L, Yue W. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Chang SW, Fann CS, Su WH, Wang YC, Weng CC, Yu CJ, Hsu CL, Hsieh AR, Chien RN, Chu CM. A genome-wide association study on chronic HBV infection and its clinical progression in male Han-Taiwanese. PLoS One. 2014;9:e99724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Tai DI, Lo SK, Kuo CH, Du JM, Chen CJ, Hung CS, Chu CM. Replication of hepatitis B in HBsAg-positive siblings. J Viral Hepat. 2002;9:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Chu CM, Chen YC, Tai DI, Liaw YF. Level of hepatitis B virus DNA in inactive carriers with persistently normal levels of alanine aminotransferase. Clin Gastroenterol Hepatol. 2010;8:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Chien RN, Yeh CT, Tsai SL, Chu CM, Liaw YF. Determinants for sustained HBeAg response to lamivudine therapy. Hepatology. 2003;38:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Thomas D, Frankenberg E. Health, nutrition and prosperity: a microeconomic perspective. Bull World Health Organ. 2002;80:106-113. [PubMed] |
| 25. | Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5062] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 26. | Chen MH, Liu X, Wei F, Larson MG, Fox CS, Vasan RS, Yang Q. A comparison of strategies for analyzing dichotomous outcomes in genome-wide association studies with general pedigrees. Genet Epidemiol. 2011;35:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Lu SN, Su WW, Yang SS, Chang TT, Cheng KS, Wu JC, Lin HH, Wu SS, Lee CM, Changchien CS. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int J Cancer. 2006;119:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009;49:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Tai DI, Tsay PK, Chen WT, Chu CM, Liaw YF. Relative roles of HBsAg seroclearance and mortality in the decline of HBsAg prevalence with increasing age. Am J Gastroenterol. 2010;105:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 31. | Lu SN, Liu JH, Wang JH, Lu CC. Secular trends of HBeAg prevalence among HBsAg-positive delivery mothers in a hepatitis B endemic area. J Trop Pediatr. 2000;46:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Lin HH, Kao JH, Chang TC, Hsu HY, Chen DS. Secular trend of age-specific prevalence of hepatitis B surface and e antigenemia in pregnant women in Taiwan. J Med Virol. 2003;69:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Lin CL, Kao JH, Chen BF, Chen PJ, Lai MY, Chen DS. Application of hepatitis B virus genotyping and phylogenetic analysis in intrafamilial transmission of hepatitis B virus. Clin Infect Dis. 2005;41:1576-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Chang HJ. 50 Years of Advancement: A Collection of Taiwan’s Medical and Public Health Records Under the Japanese Colonial Rule. National Taiwan University Press, 2012. . |
| 35. | Bertoletti A, Hong M. Age-Dependent Immune Events during HBV Infection from Birth to Adulthood: An Alternative Interpretation. Front Immunol. 2014;5:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Kallio ER, Henttonen H, Koskela E, Lundkvist A, Mappes T, Vapalahti O. Maternal antibodies contribute to sex-based difference in hantavirus transmission dynamics. Biol Lett. 2013;9:20130887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Pan Q, Zhang RN, Wang YQ, Zheng RD, Mi YQ, Liu WB, Shen F, Chen GY, Lu JF, Zhu CY. Linked PNPLA3 polymorphisms confer susceptibility to nonalcoholic steatohepatitis and decreased viral load in chronic hepatitis B. World J Gastroenterol. 2015;21:8605-8614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Ruel MT, Rivera J, Habicht JP. Length screens better than weight in stunted populations. J Nutr. 1995;125:1222-1228. [PubMed] |
| 39. | Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (1)] |