Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8660
Peer-review started: July 28, 2017
First decision: August 30, 2017
Revised: September 15, 2017
Accepted: October 17, 2017
Article in press: October 17, 2017
Published online: December 28, 2017
Processing time: 152 Days and 2.9 Hours
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) modifying agents have been involved in the development of intestinal inflammation, especially therapeutic monoclonal antibodies directed against CTLA-4. Here we report the appearance of a severe stricturing Crohn’s disease-like colitis in a patient with a kidney allograft who was treated with belatacept, a recombinant CTLA-4-Ig fusion protein.
Core tip: Belatacept is a cytotoxic T-lymphocyte-associated antigen 4 Ig fusion protein used for kidney transplant rejection prophylaxis. We report the appearance of a severe stricturing Crohn’s disease-like colitis in a patient who was treated with belatacept. After belatacept withdrawal, complete mucosal healing was observed with the persistence of a non-ulcerated left-sided colonic stricture which did not allow passage of the colonoscope. So, in patients treated with belatacept who develop digestive symptoms such as diarrhea or intestinal bleeding, we recommend performing early colonoscopy and considering belatacept withdrawal in case of suggestive endoscopic and histologic findings in order to avoid colonic sequela.
- Citation: Bozon A, Jeantet G, Rivière B, Funakoshi N, Dufour G, Combes R, Valats JC, Delmas S, Serre JE, Bismuth M, Ramos J, Le Quintrec M, Blanc P, Pineton de Chambrun G. Stricturing Crohn’s disease-like colitis in a patient treated with belatacept. World J Gastroenterol 2017; 23(48): 8660-8665
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8660
Belatacept is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) Ig fusion protein mostly used for kidney transplant rejection prophylaxis, in association with steroids and mycophenolate mofetil[1]. Belatacept selectively inhibits T cell activation and was recently demonstrated to be superior to cyclosporin in patients with renal transplants in terms of renal function with equivalent survival[2]. Frequent adverse events associated with belatacept are anemia, disturbance of bowel habits and infections, especially urinary tract infections, with no need to stop treatment in most of cases. Here, we report a case of severe stricturing Crohn’s disease (CD)-like colitis due to belatacept administration in a renal transplant recipient.
We report the case of a 62-year-old man who received a first kidney allograft from a deceased donor in September 2013. His end-stage renal disease was attributed to chronic glomerulonephritis. After an induction treatment by thymoglobulin and methylprednisolone pulses, the immunosuppression regimen consisted of tacrolimus, mycophenolate mofetil and steroids. There was no immediate complication after transplantation and the nadir of serum creatinin was 2.24 mg/dL. Rapidly, after two months, tacrolimus was withdrawn due to nephrotoxicity (histologically proven) and replaced with everolimus which was also stopped due to development of lymphocele and proteinuria. In February 2014, belatacept was started at a dose of 5 mg/kg intravenously every month in association with mycophenolate mofetil in order to decrease corticosteroids to the level of 10 mg/d.
From the start of mycophenolate mofetil treatment, the patient had anorexia and diarrhea, with liquid stools without blood. He underwent in March 2014 an upper gastrointestinal endoscopy and a colonoscopy which showed no mucosal abnormalities. Duodenal biopsies demonstrated normal mucosal histology. In October 2015, because of worsening of the diarrhea, a stool culture was performed which was positive for Campylobacter jejuni. Antibiotics course was prescribed with some efficacy but the diarrhea persisted.
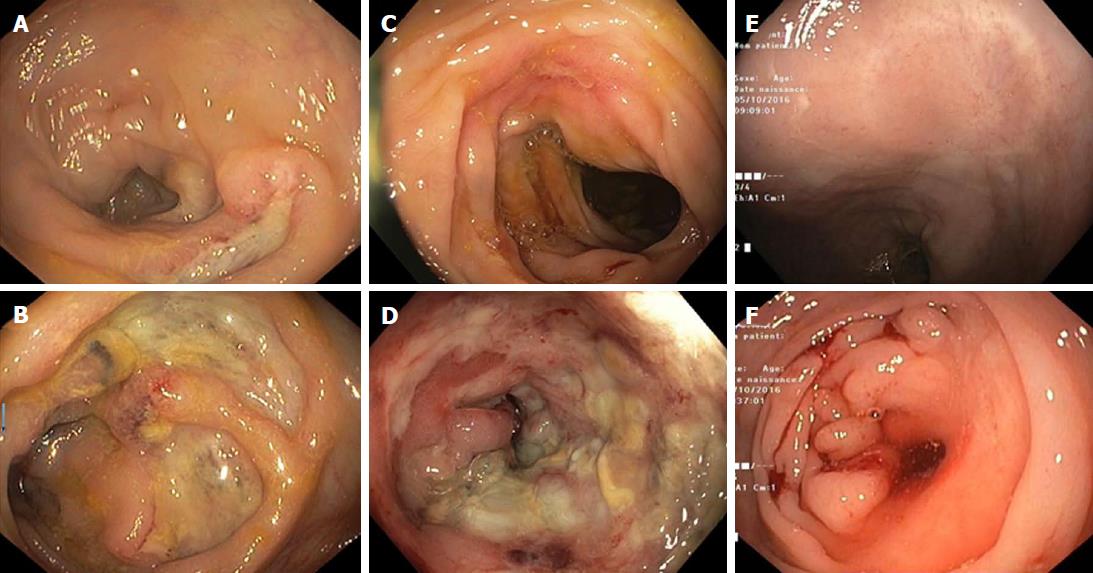
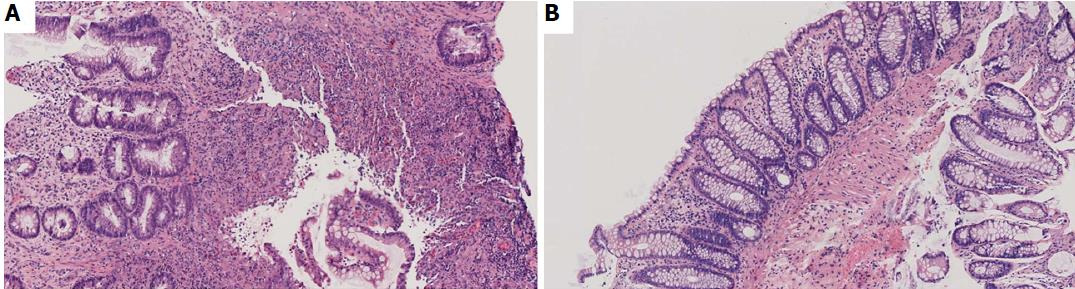
In February 2016, the patient was hospitalized for bloody stools with anemia and abdominal pain for which blood transfusion was necessary. A colonoscopy was rapidly performed showing large round shaped deep ulcers with normal surrounding mucosa. These ulcers were located in the caecum, transverse colon, left colon and sigmoid colon and were compatible with the diagnosis of CD (Figure 1). The terminal ileum and rectum were normal. Histologic examination of the colonic biopsies showed acute colitis with ulcerations, crypt abscesses, lymphocytes and neutrophil polymorphonuclear leukocyte infiltration. Neither crypt dystrophy nor granuloma was found (Figure 2). A small bowel wireless capsule endoscopy was also performed without mucosal abnormalities.
Due to suspicion of mycophenolate mofetil involvement in the acute colitis, this treatment was withdrawn in March 2016, but belatacept was pursued with an increase of steroid therapy to 20 mg/d. A follow-up colonoscopy was performed in June 2016 which showed persistence of the large ulcers previously described and the appearance of a passable ulcerated inflammatory stricture at the left colonic flexure (Figure 1). Histologic examination showed acute colitis without signs of chronic inflammation (Figure 2). No signs of cytomegalovirus colitis were found on histology, such as owl’s eye inclusion bodies. Polymerase chain reaction (PCR) analysis of colonic biopsies was positive for human herpes virus 6 (HHV-6) and negative for cytomegalovirus and herpes simplex virus. Also, PCR analysis for cytomegalovirus in the blood was negative. Belatacept was therefore withdrawn in June 2016 and the patient was treated with low dose tacrolimus for prevention of allograft rejection and an increased dose of steroids to treat colonic inflammation. In October 2016, the patient was free from diarrhea but described left-sided abdominal pain with partial obstructive symptoms probably due to the colonic stricture. The colonoscopy performed four months after belatacept withdrawal showed a complete healing of the ulcers in the left colon and a non-inflammatory stricture of the left colonic flexure which could not be passed (Figure 1). The stricture was not dilated as clinical symptoms were mild. PCR for HHV-6 was negative on colonic biopsies and only slightly positive on biopsies targeted to the colonic stricture. At the last clinical outpatient visit in January 2017 the patient was asymptomatic with no signs of acute renal rejection.
We have described here for the first time the case of a severe stricturing CD-like colitis occurring in a patient with a kidney allograft treated with belatacept. Gastrointestinal side effects are well known in renal transplant recipients receiving immunosuppressive therapy, especially mycophenolate mofetil (MMF). Indeed, MMF has multiple side effects and those affecting the gastrointestinal tract mostly occur during the first 6 mo after the onset of treatment[3]. These side effects include nausea, vomiting, abdominal pain, and diarrhea, whereas bleeding is less reported. A recent retrospective study investigated endoscopic findings in patients treated with MMF having diarrhea. In most of the cases the colonic mucosa was normal and the common lesion was simple erythema without deep ulcer or stricture[3]. Our patient had diarrhea after introduction of MMF with liquid stools but no bleeding, and this diarrhea improved after MMF withdrawal. Although acute colitis was first thought to be due to MMF, appearance of bleeding, large ulcers without erythema at colonoscopy and worsening of endoscopic lesions after MMF withdrawal led us to suspect the involvement of belatacept.
Ipilimumab and tremelimumab, two therapeutic monoclonal antibodies against CTLA-4 and prescribed in cancer patients, have previously been implicated in the development of severe and extensive forms of inflammatory bowel disease with colonic ulcerations[4]. CTLA-4 is a homologue of CD28 that binds CD80 and CD86 with higher affinity, and thereby down-regulates T cell activation. Anti CTLA-4 monoclonal antibodies block its interaction with CD80 and CD86 and favour CD28 engagement and consequently T cell activation and proliferation. The overactivation of the immune system in patients treated with anti CTLA-4 antibodies associated with a specific gut microflora may explain the development of treatment-mediated CD-like colitis[5]. Abatacept and belatacept are two recombinant fusion proteins comprising a fragment of the Fc domain of human IgG1 and the extracellular domain of human CTLAA-4. Similar to CTLA-4, abatacept and belatacept compete with CD28 for CD80 and CD86 binding to block co-stimulatory signaling, thus selectively modulating T-cell activation. In comparison to abatacept, belatacept confers higher affinity for CD 80/86 ligands and has a slower dissociation rates. It could also alter regulatory T cell development, which plays an important part in intestinal inflammation. Abatacept is effective for rheumatoid arthritis and juvenile idiopathic arthritis[6], and belatacept is currently used for kidney transplant rejection prophylaxis[1]. It has been showed that abatacept was not effective for the treatment of active ulcerative colitis and CD[6]. It may be surprising that belatacept induces CD-like colitis given it has the opposite effect from anti-CTLA-4 antibodies. However, cases of colitis have been also described in patients treated with abatacept (Table 1). A first case of ulcerative colitis was reported in 2006 in a 55 year old male patient treated with abatacept for rheumatoid arthritis[7]. The diagnosis of ulcerative colitis was made 15 mo after start of abatacept and digestive symptoms improved after abatacept withdrawal and mesalamine treatment. Two other cases of ulcerative colitis developing five and 23 mo after abatacept introduction in rheumatoid arthritis patients were reported. The first one was treated with mesalamine and infliximab and the second one with prednisolone and mesalamine[8]. Similarly to these case reports, the severe colitis occurred 23 mo after belatacept introduction in our patient. Macroscopically, the endoscopic lesions were more in favor of CD compared to abatacept-induced colitis, and the large deep ulcerations were similar to anti CTLA-4 enterocolitis. The histological findings in our patients described acute colitis with polymorph leucocyte infiltration and crypt abscesses without atrophy, distortion, branching or budding of crypts. These findings were also described in abatacept and anti CTLA-4 colitis. The most striking finding in our case is the development on belatacept treatment of an inflammatory stricture of the left colon. After belatacept withdrawal and prednisolone treatment, we observed complete healing of colonic lesions, but with persistence of a non-inflammatory colonic stricture which could be passed.
| Type of CTLA-4-Ig fusion protein | Delay between | Endoscopic findings | Histological findings | CTLA-4-Ig | Colitis treatment | Evolution of the colitis | |
| CTLA-4-Ig introduction and colitis (mo) | withdrawal | ||||||
| Amezcua-Guerra et al[7] Gut 2005 | Abatacept | 15 | UC-like colitis | Lymphoplasmocytic infiltration/cryptitis/Intraluminal abscesses | Yes | Mesalazine | Clinical remission |
| Motohashi et al[8] Case 1 Scand J Gastroenterol 2014 | Abatacept | 25 | UC-like colitis | Neutrophil infiltration/crypt abscesses | Yes | Infliximab + Mesalazine | NA |
| Motohashi et al[8] Case 2 Scand J Gastroenterol 2014 | Abatacept | 5 | UC-like colitis | Neutrophil infiltration/crypt abscesses | Yes | Mesalazine + Prednisolone + Granulocytapheresis | NA |
| Present case | Belatacept | 23 | CD-like colitis | Ulcerations/crypt abscesses/ | Yes | Prednisolone | Clinical and endoscopic remission |
| lymphocytes and neutrophil infiltration |
Although belatacept seems to be involved in the development of colitis in our patient, the exact mechanisms of this colitis are unclear. It may be a direct effect of belatacept, which could alter the development of regulatory T cells, and therefore lead to uncontrolled intestinal inflammation. Another hypothesis may be an indirect effect of belatacept which confers a profound immunosuppression leading to the development of infectious colitis. Cytomegalovirus colitis was ruled out by careful histologic examination and negative PCR analyses in the blood and in colonic biopsies. PCR in biopsies was however, strongly positive for HHV-6. HHV-6 reactivation in patients with solid organ or hematopoietic stem cell transplantation has been reported to be associated with intestinal disease[9,10]. Moreover, HHV-6 was found in colonic mucosa of inflammatory bowel disease patients in 44% of the cases and associated with disease activity and use of immunosuppressive therapy[11]. HHV-6 intensity also correlated with endoscopic severity in ulcerative colitis. After belatacept withdrawal and mucosal healing, PCR for HHV-6 in colonic biopsies was found to be negative or slightly positive in our patient.
Thus, we report here a case of CD-like colitis in a patient treated with belatacept. Despite belatacept withdrawal, the patient developed a severe colonic stricture which may impact quality of life and necessitate subsequent colonic surveillance. Therefore in patients treated with belatacept who develop digestive symptoms such as diarrhea or intestinal bleeding, we recommend performing early colonoscopy and considering belatacept withdrawal in case of suggestive endoscopic and histologic findings.
A 62-year-old man with kidney allograft treated with belatacept and mycophenolate mofetil presented a diarrhea with rectal bleeding and abdominal pain.
Abdominal tenderness associated to liquid stools and rectal bleeding.
Diarrhea associated to mycophenolate mofetil, viral enterocolitis, bacterial enterocolitis, Crohn’s disease or ulcerative colitis.
Normal stool cultures and blood tests ruled out opportunistic infections.
Colonoscopy showed large colonic ulcers with normal surrounding mucosa disseminated along the colonic tract and a passable ulcerated inflammatory stricture at the left colonic flexure.
Histologic examination of the colonic biopsies showed acute colitis with ulcerations, crypt abscesses, lymphocytes and neutrophil polymorphonuclear leukocyte infiltration. Neither crypt dystrophy nor granuloma was found. No signs of cytomegalovirus colitis were found on histology, such as owl’s eye inclusion bodies.
Withdrawal of belatacept and corticosteroid therapy.
Previous cases of colitis in patients treated with abatacept, another Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) Ig fusion protein, have been also described.
Drug-induced colitis is described with numerous agents, especially mycophenolate mofetil or antibodies against CTLA-4. Pathophysiological mechanisms are not fully understood. Endoscopic and histologic findings are not specific showing acute colitis and withdrawal of the drug which leads to complete resolution in most of the cases, confirms the diagnosis.
In patients treated with belatacept who develop digestive symptoms such as diarrhea or intestinal bleeding, early colonoscopy should be performed and belatacept withdrawal should be considered in case of suggestive endoscopic and histologic findings in order to avoid colonic sequela.
The report has high novelty, clinically important information, which is relevant in therapeutic settings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim KJ, Saniabadi AR S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Grinyó JM, Del Carmen Rial M, Alberu J, Steinberg SM, Manfro RC, Nainan G, Vincenti F, Jones-Burton C, Kamar N. Safety and Efficacy Outcomes 3 Years After Switching to Belatacept From a Calcineurin Inhibitor in Kidney Transplant Recipients: Results From a Phase 2 Randomized Trial. Am J Kidney Dis. 2017;69:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 720] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 3. | Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol. 2015;28:366-373. [PubMed] |
| 4. | Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, Zallot C, Peyrin-Biroulet L, Rahier JF, Bourdier de Beauregard M. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 920] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 6. | Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62-69.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Amezcua-Guerra LM, Hernández-Martínez B, Pineda C, Bojalil R. Ulcerative colitis during CTLA-4Ig therapy in a patient with rheumatoid arthritis. Gut. 2006;55:1059-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Motohashi R, Ikeuchi H, Hiromura K, Ohishi Y, Sakurai N, Sakairi T, Kaneko Y, Maeshima A, Nojima Y. Two cases of ulcerative colitis developing in rheumatoid arthritis patients during abatacept therapy. Scand J Gastroenterol. 2014;49:1270-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hentrich M, Oruzio D, Jäger G, Schlemmer M, Schleuning M, Schiel X, Hiddemann W, Kolb HJ. Impact of human herpesvirus-6 after haematopoietic stem cell transplantation. Br J Haematol. 2005;128:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Lamoth F, Jayet PY, Aubert JD, Rotman S, Mottet C, Sahli R, Lautenschlager I, Pascual M, Meylan P. Case report: human herpesvirus 6 reactivation associated with colitis in a lung transplant recipient. J Med Virol. 2008;80:1804-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Sipponen T, Turunen U, Lautenschlager I, Nieminen U, Arola J, Halme L. Human herpesvirus 6 and cytomegalovirus in ileocolonic mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2011;46:1324-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |