Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8452
Peer-review started: April 25, 2017
First decision: May 9, 2017
Revised: August 8, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: December 28, 2017
Processing time: 246 Days and 9.1 Hours
To assess intestinal barrier function during human intestinal ischemia and reperfusion (IR).
In a human experimental model, 6 cm of jejunum was selectively exposed to 30 min of ischemia (I) followed by 30 and 120 min of reperfusion (R). A sham procedure was also performed. Blood and tissue was sampled at all-time points. Functional barrier function was assessed using dual-sugar absorption tests with lactulose (L) and rhamnose (R). Plasma concentrations of citrulline, an amino acid described as marker for enterocyte function were measured as marker of metabolic enterocytes restoration. Damage to the epithelial lining was assessed by immunohistochemistry for tight junctions (TJs), by plasma marker for enterocytes damage (I-FABP) and analyzed by electron microscopy (EM) using lanthanum nitrate as an electrondense marker.
Plasma L/R ratio’s were significantly increased after 30 min of ischemia (30I) followed by 30 min of reperfusion (30R) compared to control (0.75 ± 0.10 vs 0.20 ± 0.09, P < 0.05). At 120 min of reperfusion (120R), ratio’s normalized (0.17 ± 0.06) and were not significantly different from control. Plasma levels of I-FABP correlated with plasma L/R ratios measured at the same time points (correlation: 0.467, P < 0.01). TJs staining shows distortion of staining at 30I. An intact lining of TJs was again observed at 30I120R. Electron microscopy analysis revealed disrupted TJs after 30I with paracellular leakage of lanthanum nitrate, which restored after 30I120R. Furthermore, citrulline concentrations closely paralleled the histological perturbations during intestinal IR.
This study directly correlates histological data with intestinal permeability tests, revealing that the human gut has the ability of to withstand short episodes of ischemia, with morphological and functional recovery of the intestinal barrier within 120 min of reperfusion.
Core tip: Using an unique experiment human intestinal ischemia and reperfusion (IR) model, this is the first study to directly correlate histological data with intestinal permeability tests. The results reveal the ability of the intestine to withstand short episodes of ischemia, with morphological and functional recovery of the intestinal barrier within 120 min of reperfusion. These results explain why there are often no signs of inflammation or bacterial translocation after short periods of intestinal ischemia. Exploration of the mechanisms responsible for this rapid recovery might impact understanding and treatment of intestinal diseases. Data from the dual-sugar absorption tests and citrulline reflect the histological perturbations during intestinal IR, highlighting the potential diagnostic value of these tests in patients with intestinal diseases associated with intestinal barrier loss.
- Citation: Schellekens DH, Hundscheid IH, Leenarts CA, Grootjans J, Lenaerts K, Buurman WA, Dejong CH, Derikx JP. Human small intestine is capable of restoring barrier function after short ischemic periods. World J Gastroenterol 2017; 23(48): 8452-8464
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8452.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8452
The intestinal epithelial lining is one of the largest sites of the human body exposed to the external environment and holds a dual function: the absorption of nutrients and water from the lumen and at the same time providing an effective barrier against potentially harmful compounds such as bacteria, toxins and antigens[1,2].
This is achieved by various lines of defense, including a physical barrier, formed by a mucus layer and a single rigid layer of enterocytes which are interconnected by tight junctions (TJs) and the subjacent adherens junctions (AJs)[3]. The TJs act as a fence, regulating the passive diffusion of solutes across the paracellular pathway[4]. They are composed of transmembrane proteins such as occludin and members of the claudin family, which are anchored to the cell cytoskeleton by zonula occludens proteins (ZO-1, ZO-2 and ZO-3)[5-8]. AJs are important for connecting neighbouring cells and consist, amongst others, of the transmembrane protein E-cadherin. Formation of AJs is required prior to the assembly of the TJs[9].
Loss of intestinal barrier function is considered to play a key role in the development and/or progression of intestinal inflammation and the onset of systemic inflammatory response syndrome, and is associated with intestinal diseases including celiac disease, inflammatory bowel disease (IBD) and intestinal ischemia-reperfusion (IR)[1,10]. Intestinal IR is a frequently occurring phenomenon associated with high morbidity and mortality. It is caused by various pathological conditions that involve a critical reduction of blood flow to the intestine, such as acute obstruction of mesenteric arterial blood flow and hypoperfusion associated with major vascular or abdominal surgical procedures, hemorrhagic shock, major trauma and sepsis[11-15].
Previous research of our group revealed the striking ability of the human small intestine to reduce and restore IR-induced epithelial lining damage. Using an experimental human intestinal IR model[16], it was observed that after periods of ischemia of up to 30 min, villous sloughing with epithelial lining disruption was rapidly followed by complete histological recovery during reperfusion[17,18]. The important remaining question however is whether functional barrier restoration with closure of paracellular permeability is achieved within this time span. This is in particular important as few human experimental studies exist that directly correlate barrier function to histological appearance. Here, we set out to study the relation between histological and functional barrier recovery using a human experimental small intestinal IR model. Damage to the epithelial lining was assessed by immunohistochemistry, plasma measurement of Intestinal Fattty Acid Binding Protein (I-FABP) and electron microscopy (EM). Functional intestinal barrier loss was evaluated using lanthanum nitrate as an electrondense marker together with the currently used gold standard for the measurement of intestinal permeability in clinical practice, the dual-sugar absorption tests (DST)[19]. These tests rely on the differential intestinal paracellular and cellular permeability of larger (lactulose) and smaller (L-rhamnose) molecules. Simultaneous measurement of lactulose and L-rhamnose are used as controls for gastric emptying, intestinal fluid volume, gastrointestinal transit time, and renal excretion which are thought to affect each molecule equally. The ratio of plasma concentrations reflects small intestinal permeability. Furthermore, plasma concentrations of citrulline, an amino acid produced by enterocytes that has been described as a marker for enterocyte mass/function[20], were measured as a marker of functional, metabolic enterocyte restoration following 30 minutes of ischemia.
The study was approved by the Medical Ethics Committee of the Maastricht University Medical Center (METC 06-3-044) and was conducted according to the revised version of the Declaration of Helsinki (October 2008, Seoul). Written informed consent of all patients was obtained.
Patients: 23 patients undergoing pancreatico-duodenectomy for benign or malignant disease were included in this study. DST data were obtained from 13 patients (10F:3M) with a median age of 69 (range 48 to 85) years included in the experimental human intestinal IR model of these 3 patients underwent a sham procedure; their intestinal barrier function was assessed using DST as described below without being exposed to ischemia. DST data were compared to histology from 10 other patients (3F:7M), with a median age of 63 (range 47 to 78) years undergoing the same human intestinal IR model without applying the DST.
Human intestinal IR protocol: The experimental protocol was performed as previously described[16]. In short, during pancreatico-duodenectomy, a variable length of jejunum is routinely resected as part of the surgical procedure. The terminal 6 cm of this jejunal segment was isolated and subjected to 30 min of ischemia by placing two atraumatic vascular clamps across the mesentery (n = 20 of which 10 patients were included in the DST-protocol (see below)). Meanwhile surgery proceeded as planned. After ischemia, one third (2 cm) of the isolated ischemic jejunum was resected using a linear cutting stapler (30I) (GIAtm, Covidien, Mansfield, MA). Next, clamps were removed to allow reperfusion, as confirmed by regaining of normal pink color and restoration of gut motility. Another segment of the isolated jejunum (2 cm) was resected similarly after 30 min of reperfusion (30I30R) and 120 min of reperfusion (30I120R). Simultaneously, 2 cm of jejunum which remained untreated during surgery was resected and served as internal control tissue. Tissue samples were immediately snap frozen or formalin fixed for future analysis.
DST protocol: To study the consequences of IR on intestinal barrier function loss and recovery, a bolus of 10 mL 0.9% NaCl containing the saccharides lactulose (1 mg/mL Lactulose, Centrafarm B.V. Etten-Leur, The Netherlands) and L-Rhamnose (0.5 mg/mL, Danisco Sweeteners, Copenhagen, Denmark) was directly injected into the lumen of the isolated part of intestine of 10 patients before the induction of ischemia. The piece of jejunum with the injection site was stapled off, to prevent any possible leakage of intraluminal content towards the abdominal cavity. After the first blood sample was obtained (5 min after injecting the saccharides), the human IR protocol was initiated (see detailed protocol above). The only difference with the regular IR protocol was that no tissue was resected during IR to eliminate potential confounding effects of absorptive surface reduction and decrease in luminal presence of the saccharide solution on the outcome parameters. Three patients underwent a sham procedure during which the saccharide solution was injected in the isolated jejunum segment, without this being exposed to IR. Blood from all patients was drawn, centrifuged, aliquoted and stored according to the regular intestinal IR protocol (as mentioned below) until further analysis for plasma saccharide concentrations. In addition, luminal debris from the isolated segment was sampled at the end of the IR protocol to measure the remaining saccharide concentration and compare this to the concentration at the beginning of the experiment.
Blood sampling: Arterial blood was sampled before ischemia, immediately on reperfusion (30I), and at 30 (30I30R) and 120 min (30I120R) after start of reperfusion. Simultaneous with each respective arterial blood sample, blood was drawn from the venule draining the isolated jejunal segment by direct puncture to assess concentration gradients across the isolated jejunal segment. All blood samples were centrifuged at 3500 rpm, 4 °C for 15 min to obtain plasma. Plasma was immediately stored in aliquots at -80 °C until analysis.
Arterial and venous plasma concentrations of lactulose and L-rhamnose were measured using High Performance Liquid Chromatography combined with Mass Spectrometry (HPLC-MS). In brief, sugars were separated using ion-exchange chromatography as described previously[21]. After separation, the column effluent was mixed with an ammonia containing solvent, which enabled the electrospray ionisation to ammonia adducts. Detection, based on the mass to charge value (m/z value) of the ammonia adducts was then performed using mass spectrometry in positive mode. Arterial-venous concentrations differences and lactulose/L-rhamnose ratios (L/R-ratio) were calculated to investigate intestinal barrier function during IR.
Histology: Tissue specimens obtained during the experimental protocol were immediately immersed in 4% formaldehyde fixative (Unifix, Klinipath, Duiven, the Netherlands) and incubated overnight at room temperature. Next, tissue samples were embedded in paraffin and 4 μm sections were cut. Sections were deparaffinized in xylene and rehydrated in graded ethanol to distilled water and stained with haematoxylin and eosin (H&E).
Immunofluorescence: Cryostat sections (4 μm) were cut and stained for ZO-1, occludin and E-cadherin. Briefly, slides were dried, fixed in 10% trichloroacetic acid for 15 min and permeabilized using 30 mmol/L glycine and 1% triton X-100 in phosphate buffered saline (PBS). Non-specific antibody binding was blocked using 30 mmol/L glycine and 3% Fetal Calf Serum in PBS for one hour. Next, slides were incubated with mouse anti-human ZO-1, rabbit anti human occludin (both Invitrogen, Eugene, OR, United States) or mouse anti human E-cadherin (Abcam, Cambridge, United Kingdom). After washing, slides were incubated with goat anti-mouse Alexa 488, goat anti-rabbit CY3, or goat anti-mouse CY3 (all Invitrogen) secondary antibodies, followed by incubation with 4’,6-diamino-2-phenylindole dihydrochloride (DAPI). Next, slides were washed and mounted in VECTASHIELD® Mounting Media (Vector Laboratories, Burlingame, CA, United States) and visualized with an immunofluorescence microscope.
Electron microscopy: For electron microscopy (EM), jejunal tissue was directly immersed in 2.5% glutaraldehyde and 2% paraformaldehyde fixative buffered in 0.1M cacodylate buffer at a pH of 7.4 for at least 3 d. Samples were then washed overnight in 0.1 mol/L cacodylate buffer with 7.5% sucrose and postfixed for 1 h at 4 °C in 1% osmium tetroxide, containing 1% lanthanum nitrate buffered to pH 7.4 with 0.1 mol/L cacodylate. After washing in cacodylate buffer containing 7% sucrose at pH 7.4, dehydration was carried out rapidly in graded ethanol series followed by routine embedding in Epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate. A Philips CM 100 electron microscope was used to visualize the ultrastructure of the intestinal epithelial lining and the distribution of lanthanum nitrate particles. The presence of lanthanum nitrate inside the paracellular space of two adjacent enterocytes is associated with TJ and AJ function loss.
Measurement of intestinal mucosal cell damage: Damage to the epithelial lining was quantified using plasma levels of Intestinal fatty acid–binding protein (I-FABP). I-FABPs are small (14 kDa) cytosolic proteins specifically present in mature enterocytes at the tip of the villus[22]. They are released upon enterocyte membrane integrity loss into the circulation, which makes them useful as markes for enterocyte damage[23]. I-FABP-levels were measured in both arterial and venous plasma samples, to allow for calculation of arteriovenous (V-A) concentration differences, by means of an in-house enzyme-linked immunosorbent assay (ELISA). This assay was developed to measure I-FABP in human plasma samples with rabbit polyclonal antibodies, using purified human I-FABP as standard (detection window: 12.5 to 800 pg/mL). Samples were run in duplicate, and a variability of 5% between sample duplicates was accepted.
To study the metabolic activity of enterocytes following 30 min of ischemia and subsequent reperfusion, arterial and venous plasma concentrations of citrulline and glutamine were measured using HPLC (Model PU-1980 pump, Jasco Easton, MD, United States) combined with MS (Model LTQ XL, Thermo Fisher Scientific, Waltham, MA, United States) as previously described[24]. A 100 μL plasma aliquot was pipetted into a 1.5 mL Eppendorf tube that already contained 5.5 mg solid sulfosalicylic acid (SSA), vortex-mixed immediately to deproteinize the plasma samples, snap frozen in liquid nitrogen and stored at -80 °C until analysis.
Before analysis, deproteinized plasma samples were thawed, vortex-mixed and centrifuged for 10 min at 50000 × g at 4 °C in a Biofuge Stratos centrifuge (Heraeus, Haarlem, the Netherlands). Next, 5 μL of the clear supernatant was diluted 100-fold in ice-cold water into a 1 mL WISP-style vial (Waters, Etten-Leur, the Netherlands). Amino acid analysis was performed by HPLC after automated pre-column derivatization using ophthaldialdehyde (OPA). At the start of each cycle, 5 μL of the diluted sample, stored in the pre-chilled sample compartment of a WISP autosampler, was automatically mixed with 5 μL of OPA reagent. The resulting OPA-amino acid derivatives were separated on a 150 mm × 4.6 mm (inner diameter) Allsphere ODS 2 3 μmol/L High-Performance Liquid Chromatography (HPLC) column (Grace, Breda, the Netherlands), using an acetonitrile gradient against an aqueous citric acid buffer (25 mmol/L, pH = 6.8) and detected by fluorescence (330 nm excitation, 440 nm emission). Arterial-venous concentration differences of citrulline divided by the arterial concentration of glutamine (Cit V-A/Gln A) reflect enterocyte functional and metabolic capacity.
Statistical analysis was performed using GraphPad Prism 5.0 for Windows (GraphPad Software Inc. San Diego, CA, United States) and SPSS 23.0 (SPSS, Inc., Chicago, IL, United States). Normality was verified by using the Kolmogorov-Smirnov test. None of the parameters showed a normal distribution. A Dunn multiple comparison test was used (after significant one-way analysis of variance) to compare DST values and amino acid ratios over time. For between-group comparisons, the Mann Whitney U test was used. All data are expressed as mean ± SE.
Linear mixed model regression was used to analyze correlations over time between plasma I-FABP and plasma L/R ratio. Within-person correlations were computed by normalizing both data sets. This enables the assessment of the pure association of both variables by calculating the correlation coefficient. Linear regression was used to visualize the correlation. A P-value below 0.05 was considered statistically significant.
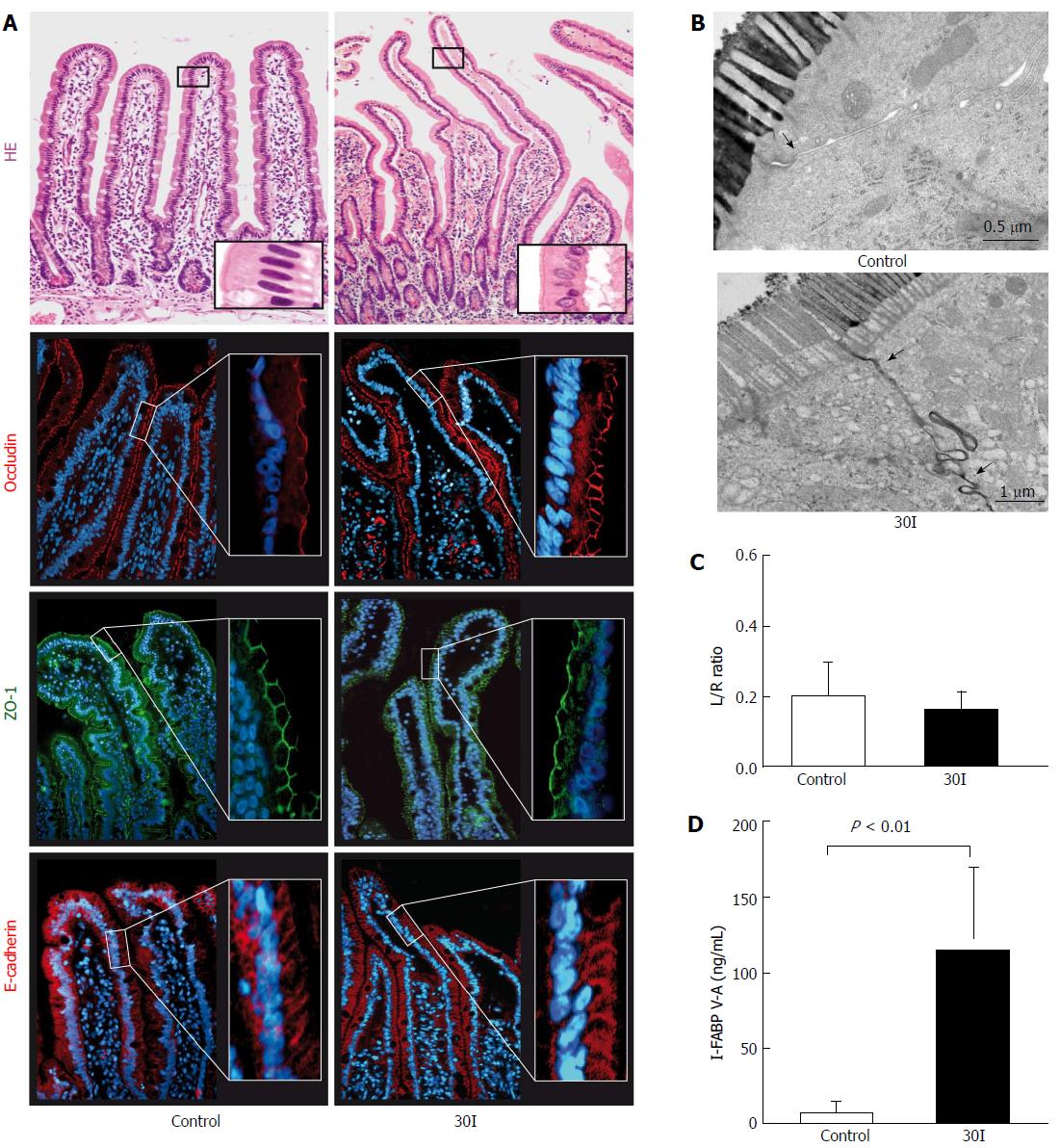
As described previously, the epithelial lining was microscopically intact directly after 30 min ischemia (30I) (Figure 1A, right panel)[18]. Immunofluorescence staining of ZO-1, occludin and E-cadherin revealed structured ZO-1 and occludin staining at the apical pole of the epithelial cells and evenly distributed E-cadherin between the epithelial cells over the tips of the villi in both control tissue and jejunum after an ischemic period of 30 min (Figure 1A). To assess whether the ischemia-induced enterocyte damage resulted in increased intestinal permeability, the plasma Lactulose/L-rhamnose (L/R) ratio was determined. No statistically significant changes were observed in the plasma L/R ratio directly after 30I compared to control (0.16 ± 0.05 vs 0.20 ± 0.09, P = 0.80; Figure 1C) or sham-operated patients (0.10 ± 0.07 vs 0.20 ± 0.09, P = 0.72; supplementary Figure 1). These data suggest that a short period of ischemia alone does not result in increased intestinal permeabi lity. EM images of two adjacent enterocytes with microvilli showed that in control tissue the interconnecting TJs were intact (Figure 1B left panel, arrowhead) and the contrast dye lanthanum remains intraluminally. However, in contrast to the histological analysis, as shown by EM after 30I, lanthanum was able to penetrate the paracellular space, indicating intestinal barrier integrity loss (Figure 1B, right panel, arrowheads).
Enterocyte damage was quantified by measuring arteriovenous I-FABP concentration differences across the studied jejunum. I-FABP arteriovenous concentration differences significantly increased from 1.75 ng/mL ± 0.78 ng/mL before ischemia to 126.6 ng/mL ± 65.53 ng/mL on reperfusion (P < 0.01; Figure 1D).
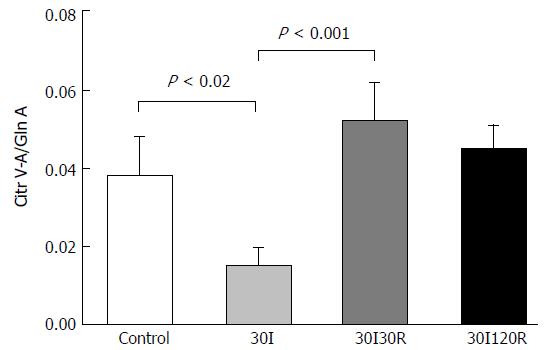
The arterial-venous concentration differences of citrulline divided by the arterial concentration of glutamine (Cit V-A/Gln-A) ratio in 10 patients at control was 0.04 ± 0.009. During the ischemic period, a significant decline in the Cit V-A/Gln-A ratio was observed (0.02 ± 0.004, P < 0.02 compared to control, Figure 2).
This decreased plasma Cit-V-A/Gln-A ratio was the result of a decline in venous citrulline concentrations after ischemia (see Supplementary Table 1).
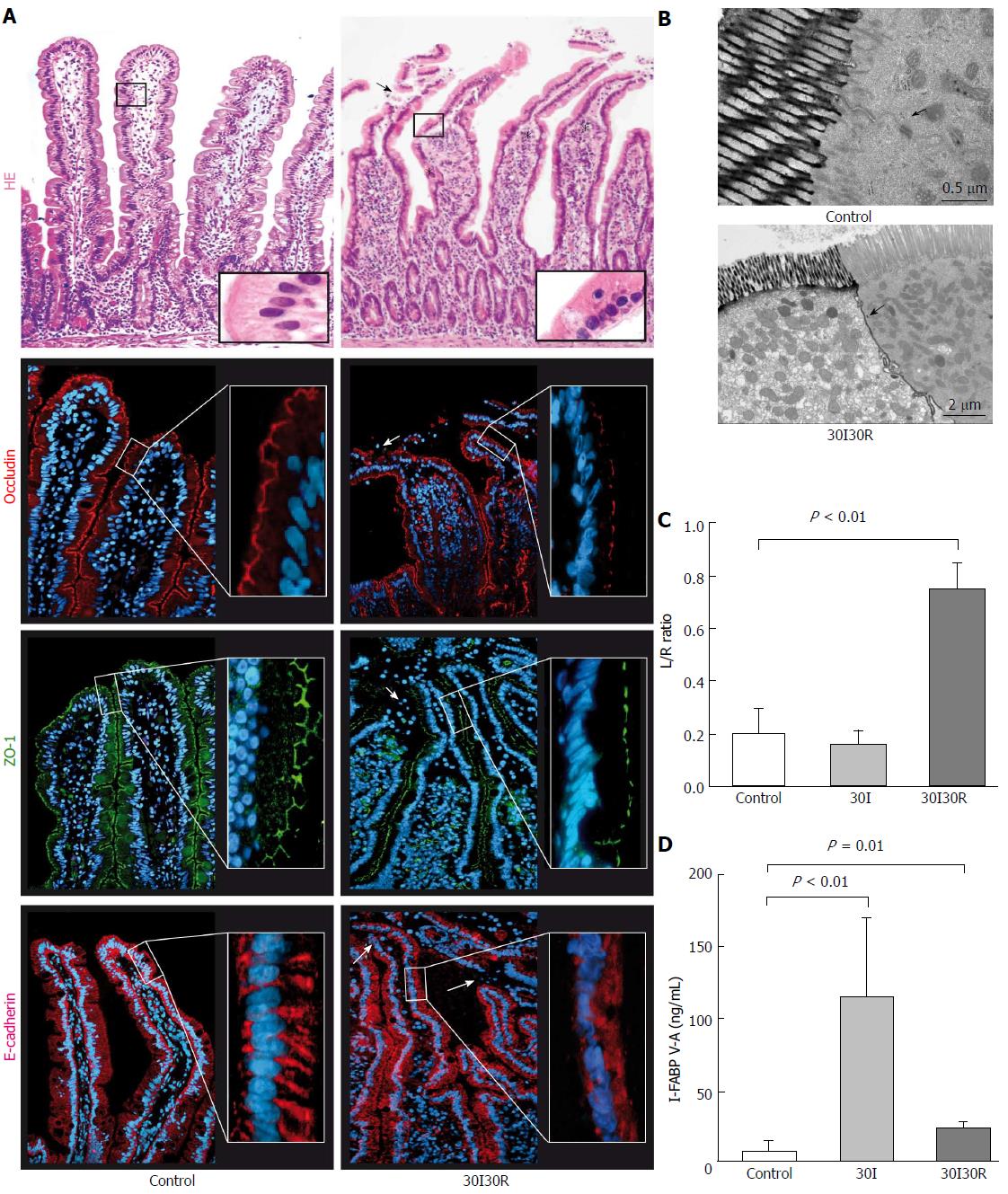
After 30 min of ischemia followed by 30 min of reperfusion (30I30R), shedding of IR-damaged enterocytes from the villus tips towards the intestinal lumen was observed (Figure 3A, right panel, arrowhead). Short reperfusion led to compromised TJ integrity as immunofluorescence for ZO-1 and occludin showed an irregular staining pattern and loss of protein expression in the epithelial sheets that had lost contact with the basal membrane at the tips of the villi (Figure 3A, right panels, arrowheads). This was accompanied by disruption of E-cadherin with diffuse staining in the cell cytoplasm across the villi (Figure 3A, right panel, arrowheads). The plasma L/R ratio significantly increased after 30I30R compared to control (0.75 ± 0.10 vs 0.20 ± 0.09, P < 0.01; Figure 3C). Analysis also showed an increase of the plasma L/R ratio after 30I30R compared to sham (0.75 ± 0.10 vs 0.04 ± 0.01, P < 0.02; supplementary Figure 1). EM analysis revealed that lanthanum was still able to penetrate between two adjacent enterocytes demonstrating disruption of TJs and AJs after 30I30R (Figure 3B). These results show that the intestinal barrier function gets compromised during the early stages of reperfusion after a short ischemic hit.
I-FABP V-A concentrations declined after 30I30R but were still significantly increased compared to control (27.06 ± 2.73 ng/mL vs 1.75 ± 0.78 ng/mL, P = 0.01; Figure 3D), still indicating loss of enterocyte membrane integrity during the early reperfusion phase.
The Cit V-A/Gln A ratio however, was increased after a short period of 30 minutes of reperfusion when compared with 30 min of ischemia (0.05 ± 0.09 vs 0.02 ± 0.004, P < 0.001, (Figure 2) and was no longer significantly different compared with control. This may indicate that the remaining enterocytes are already viable despite the compromised intestinal barrier.
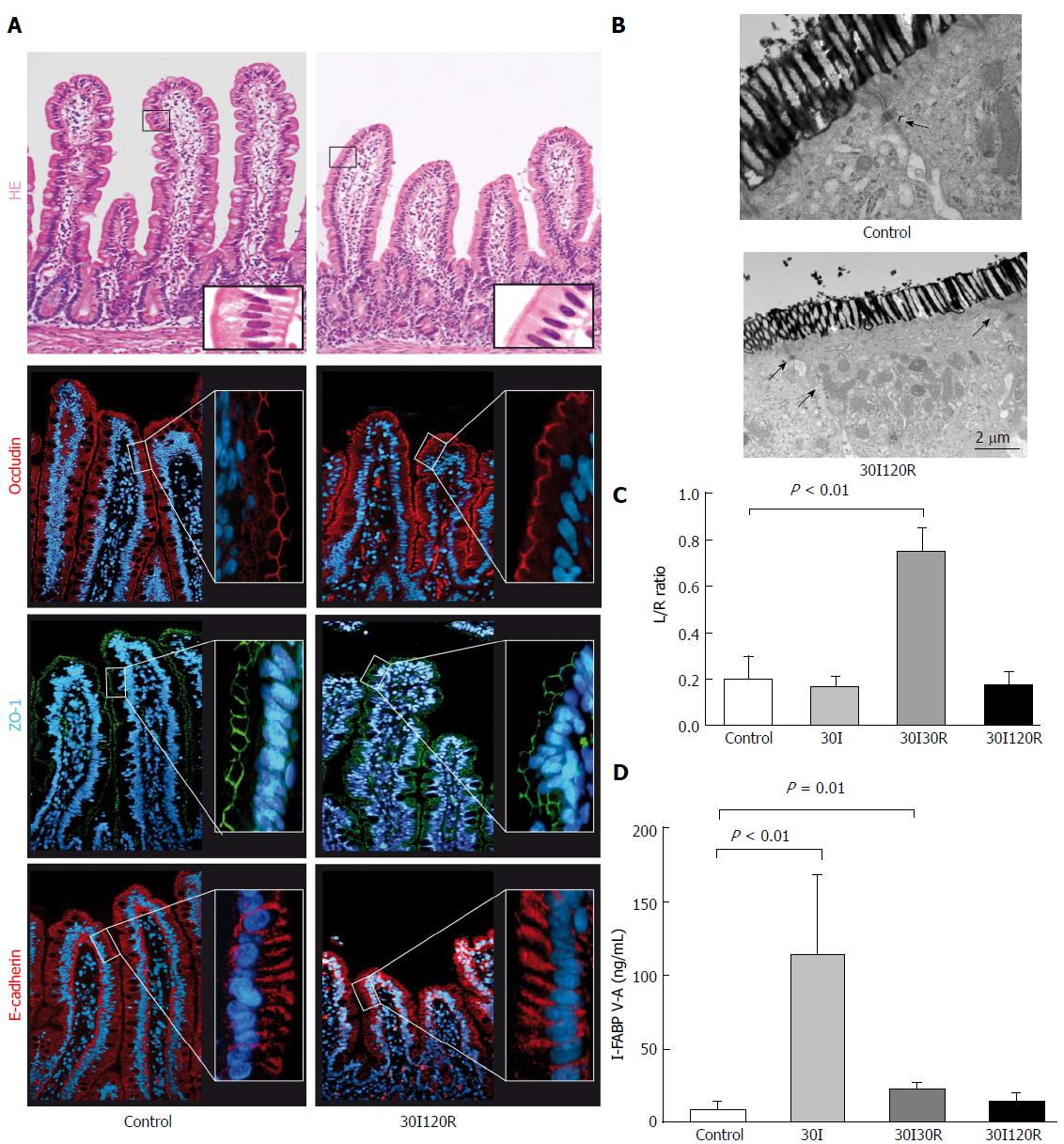
After 30I120R, shedding of IR-damaged cells led to shortening of the villi, with an intact epithelial lining (Figure 4A, right panel). Immunofluorescence showed that both ZO-1 and occludin again formed a continuous staining pattern around the epithelial lining near the apical region of the enterocytes. In addition, E-cadherin staining appeared normal and was visible along the entire epithelium of each villus, rebuilding epithelial cell-cell integrity (Figure 4A, right panel). In line, the L/R ratio normalized at 30I120R to 0.17 ± 0.06 and was not significantly different from control or sham (Figure 4C). In EM images, lanthanum was no longer present in paracellular spaces after 30I120R (Figure 4B, right panel) indicating restored tight junction integrity.
I-FABP levels rapidly decreased over the course of a period of 120 min reperfusion to 14.77 ng/mL ± 5.46 ng/mL and were no longer significantly elevated compared to control, demonstrating rapid and full recovery of epithelial membrane integrity (Figure 4D).
Remarkably, at 120 min of reperfusion the Cit V-A/Gln A ratio no longer differed from the value measured at control (Figure 2). The results demonstrate that, despite IR-induced reduction of enterocyte mass, the remaining epithelial cells are functional again after a short period of ischemia followed by 120 min of reperfusion
To rule out the possibility that the low concentrations of saccharides measured at later time points were due to significantly decreased intraluminal saccharides as a consequence of uptake/leakage during early ischemia and reperfusion, we measured intraluminal concentrations of saccharides at 30I120R. Although the concentrations measured at 120R were approximately 3 times lower than the administered stock (29.16 ± 0.31 vs 11.47 ± 1.01 P < 0.002) and L-rhamnose (30.62 ± 1.80 vs 8.22 ± 1.30 P < 0.002, Supplementary Figure 2), the luminal concentrations of both sugars were still 100x higher than the observed plasma lactulose and L-rhamnose concentrations at 30I120R.
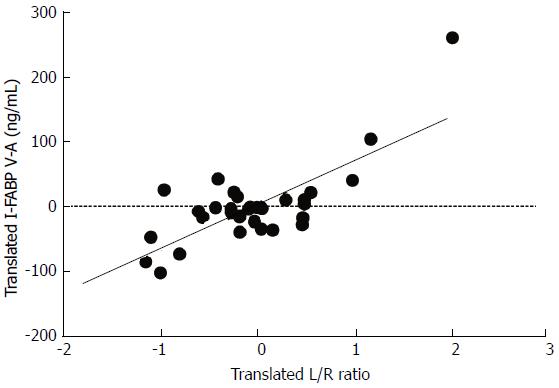
The IR-induced damage to the epithelial lining was measured using plasma I-FABP. This sensitive plasma parameter of enterocyte damage is rapidly released upon intestinal ischemia. This allows us to relate the structural damage to the mucosa with the permeability changes during intestinal IR. To this end within-person correlations were studied between arteriovenous concentration differences of plasma I-FABP and L/R ratios. Plasma levels of I-FABP correlated with plasma L/R ratio’s measured at the same time points [correlation: 0.467, (P < 0.01)], indicating a relationship between structural damage to the intestinal mucosa and the observed changes in permeability (Figure 5).
The current study demonstrated that the human intestinal epithelial barrier has the remarkable capacity to rapidly and functionally restore IR-induced tissue damage within 120 min of reperfusion. Second, we show in this study that the currently used golden standard to measure intestinal epithelial permeability i.e. the dual sugar test, reflects intestinal barrier function as objectified by electron microscopy in our model of acute intestinal damage and repair. These results are important for our understanding of the pathophysiology of human intestinal ischemia-reperfusion in general, and have implications for clinical practice. There is increasing evidence that the loss of intestinal barrier function after a period of ischemia or hypoperfusion is associated with the development of sepsis and multiple organ dysfunction in the acute setting, including trauma, hemorrhagic shock, following major (vascular) surgery or acute pancreatitis[2,25]. Furthermore, exposure of the intestinal epithelium to periods of ischemia followed by reperfusion results in sloughing of enterocytes at the tips of villi causing a breach in the gut barrier[12,26,27]. Disruption of epithelial integrity is associated with intestinal inflammation, bacterial translocation, the development of sepsis and multiple organ dysfunction[4,28,29]. In addition, loss of intestinal barrier function plays a role in the pathophysiology of various intestinal diseases including IBD and celiac disease[3,30]. Epithelial hypoxia can occur secondary to the inflammation, and might play a role in the pathophysiology[31] and etiology[32] of IBD.
The intestinal barrier function is dependent on an intact epithelial lining which is formed by epithelial cells, interconnected by cell to cell adhesion proteins at different levels of the intercellular junction[3]. TJ complexes consist of transmembrane proteins, including occludin and claudins linked to the cell cytoskeleton by intracellular proteins, such as ZO-1. The TJ barrier controls the paracellular pathway via at least two routes allowing selective transport across the intestinal barrier. The pore pathway is a size- and charge-selective route for ions which is regulated by claudins. The leak pathway allows paracellular transport of large molecules, including proteins and bacterial lipopolysaccharides. Studies have shown that occludin and ZO-1 are mainly involved in the regulation of the leak pathway. Mainly defects in the leak pathway lead to increased epithelial permeability allowing leakage of bacterial products and antigens from the lumen into the systemic circulation[4,8].
At 30 min of ischemia, ZO-1 and occludin appeared as continuous bands along the villous tips, similar as the immunofluorescent staining of control tissue. In line, the DST showed that the intestinal barrier was functionally still intact. Similar to the human setting animal studies have shown that the appearances of sub epithelial spaces during ischemia are not accompanied by increased permeability for lactulose[33].
In contrast, shortly after reperfusion desquamation of the intestinal epithelium at the tips of the villi occurred. Although the gut possesses elegant pathways that maintain the intestinal barrier during cell shedding[34,35], excessive rates of apoptosis make these pathways to fall short resulting in intestinal barrier loss[26,36]. Indeed, TJ derangements were observed and accompanied by increased L/R ratios.
These human data are in line with previous data from animal models demonstrating that the magnitude of ischemia and reperfusion-induced intestinal mucosal damage is a result of the duration of occlusion time. This was evaluated in standardized animal models of complete segmental arterial ischemia demonstrating that 30 min of ischemia followed by 30 min of reperfusion results in massive epithelial lifting with a few denuded villi[37]. Moreover, the mechanism of intestinal barrier repair during intestinal IR is also previously described[26]. This is a highly regulated event involving villus contraction, epithelial restitution and closure of the paracellular space. The latter is considered to account for the majority of barrier recovery after intestinal injury. The net result of the above mentioned repair mechanisms is a remarkably rapid closure of mucosal wounds in the mammalian intestinal epithelial lining to prevent the onset of sepsis. The observed time scale of intestinal barrier restoration within hours after ischemia, is also in agreement with previous studies[26].
To study if the intestinal barrier is indeed functionally repaired, it is necessary to be able to actively and reliably measure intestinal barrier function. However, this has been a major challenge in clinical practice. Classically, DST are used based on the differential absorption of intraluminal sugars of different molecular weight across the intestinal barrier. Using these tests it has been shown that intestinal barrier function is involved in intestinal diseases including patients with IBD and celiac disease[38,39]. However, a pitfall of the DST is the lack of knowledge on how these tests correlate to epithelial cell damage and tight junction loss in vivo. In intestinal IR, loss of intestinal barrier function is seen as key explanation of intestinal dysfunction in patients leading to increased incidence of bacteria and toxin translocation from the intestinal lumen to the systemic circulation, causing infectious complications including sepsis and the multiple organ failure syndrome[28]. Using our in vivo human model, we were able to demonstrate the direct relationship between morphological barrier loss (epithelial cells and TJ proteins) and functional intestinal barrier loss (dual-sugar absorption test and leakage of lanthanum nitrate through defective tight junction complexes as visualized by EM).
I-FABP concentrations and release-patterns were comparable with previous published data from our group[23]. This shows that 30 min of ischemia is associated with rapid reversal of IR-induced structural tissue damage of the epithelial lining. Interestingly, the extent of intestinal damage, reflected by an increase in plasma I-FABP, significantly correlated with the permeability changes in the small intestine. This is in line with previous studies performed in humans undergoing splanchnic hypoperfusion because of moderate-to-high intensity exercise demonstrating that intestinal cell damage result in permeability changes including restoration of the epithelial lining[40].
Our results showed that the DST data closely reflect the morphological findings during intestinal IR. This is of importance as it supports the usefulness of DST[10,41,42].
In addition, we provide support for the use of citrulline as a marker for enterocyte function. Measurement of gastrointestinal dysfunction, following small bowel diseases, is hard to establish[43]. Citrulline has been used for this purpose as it is an amino acid mainly produced by small intestinal enterocytes by the conversion of glutamine through the glutamate to ornithine pathway, without incorporation into proteins[44]. Loss of the intestinal epithelial lining has been shown to result in declined circulating levels of citrulline[45]. Several studies showed that there is a link between low plasma citrulline concentration and intestinal barrier function[46-48]. Supply of glutamine to the enterocyte occurs from both arterial blood as well as from the intestinal lumen. As all patients were fasted, we hypothesized that the arterial-venous concentrations of citrulline divided by the arterial concentration of glutamine (Cit V-A/Gln A) ratios reflect enterocyte functional capacity. It is striking that the DST is at baseline level at 30I and only increased at 30I30R, whereas the Citr/Gln ratio is only decreased at the 30I time point and not during reperfusion. This discrepancy is in agreement with previous studies in patients with chemotherapy-induced mucositis and patients suffering from IBD, where permeability was not correlated with changes in plasma citrulline concentrations[49,50]. This may indicate that enterocytes do not require an intact intestinal barrier to be metabolically viable. Citrulline performed better as a marker for functional epithelial cell mass in these studies where it detected impairment of intestinal epithelium and small intestinal barrier integrity earlier compared with the DST and indicated recovery more accurate.
Potential limitations of this study may include the fact that the intraluminal concentration of lactulose and L-rhamnose show a decrease during the IR protocol. This drop in concentration however, is only partly relevant since the remaining concentrations in the lumen were still 100x higher than the observed plasma lactulose and L-rhamnose concentrations at 30I120R. Furthermore the hyperosmolarity of the saccharide solution may affect the intestinal permeability for L-rhamnose directing the flux towards the intestinal lumen. To correct for this diffusion, the experimental IR protocol started 5 min after injection of the saccharides. In addition, dual-sugar absorption tests can be influenced by the presence of food-derived sugars in plasma and most of the studies of intestinal permeability do not report baseline excretion of the saccharides, which is perhaps most relevant with the use of DST. As baseline measurements were taken in the current study, this should have been accounted for.
Next, the larger part of the total paracellular conductance of intestinal epithelial lining is via a high linear density of TJs residing in the crypts[4]. After villus contraction, caused by the IR injury, crypt epithelium accounts for the majority of surface area remaining. As we did not focus on the role of the crypts in this study, the latter could be of influence on the results. Future studies, measuring mucosal barrier function within the crypts are warranted to investigated villus/crypt differences during IR injury.
Also, one should be careful generalizing the current findings to the whole length of the human gastro-intestinal tract. First, it is important to understand that epithelial permeability of the gastrointestinal tract needs to be evaluated in a site specific manner. Several saccharide probes are destroyed by digestion processes that take place in the lumen of different parts of the gut, which limits their capabilities to detect permeability changes throughout the hole intestinal tract. For example lactulose and L-rhamnose are destroyed in the caecum and therefore provide only information regarding the small intestinal epithelium[3]. Also the expression of epithelial tight junction proteins is region-specific along the gastrointestinal tract, which determine the properties of permeability in different regions. The proximal segments of the jejunum have a higher permeability than the distal ileum segments. Next, Takeyoshi et al[51]. evaluated the mucosal regeneration of different parts of the small intestine during transplantation in dogs. They showed that the regenerative capacity was twice as fast in the jejunum than in the ileum. This more pronounced recovery in jejunal tissue could have a beneficial effect on our data.
Last, important to note is that the dual-sugar tests have not yet gained a place in everyday practice for diagnosis and follow up of the several patients groups, mainly because the detection methods are complex and not widely available. Taken together, these findings reflect the ability of the intestine to withstand short episodes of ischemia, with morphological and functional recovery of the intestinal barrier within 120 minutes of reperfusion. These results explain why there are often no signs of inflammation or bacterial translocation after short periods of intestinal ischemia. Further exploration of the mechanisms responsible for this rapid morphological and functional recovery might impact treatment of intestinal diseases associated with barrier recovery loss. Next, data from the DST and citrulline glutamine ratios closely reflect the histological perturbations during intestinal IR highlighting the potential diagnostic value of these tests in the follow-up of patients with intestinal disease associated with intestinal barrier loss.
Human small intestine is frequently exposed to ischemia without severe complications. Intestinal hypoxia and loss of intestinal barrier function are associated with the onset of sepsis and multiorgan failure or intestinal diseases including celiac disease and inflammatory bowel disease. Rapid morphological recovery occurs in human ischemia/reperfusion-exposed small intestine by a zipper like constriction of the epithelium.
Patients with intestinal ischemia may suffer from sepsis, however we showed in previous human studies that short periods of ischemia led to fast structural recovery of damaged mucosa. Animal studies suggest that this is accompanied by barrier function recovery. In humans, we still have to elucidate whether structural ischemic damage and recovery correlates with barrier function. This is of importance, since complete knowledge of pathophysiological sequelae of human intestinal ischemia-reperfusion will help us to guide new therapeutic options and develop better diagnostic possibilities for the patients suffering from intestinal ischemia.
The author’s results show that short periods of human intestinal ischemia are followed by mucosal damage, which is rapidly followed by herstel. Most important, we add to this knowledge that the gut has the remarkable capacity of functional recovery after short periods of ischemia. Furthermore, our results indicate that the dual-sugar absorption tests and plasma citrulline may have a potential diagnostic value in detecting and monitoring patients with intestinal diseases associated with intestinal barrier loss.
There is great need for better insight in the pathophysiology of small intestinal IR, because of the high morbidity and mortality rates, while preventive and/or therapeutic approaches are lacking. Results of this study will add important knowledge to our understanding of the pathophysiology of intestinal ischemia. This will also help to early detect intestinal ischemia and its serious consequences. Next it will help open new opportunities to develop therapeutic interventions.
Intestinal ischemia-reperfusion: Pathological condition characterized by an initial undersupply of blood to the intestine followed by a restoration of perfusion and reoxygenation Reperfusion and reoxygenation is commonly associated with an exacerbation of tissue injury and a profound inflammatory response. This often leads to systemic inflammatory response syndrome (SIRS), sepsis and multiple organ failure (MOF), causing the high morbidity and mortality rates associated with intestinal IR. Intestinal barrier: The protective component of the intestine shielding us against the invasion of bacteria toxins and antigens, but on the other hand enabling us to absorb nutrients
The study is well designed and sound, the manuscript is interesting. Maintenance of the intestinal barrier is an important defense against invasion of luminal pathogens, and assessment of barrier function is relevant in a number of diseases of the gut where the barrier is compromised. The paper presents some interesting in vivo data showing the ability of the jejunal epithelium to recover rapidly following ischemic injury. Overall, the study is well designed and sound.
This is a straight-forward paper associating results from lactulose/rhamnose ratios with histology and microscopic observations of the intestine following ischemia reperfusion. Much of what is reported has been demonstrated in animal models, but not before in human tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: the Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Boros M, Capasso R, Danielsen EM, Singer SM S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
| 1. | DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 463] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2707] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 5. | Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1851] [Cited by in RCA: 1903] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 6. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 1061] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 7. | Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA. 2010;107:8237-8241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 659] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 9. | Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 711] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 10. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 630] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Derikx JP, Poeze M, van Bijnen AA, Buurman WA, Heineman E. Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock. 2007;28:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Hanssen SJ, Derikx JP, Vermeulen Windsant IC, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | de Haan JJ, Lubbers T, Derikx JP, Relja B, Henrich D, Greve JW, Marzi I, Buurman WA. Rapid development of intestinal cell damage following severe trauma: a prospective observational cohort study. Crit Care. 2009;13:R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Derikx JP, van Waardenburg DA, Thuijls G, Willigers HM, Koenraads M, van Bijnen AA, Heineman E, Poeze M, Ambergen T, van Ooij A. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PLoS One. 2008;3:e3954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Derikx JP, Matthijsen RA, de Bruïne AP, van Dam RM, Buurman WA, Dejong CH. A new model to study intestinal ischemia-reperfusion damage in man. J Surg Res. 2011;166:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, Heineman E, van Dam RM, Dejong CH, Buurman WA. Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation. PLoS One. 2008;3:e3428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Grootjans J, Thuijls G, Derikx JP, van Dam RM, Dejong CH, Buurman WA. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol. 2011;224:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Fink MP. Interpreting dual-sugar absorption studies in critically ill patients: what are the implications of apparent increases in intestinal permeability to hydrophilic solutes? Intensive Care Med. 1997;23:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | van Wijck K, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Novel analytical approach to a multi-sugar whole gut permeability assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2794-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Derikx JP, Schellekens DH, Acosta S. Serological markers for human intestinal ischemia: A systematic review. Best Pract Res Clin Gastroenterol. 2017;31:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Schellekens DH, Grootjans J, Dello SA, van Bijnen AA, van Dam RM, Dejong CH, Derikx JP, Buurman WA. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | van Eijk HM, Rooyakkers DR, Deutz NE. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr. 1993;620:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1218] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 26. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 27. | American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000;118:951-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Fukatsu K, Sakamoto S, Hara E, Ueno C, Maeshima Y, Matsumoto I, Mochizuki H, Hiraide H. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after severe surgical insults. Crit Care Med. 2006;34:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1682] [Cited by in RCA: 2137] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 31. | Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 32. | Ibrahim CB, Aroniadis OC, Brandt LJ. On the role of ischemia in the pathogenesis of IBD: a review. Inflamm Bowel Dis. 2010;16:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Khanna A, Rossman JE, Fung HL, Caty MG. Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J Surg Res. 2001;99:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 34. | Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 624] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 35. | Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology. 2011;140:1208-1218.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 36. | Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 37. | Boros M, Takaichi S, Hatanaka K. Ischemic time-dependent microvascular changes and reperfusion injury in the rat small intestine. J Surg Res. 1995;59:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn’s disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Mauriño E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | van Wijck K, Lenaerts K, van Loon LJ, Peters WH, Buurman WA, Dejong CH. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One. 2011;6:e22366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 41. | Sequeira IR, Lentle RG, Kruger MC, Hurst RD. Standardising the lactulose mannitol test of gut permeability to minimise error and promote comparability. PLoS One. 2014;9:e99256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | van Wijck K, Bessems BA, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Polyethylene glycol versus dual sugar assay for gastrointestinal permeability analysis: is it time to choose? Clin Exp Gastroenterol. 2012;5:139-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Peters JH, Wierdsma NJ, Teerlink T, van Leeuwen PA, Mulder CJ, van Bodegraven AA. The citrulline generation test: proposal for a new enterocyte function test. Aliment Pharmacol Ther. 2008;27:1300-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids. 2005;29:177-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 389] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 45. | Piton G, Capellier G. Plasma citrulline in the critically ill: intriguing biomarker, cautious interpretation. Crit Care. 2015;19:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Herbers AH, Blijlevens NM, Donnelly JP, de Witte TJ. Bacteraemia coincides with low citrulline concentrations after high-dose melphalan in autologous HSCT recipients. Bone Marrow Transplant. 2008;42:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Piton G, Belon F, Cypriani B, Regnard J, Puyraveau M, Manzon C, Navellou JC, Capellier G. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Blijlevens NM, Lutgens LC, Schattenberg AV, Donnelly JP. Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant. 2004;34:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Papadia C, Sherwood RA, Kalantzis C, Wallis K, Volta U, Fiorini E, Forbes A. Plasma citrulline concentration: a reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol. 2007;102:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer. 2005;103:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Takeyoshi I, Zhang S, Nomoto M, Zhu Y, Kokudo Y, Suzuki T, Hamada N, Nemoto A, Starzl TE, Todo S. Mucosal damage and recovery of the intestine after prolonged preservation and transplantation in dogs. Transplantation. 2001;71:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |