Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.743
Peer-review started: August 31, 2016
First decision: October 20, 2016
Revised: November 17, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: February 7, 2017
Processing time: 148 Days and 10.7 Hours
In addition to its contributing role in the development of chronic liver diseases, chronic hepatitis C virus (HCV) infection is associated with extrahepatic manifestations, particularly, cutaneous-based disorders including those with pruritus as a symptom. Pruritus is frequently associated with the development of chronic liver diseases such as cholestasis and chronic viral infection, and the accumulation of bile acids in patients’ sera and tissues as a consequence of liver damage is considered the main cause of pruritus. In addition to their role in dietary lipid absorption, bile acids can trigger the activation of specific receptors, such as the G protein-coupled bile acid receptor (GPBA/ TGR5). These types of receptors are known to play a crucial role in the modulation of the systemic actions of bile acids. TGR5 expression in primary sensory neurons triggers the activation of the transient receptor potential vanilloid 1 (TRPV1) leading to the induction of pruritus by an unknown mechanism. Although the pathologic phenomenon of pruritus is common, there is no uniformly effective therapy available. Understanding the mechanisms regulating the occurrence of pruritus together with the conduction of large-scale clinical and evidence-based studies, may help to create a standard treatment protocol. This review focuses on the etiopathogenesis and treatment strategies of pruritus associated with chronic HCV infection.
Core tip: Pruritus is a frequent symptom of chronic liver diseases. Chronic hepatitis C virus (HCV) infection can cause pruritus through both direct and indirect mechanisms. The direct mechanisms include induction of pro-inflammatory cytokines and chemokines as a consequence of the chronic HCV infection. Indirect mechanisms are associated with HCV-induced cholestasis leading to the accumulation of autotaxin, which is responsible for the conversion of lysophosphatidic choline into lysophosphatidic acid. This stimulates epidermal nerve endings leading to pruritus.
- Citation: Alhmada Y, Selimovic D, Murad F, Hassan SL, Haikel Y, Megahed M, Hannig M, Hassan M. Hepatitis C virus-associated pruritus: Etiopathogenesis and therapeutic strategies. World J Gastroenterol 2017; 23(5): 743-750
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/743.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.743
In addition to hepatic damage, chronic hepatitis C virus (HCV) infection is associated with the development of extrahepatic manifestations, particularly, cutaneous-based disorders including pruritus[1,2]. The occurrence of pruritus is associated with skin disorders (e.g., atopic dermatitis and psoriasis) as well as chronic liver disease[3,4]. Pruritus is a the common dermatologic manifestations that is recognized as an early sign of chronic HCV infection[5,6], particularly infections associated with the development of cholestasis[7]. Hepatic disorders such as cholestasis can impair the bile flow from the liver to the duodenum. Consequently, the accumulation of bile acids and bilirubin in patients’ plasma and tissues can result in the development of pruritus, which is recognized as a common pathological phenomenon in patients with jaundice[8]. Thus, pruritus in patients with chronic liver disease, particularly those associated with chronic HCV infection, is thought to be a consequence of the molecular action of bile salts. In cholestatic HCV patients, the activation of signaling pathways mediating the occurrence of pruritus is attributed to the toxic compound of the accumulated bile salts both in patient sera and tissues. In addition to its occurrence in patients with chronic viral hepatitis infection, pruritus has also been recognized as a common adverse effect of the treatment of viral hepatitis[9]. Thus, the induction of pruritus in patients with chronic HCV infection is not only the consequences of the infection, but also result from the treatment[10]. Bile salts are known as potent signaling molecules in gastrointestinal organs including liver, bile ducts, and intestine[11]. The accumulation of the toxic bile compounds is thought to trigger the activation of signaling pathways mediating the induction of chronic liver disease-associated symptoms including pruritus. This review focuses on the etiopathogenesis and treatment strategies of pruritus associated with chronic HCV infection.
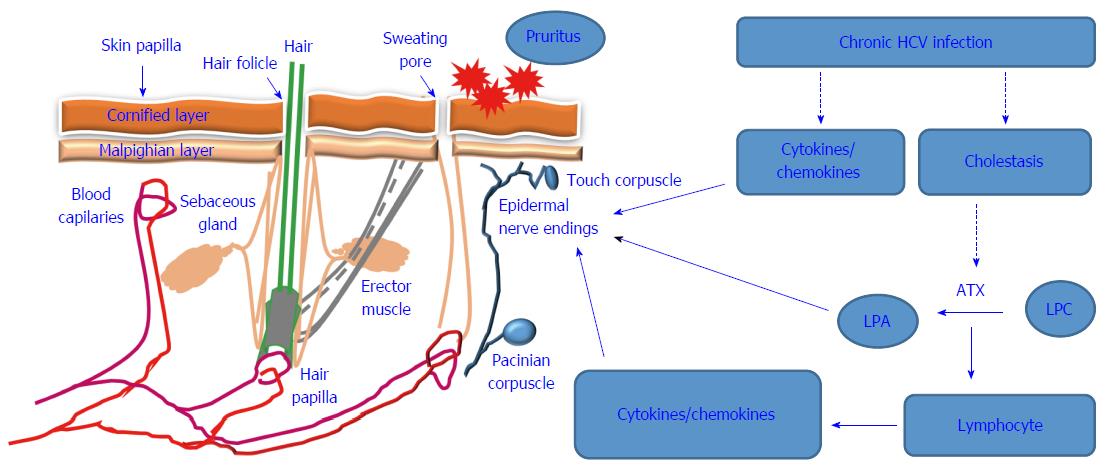
Pruritus in HCV infected patients may be the results of HCV-induced mechanisms, particularly those associated with the induction of cholestasis as well as those associated with the alteration of chemokine and cytokines profile in patients with chronic HCV infection. HCV-associated cholestasis is well described in different reports on liver transplantation. Its occurrence is attributed to viral overload and the continuous suppression of host immune response as a consequence of anti- viral agents[12]. Accordingly, the mechanisms of HCV-associated pruritus are attributed to HCV-induced cholestasis and the induction of interferon-stimulated genes (ISGs) as a result of viral overload[13]. The elevated production of cytokines (e.g., IL-8) and chemokines (e.g., CCL2, CXCL1 and CXCL5) during the course of cholestatic hepatitis C[14-17], is expected to be the main mediators for the induction of HCV-associated pruritus.
Cholestasis like features in patients with chronic HCV infection were first described by Poulson and Christoffersen[18], Who found that the occurrence of pruritus in HCV patients is correlated with significant damage in small or medium sized bile ducts causing the formation of hepatic type lesions, which offer a favorable environment for virus propagation[19]. Bile duct lesions are recognized as a histopathological sign for chronic infection with viral hepatitis[20]. The formation of bile duct lesions is common among patients with chronic HCV infection; however, the pattern of their formation is progressive, and the mechanism is bile duct damage-independent[19]. Although they share common features, histological examination reveals that the bile duct damage related to HCV infection is significantly different than damage related to the primary biliary cirrhosis (PBC)[21,22]. To that end, both the occurrence and the outcome of HCV-associated cholestasis are expected to differ from those associated with PBC. Unlike inflammatory bile duct lesions, HCV-associated lesions are characterized by the appearance of specific-pathological features such as swelling, vacuolization, nuclear irregularity, and stratification of the epithelial cells[23].
The frequency of the bile duct lesions associated with chronic HCV infection is more significant than that lesions induced by chronic hepatitis B virus (HBV) infection[24]. Even the induction mechanisms of pruritus in HCV-infected patients developing cholestasis differ from those implicated in the regulation of pruritus in non-cholestatic HCV infected patients[25]. Of note, the most described HCV-associated cholestasis was reported in liver transplant recipients as a consequence of the viral overload[26], however, chronic HCV infection-associated pruritus has not yet been described in detail. Although most reported data support an association between HCV-induced cholestasis and the occurrence of pruritus[27-30], the mechanisms remain unknown. The investigation of the clinicodemographic and histological features of HCV-associated cholestasis may help to address the mechanistic role of chronic HCV infection in the development of pruritus.
Although the pruritogenic mediators and their receptors have been identified, there is no consensus regarding the mechanisms regulating the etiopathogenesis of pruritus. Apart from the suspected mediators and their origin, the onset of pruritus is closely associated with the development of intrahepatic cholestasis[31]. Although hepatic disorders such as chronic infection with viral hepatitis, primary biliary cirrhosis or primary sclerosing cholangitis are considered the main cause of intrahepatic cholestasis[32,33], the elevation of bile salts and μ-opioids levels in patients with intrahepatic cholestasis is largely associated with pruritus[34]. To date, the causative link between bile salts levels and severity of pruritus has not yet been investigated. However, pruritus in patients with chronic HCV infection is thought to be a consequences of HCV-induced cholestasis[35,36].
The development of pruritus during the course of liver diseases is regulated by various pruritogenic mediators such as autotaxin (ATX)[37,38]. Thus, in addition to its role in the synthesis of lysophosphatidic acid, ATX may play a role in the regulation of vascular and neural development, wound healing, neuropathic pain, and cancer development[39,40].
Although the ATX- lysophosphatidic acid (LPA)-axis has been identified as a key mechanism in the development of chronic liver diseases, particularly those associated with pruritus, the source of serum ATX has not been determined. To that end, Ikeda et al[41] assumed that the elevated activity of ATX in patients’ sera may be a consequence of HCV-induced chronic liver diseases. However, the contribution of ATX to the pathogenesis of hepatic fibrosis and the subsequent induction of pruritus suggests that ATX acts as a mediator in the modulation of HCV-induced liver damage leading, to the development of pruritus.
The ATX protein is encoded by the ecto-nucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2) gene, whose expression is regulated by the transcription factors v-Jun and signal transducer and activator of transcription 3 (STAT3)[42,43]. The activation of STAT3 by HCV suggests an essential role for HCV-induced STAT3 activation in the regulation of ATX during HCV infection[44,45]. ATX also is a lysophospholipase that mediates the formation of the bioactive lipid mediators such as LPA to form lysophosphatidylcholine (LPC)[46]. Of note, LPA has been suggested to be a highly potent signaling molecule that is involved in the regulation of various cellular functions via mechanism mediated by a family of G-protein-coupled receptors[47]. Thus, the ubiquitous and highly expressed level of LPA1 receptor on neurons is thought to play an essential role in the modulation of pruritus during chronic liver diseases including cholestasis and viral infection[48]. The role of LPA in the promotion of pruritus has been demonstrated by the development of dose-dependent scratch in response to the injection of mice with LPA[49]. LPA is a highly potent signaling molecule with the ability to trigger the activation of the transient receptor potential cation channel subfamily V member 1 (TrpV1), known as the capsaicin receptor[50]. The regulation of LPA by PI3k, protein kinase A (PKA) and C (PKC)-dependent mechanisms has been reported[51]. Activation of PI3k, PKA and PKC in response to HCV infection was noted both in vitro and in vivo[52-54], suggesting a central role for PI3 kinase, PKA and PKC pathways in the regulation of HCV-associated pruritus. More importantly, the role of PKC in the modulation of phorbol ester-induced phosphorylation of LPA1 receptor suggests a contributing role for PKC in the modulation of HCV-associated pruritus[55]. Moreover, in addition to its role in the regulation of TRPV receptor, PI3k has been implicated in the regulation of ATX. Li et al[56] demonstrated the importance of the PI3K-AKT-β-catenin pathway in the induction of ATX following the stimulation of THP-1 cells with lipopolysaccharides (LPS). In addition, the involvement of the mitogen activated protein kinase (MAPK) in the regulation of ATX has been established. The activation of c-Jun-N-terminal (JNK) and p38 MAPK has been shown to be essential for the induction of ATX[56], and the activation of mitogen activated protein kinase (MAPK) signaling pathways in response to infection with HCV has been reported in several studies. These include the activation of c-jun-N-terminal kinase (JNK), p38 and extracellular regulated kinase (ERK) pathways[57,58]. Similar to its role in modulation of cholesteric pruritus, the LPA- ATX -axis seems to be essential for the initiation and progression of pruritus in patients with chronic HCV infection[59].
Under pathological conditions elevated bile salts in patient’s tissues and sera such as taurourosodeoxycholate (TUDCA), glycochenodeoxycholate are able to signal the activation of ATX-LPA signaling[34], leading to the accumulation of LPA, which in turn can initiate or potentiate pruritus. The association between ATX-LAP and cholestatic pruritus was first reported by Kremer et al[34] who demonstrated that the intradermal injection of LPA induces itching response in mice. Thus, it is expected that the occurrence of HCV-associated pruritus is a consequence of HCV-induced cholestasis leading to the activation of the ATX-LPA signaling pathway. A proposed diagram is outlined in Figure 1 and describes the possible mechanisms that are thought to be involved in the modulation of HCV-associated pruritus.
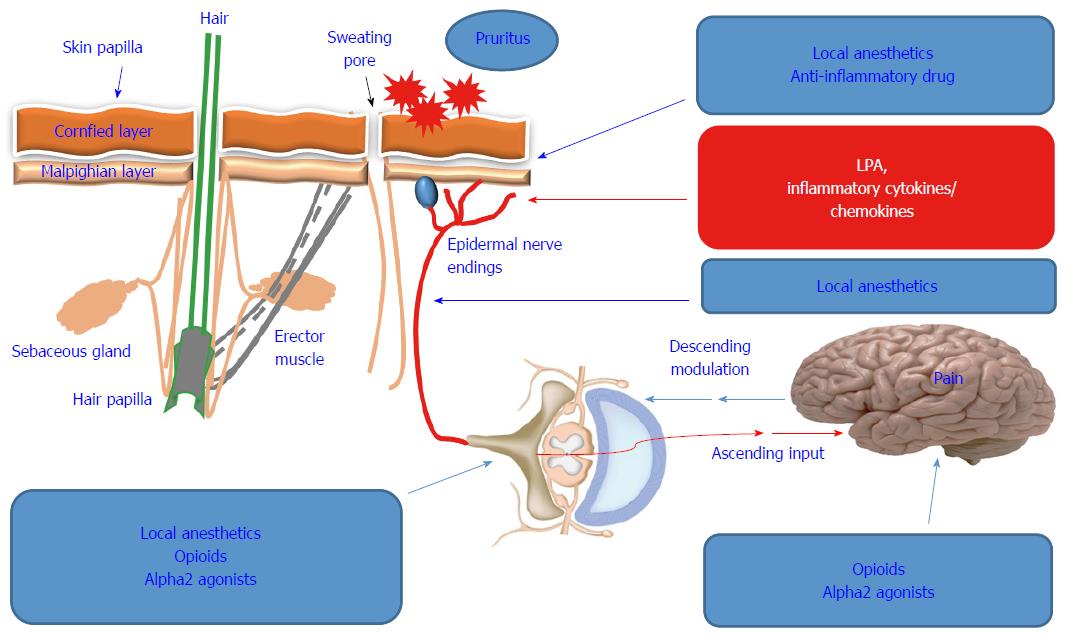
Pruritus is a common symptom that cannot be uniformly classified or quantified to date. As a consequence, its treatment, particularly, in patients with chronic liver disease is a challenge for clinicians and patients. Although there are similarity between pruritus occurrence in patients with cholestatic vs noncholestatic liver diseases, no uniformly treatment protocol has been established[60]. Current treatment strategies include topical therapies for mild and localized pruritus as well as systemic therapies for patients with severe or generalized pruritus[61,62].
Based on the fact that histamine-dependent mechanisms are responsible for the occurrence of pruritus associated with urticaria, the clinical utilization of antihistamines has been suggested as a therapeutic option for prurigo nodularis or aqua genic pruritus[63-66]. Thus, once the cause of pruritus has been identified, the implementation of the therapeutic modalities can be determined. The current guidelines suggest the application of topical substances such as capsaicin and calcineurin inhibitors, particularly in patients with chronic pruritus[64]. These substances have been approved for their effects on cutaneous neurons, where they serve as suppressors for chronic pruritus[67,68].
Substances like opioid receptor antagonists, anticonvulsants, selective serotonin re-uptake inhibitors and antidepressants have been recommended[69,70]. Although therapeutic options of pruritus are available, the lack of well-conducted, randomized, controlled studies is an obstacle for the development of an effective and uniform treatment protocol. Cholestyramine is the most recommended first line therapy for pruritus[71], while rifampicin, opiate antagonists and sertraline have been utilized as second-, third-, and fourth-line therapies, respectively[72,73].
In addition to the poor prognosis of patients with pruritus, topical and systemic therapies can offer only short term relief and most are associated with complicated adverse effects, particularly, in patients with chronic HCV infection[3].
Although antiviral therapeutics have improved in recent years, the treatment of HCV patients is associated with a marked increase in dermatological adverse effects, particularly pruritus[74]. In addition, it is difficult to distinguish between treatment- and HCV-induced pruritus in terms of causality. Even the consequences of the interference of anti-viral therapy with HCV-induced extrahepatic manifestations are not predictable. Although the treatment of HCV patients with interferon is commonly associated with local and generalized dermatological side effects, including pruritus[75], the combination of interferon with ribavirin increases the risk of pruritus occurrence[76,77]. For example, the frequency of dermatological adverse effects including pruritus associated with HCV protease inhibitors as combinatory part of the triple therapy regimen (telaprevir/boceprevir with peginterferon/ribavirin), are higher than those associated with peg interferon/ribavirin regimen alone[78,79]. Some possible therapeutic strategies for pruritus are shown in Figure 2.
Both HCV infection and its treatment are associated with significant dermatological manifestations, particularly pruritus. In order to treat pruritus in patients with HCV infection an effective management strategy is needed to limit the severity of HCV-associated pruritus. The elevation of ATX in patients’ sera may be the cause for the increase of LPA levels thought to be responsible for the simultaneous promotion of itching signaling and inhibition of the pain signaling. Thus, the investigation of the molecular mechanisms, essential for modulating the cross-talk between the activation of ATX- LPA receptor axis and the occurrence of pruritus during infection and treatment of HCV patients will drive the development of novel therapies for neglected symptoms of HCV including pruritus. Larger clinical studies will help to outline the efficacy of available anti-pruritic therapeutics.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Marin JJG, Lee HC S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Hassan M, Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O. Hepatitis C virus-host interactions: Etiopathogenesis and therapeutic strategies. World J Exp Med. 2012;2:7-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Dervis E, Serez K. The prevalence of dermatologic manifestations related to chronic hepatitis C virus infection in a study from a single center in Turkey. Acta Dermatovenerol Alp Pannonica Adriat. 2005;14:93-98. [PubMed] |
| 3. | Cacoub P, Bourlière M, Lübbe J, Dupin N, Buggisch P, Dusheiko G, Hézode C, Picard O, Pujol R, Segaert S. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Hung KY, Shyu RS, Tsai TJ, Chen WY. Viral hepatitis infection should be considered for evaluating uremic pruritus in continuous ambulatory peritoneal dialysis patients. Blood Purif. 1998;16:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Halawani M. Screening of hepatitis C virus genotypes in urticaria patients in Saudi Arabia. Genet Test Mol Biomarkers. 2012;16:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Cordel N, Chosidow O, Francès C. Cutaneous disorders associated with hepatitis C virus infection. Ann Med Interne (Paris). 2000;151:46-52. [PubMed] |
| 7. | Pellicelli AM, D’Ambrosio C, Dessanti ML, Villani R, Fondacaro L, Miglioresi L, Grillo LR, Andreoli A. Cholestatic hepatitis C after chemotherapy containing rituximab in diffuse large B cell lymphoma. Ann Hepatol. 2015;14:756-761. [PubMed] |
| 8. | Eisendle K, Müller H, Ortner E, Talasz H, Graziadei I, Vogel W, Höpfl R. Pruritus of unknown origin and elevated total serum bile acid levels in patients without clinically apparent liver disease. J Gastroenterol Hepatol. 2011;26:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Rahman A, Rizvi SD, Sheikh ZI. Frequency of HCV infection in different dermatological disorders. J Ayub Med Coll Abbottabad. 2012;24:58-61. [PubMed] |
| 10. | Bosonnet L. Pruritus: scratching the surface. Eur J Cancer Care (Engl). 2003;12:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50:1721-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Villaverde AF, Benlloch S, Berenguer M, Miguel Rayón J, Pina R, Berenguer J. Acute hepatitis of cholestatic type possibly associated with the use of glucomannan (amorphophalus konjac). J Hepatol. 2004;41:1061-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Deshpande V, Burd E, Aardema KL, Ma CK, Moonka DK, Brown KA, Abouljoud MS, Nakhleh RE. High levels of hepatitis C virus RNA in native livers correlate with the development of cholestatic hepatitis in liver allografts and a poor outcome. Liver Transpl. 2001;7:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Antonelli A, Ferri C, Fallahi P, Ferrari SM, Frascerra S, Pampana A, Panicucci E, Carpi A, Nicolini A, Ferrannini E. CXCL10 and CCL2 chemokine serum levels in patients with hepatitis C associated with autoimmune thyroiditis. J Interferon Cytokine Res. 2009;29:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Nischalke HD, Berger C, Luda C, Müller T, Berg T, Coenen M, Krämer B, Körner C, Trebicka J, Grünhage F. The CXCL1 rs4074 A allele is associated with enhanced CXCL1 responses to TLR2 ligands and predisposes to cirrhosis in HCV genotype 1-infected Caucasian patients. J Hepatol. 2012;56:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zekri AR, Bahnassy AA, Mohamed WS, Alam El-Din HM, Shousha HI, Zayed N, Eldahshan DH, Abdel-Aziz AO. Dynamic interplay between CXCL levels in chronic hepatitis C patients treated by interferon. Virol J. 2013;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Akbar H, Idrees M, Butt S, Awan Z, Sabar MF, Rehaman Iu, Hussain A, Saleem S. High baseline interleukine-8 level is an independent risk factor for the achievement of sustained virological response in chronic HCV patients. Infect Genet Evol. 2011;11:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Poulsen H, Christoffersen P. Abnormal bile duct epithelium in liver biopsies with histological signs of viral hepatitis. Acta Pathol Microbiol Scand. 1969;76:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Giannini E, Botta F, Fasoli A, Romagnoli P, Mastracci L, Ceppa P, Comino I, Pasini A, Risso D, Testa R. Increased levels of gammaGT suggest the presence of bile duct lesions in patients with chronic hepatitis C: absence of influence of HCV genotype, HCV-RNA serum levels, and HGV infection on this histological damage. Dig Dis Sci. 2001;46:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Torbenson M, Yeh MM, Abraham SC. Bile duct dysplasia in the setting of chronic hepatitis C and alcohol cirrhosis. Am J Surg Pathol. 2007;31:1410-1413. [PubMed] |
| 21. | Vespasiani-Gentilucci U, Carotti S, Onetti-Muda A, Perrone G, Ginanni-Corradini S, Latasa MU, Avila MA, Carpino G, Picardi A, Morini S. Toll-like receptor-4 expression by hepatic progenitor cells and biliary epithelial cells in HCV-related chronic liver disease. Mod Pathol. 2012;25:576-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kimura Y, Leung PS, Kenny TP, Van De Water J, Nishioka M, Giraud AS, Neuberger J, Benson G, Kaul R, Ansari AA. Differential expression of intestinal trefoil factor in biliary epithelial cells of primary biliary cirrhosis. Hepatology. 2002;36:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Fillipowicz EA, Xiao Sy, Sower LE, Weems J, Payne DA. Detection of HCV in bile duct epithelium by laser capture microdissection (LCM). In Vivo. 2005;19:737-739. [PubMed] |
| 24. | Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, Chiarla C, Giovannini I. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg. 2010;62:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Dega H, Francès C, Dupin N, Lebre C, Simantov A, Callot C, Laporte JL, Blot C, Opolon P, Poynard T. [Pruritus and the hepatitis C virus. The MULTIVIRC Unit]. Ann Dermatol Venereol. 1998;125:9-12. [PubMed] |
| 26. | Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation. 2014;98:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Belay T, Woldegiorgis H, Gress T, Rayyan Y. Intrahepatic cholestasis of pregnancy with concomitant hepatitis C virus infection, Joan C. Edwards SOM, Marshall University. Eur J Gastroenterol Hepatol. 2015;27:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Abu-Hayyeh S, Ovadia C, Lieu T, Jensen DD, Chambers J, Dixon PH, Lövgren-Sandblom A, Bolier R, Tolenaars D, Kremer AE. Prognostic and mechanistic potential of progesterone sulfates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology. 2016;63:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Dehghani SM, Bahador A, Nikeghbalian S, Salahi H, Geramizadeh B, Malekpour A, Malek-Hosseini SA. Liver transplant in a case of arthrogryposis-renal tubular dysfunction-cholestasis syndrome with severe intractable pruritus. Exp Clin Transplant. 2013;11:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Kumar N, Garg N, Bailey A. Opiate receptor antagonists for treatment of severe pruritus associated with advanced cholestatic liver disease. J Palliat Med. 2013;16:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Ołdakowska-Jedynak U, Jankowska I, Hartleb M, Jirsa M, Pawłowska J, Czubkowski P, Krawczyk M. Treatment of pruritus with Prometheus dialysis and absorption system in a patient with benign recurrent intrahepatic cholestasis. Hepatol Res. 2014;44:E304-E308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Postnikova OA, Nepomnyashchikh GI, Yudanov AV, Nepomnyashchikh DL, Kapustina VI, Isayenko VI. Intracellular cholestasis in HCV and HBV infection. Bull Exp Biol Med. 2012;153:898-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Nguyen KD, Sundaram V, Ayoub WS. Atypical causes of cholestasis. World J Gastroenterol. 2014;20:9418-9426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 34. | Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008-1018, 1018.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 35. | Delgado J, Muñoz de Bustillo E, Ibarrola C, Colina F, Morales JM, Rodriguez E, Aguado JM, Fuertes A, Gomez MA. Hepatitis C virus-related fibrosing cholestatic hepatitis after cardiac transplantation: is azathioprine a contributory factor? J Heart Lung Transplant. 1999;18:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Rosenberg PM, Farrell JJ, Abraczinskas DR, Graeme-Cook FM, Dienstag JL, Chung RT. Rapidly progressive fibrosing cholestatic hepatitis--hepatitis C virus in HIV coinfection. Am J Gastroenterol. 2002;97:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Cooper AB, Wu J, Lu D, Maluccio MA. Is autotaxin (ENPP2) the link between hepatitis C and hepatocellular cancer? J Gastrointest Surg. 2007;11:1628-1634; discussion 1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis. 2011;29:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y, Kontoyiannis DL, Remboutsika E. ATX expression and LPA signalling are vital for the development of the nervous system. Dev Biol. 2010;339:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Yukiura H, Hama K, Nakanaga K, Tanaka M, Asaoka Y, Okudaira S, Arima N, Inoue A, Hashimoto T, Arai H. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J Biol Chem. 2011;286:43972-43983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Ikeda H, Watanabe N, Nakamura K, Kume Y, Nakai Y, Fujishiro M, Omata M, Igarashi K, Yokota H, Yatomi Y. [Significance of serum autotaxin activity in gastrointestinal disease]. Rinsho Byori. 2009;57:445-449. [PubMed] |
| 42. | Black EJ, Clair T, Delrow J, Neiman P, Gillespie DA. Microarray analysis identifies Autotaxin, a tumour cell motility and angiogenic factor with lysophospholipase D activity, as a specific target of cell transformation by v-Jun. Oncogene. 2004;23:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Azare J, Doane A, Leslie K, Chang Q, Berishaj M, Nnoli J, Mark K, Al-Ahmadie H, Gerald W, Hassimi M. Stat3 mediates expression of autotaxin in breast cancer. PLoS One. 2011;6:e27851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196:641-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Zhao LJ, He SF, Wang W, Ren H, Qi ZT. Interferon alpha antagonizes STAT3 and SOCS3 signaling triggered by hepatitis C virus. Cytokine. 2016;80:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Teo K, Brunton VG. The role and therapeutic potential of the autotaxin-lysophosphatidate signalling axis in breast cancer. Biochem J. 2014;463:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Fincher J, Whiteneck C, Birgbauer E. G-protein-coupled receptor cell signaling pathways mediating embryonic chick retinal growth cone collapse induced by lysophosphatidic acid and sphingosine-1-phosphate. Dev Neurosci. 2014;36:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Foschi FG, Dall’aglio AC, Lanzi A, Marano G, Savini S, Andreone P, Bernardi M, Stefanini GF. Cryoglobulinemia in elderly patients with HCV-related chronic hepatitis. World J Gastrointest Pharmacol Ther. 2010;1:72-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Hashimoto T, Ohata H, Momose K. Itch-scratch responses induced by lysophosphatidic acid in mice. Pharmacology. 2004;72:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Nieto-Posadas A, Picazo-Juárez G, Llorente I, Jara-Oseguera A, Morales-Lázaro S, Escalante-Alcalde D, Islas LD, Rosenbaum T. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2011;8:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 51. | Kassmann M, Harteneck C, Zhu Z, Nürnberg B, Tepel M, Gollasch M. Transient receptor potential vanilloid 1 (TRPV1), TRPV4, and the kidney. Acta Physiol (Oxf). 2013;207:546-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Banerjee A, Meyer K, Mazumdar B, Ray RB, Ray R. Hepatitis C virus differentially modulates activation of forkhead transcription factors and insulin-induced metabolic gene expression. J Virol. 2010;84:5936-5946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Fimia GM, Evangelisti C, Alonzi T, Romani M, Fratini F, Paonessa G, Ippolito G, Tripodi M, Piacentini M. Conventional protein kinase C inhibition prevents alpha interferon-mediated hepatitis C virus replicon clearance by impairing STAT activation. J Virol. 2004;78:12809-12816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Borowski P, Oehlmann K, Heiland M, Laufs R. Nonstructural protein 3 of hepatitis C virus blocks the distribution of the free catalytic subunit of cyclic AMP-dependent protein kinase. J Virol. 1997;71:2838-2843. [PubMed] |
| 55. | Hernández-Méndez A, Alcántara-Hernández R, Acosta-Cervantes GC, Martínez-Ortiz J, Avendaño-Vázquez SE, García-Sáinz JA. Conventional protein kinase C isoforms mediate phorbol ester-induced lysophosphatidic acid LPA1 receptor phosphorylation. Eur J Pharmacol. 2014;723:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Li S, Xiong C, Zhang J. ATX and LPA receptor 3 are coordinately up-regulated in lipopolysaccharide-stimulated THP-1 cells through PKR and SPK1-mediated pathways. FEBS Lett. 2012;586:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Hassan M, Selimovic D, Ghozlan H, Abdel-Kader O. Induction of high-molecular-weight (HMW) tumor necrosis factor(TNF) alpha by hepatitis C virus (HCV) non-structural protein 3 (NS3) in liver cells is AP-1 and NF-kappaB-dependent activation. Cell Signal. 2007;19:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Hassan M, Ghozlan H, Abdel-Kader O. Activation of c-Jun NH2-terminal kinase (JNK) signaling pathway is essential for the stimulation of hepatitis C virus (HCV) non-structural protein 3 (NS3)-mediated cell growth. Virology. 2005;333:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Street A, Macdonald A, Crowder K, Harris M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232-12241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012;32 Suppl 1:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 61. | Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Cheer SM, Plosker GL. Tacrolimus ointment. A review of its therapeutic potential as a topical therapy in atopic dermatitis. Am J Clin Dermatol. 2001;2:389-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Lim VM, Maranda EL, Patel V, Simmons BJ, Jimenez JJ. A Review of the Efficacy of Thalidomide and Lenalidomide in the Treatment of Refractory Prurigo Nodularis. Dermatol Ther (Heidelb). 2016;6:397-411. [PubMed] |
| 64. | Weisshaar E, Szepietowski JC, Darsow U, Misery L, Wallengren J, Mettang T, Gieler U, Lotti T, Lambert J, Maisel P. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92:563-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 65. | Shintani T, Ohata C, Koga H, Ohyama B, Hamada T, Nakama T, Furumura M, Tsuruta D, Ishii N, Hashimoto T. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Herman-Kideckel SM, Binkley K. Successful treatment of aquagenic pruritus with montelukast. J Cutan Med Surg. 2012;16:151-152. [PubMed] |
| 67. | Pereira U, Boulais N, Lebonvallet N, Pennec JP, Dorange G, Misery L. Mechanisms of the sensory effects of tacrolimus on the skin. Br J Dermatol. 2010;163:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Senba E, Katanosaka K, Yajima H, Mizumura K. The immunosuppressant FK506 activates capsaicin- and bradykinin-sensitive DRG neurons and cutaneous C-fibers. Neurosci Res. 2004;50:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Mettang T, Ständer S, Kremer AE. [Treatment of chronic itch in systemic disease. Current standards]. Internist (Berl). 2015;56:1369-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Zeidler C, Ständer S. [Therapy of prurigo nodularis]. Hautarzt. 2014;65:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Ala S, Eshghi F, Enayatifard R, Fazel P, Rezaei B, Hadianamrei R. Efficacy of cholestyramine ointment in reduction of postoperative pain and pain during defecation after open hemorrhoidectomy: results of a prospective, single-center, randomized, double-blind, placebo-controlled trial. World J Surg. 2013;37:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Fuhrmann V, Drolz A, Trauner M. Extracorporeal artificial liver support systems in the management of intractable cholestatic pruritus. Liver Int. 2011;31 Suppl 3:31-33. [PubMed] |
| 73. | Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg. 2012;75:399-404. [PubMed] |
| 74. | Cunha VS, Meotti C, Oliveira JH, Sprinz E, Alvares-da-Silva MR, Goldani LZ. Different patterns of dermatological presentations in patients co-infected with human immunodeficiency virus and hepatitis C virus (HCV), and those infected with HCV alone. Clin Exp Dermatol. 2012;37:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Perry CM, Wagstaff AJ. Interferon-alpha-n1: a review of its pharmacological properties and therapeutic efficacy in the management of chronic viral hepatitis. BioDrugs. 1998;9:125-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Ghosh S, Duseja A, Dhiman RK, Chawla YK. Tongue hyperpigmentation resulting from peginterferon alfa-2b and ribavirin treatment in a patient with chronic hepatitis C. Dig Dis Sci. 2012;57:820-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Qin H, Li H, Zhou X, Feng F, Shen Y, Tan H, Ye F, Xie Y. Safety of telaprevir for chronic hepatitis C virus infection: a meta-analysis of randomized controlled trials. Clin Drug Investig. 2012;32:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 79. | Jorquera F, Monte MJ, Guerra J, Sanchez-Campos S, Merayo JA, Olcóz JL, González-Gallego J, Marin JJ. Usefulness of combined measurement of serum bile acids and ferritin as additional prognostic markers to predict failure to reach sustained response to antiviral treatment in chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |