Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8207
Peer-review started: September 10, 2017
First decision: September 20, 2017
Revised: October 13, 2017
Accepted: November 7, 2017
Article in press: November 7, 2017
Published online: December 14, 2017
Processing time: 93 Days and 1.9 Hours
To establish a classification method for differential diagnosis of colorectal ulcerative diseases, especially Crohn’s disease (CD), primary intestinal lymphoma (PIL) and intestinal tuberculosis (ITB).
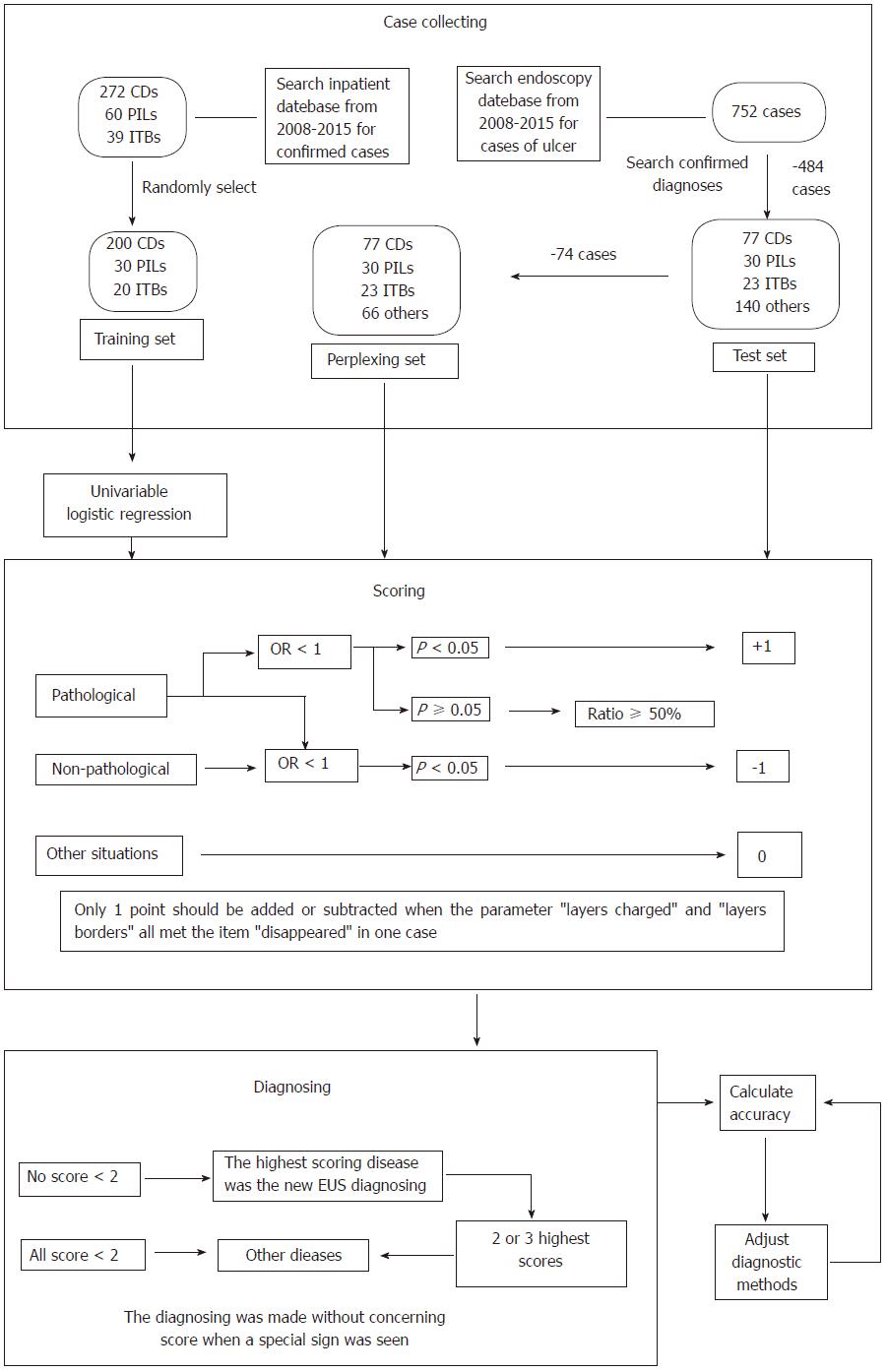
We searched the in-patient medical record database for confirmed cases of CD, PIL and ITB from 2008 to 2015 at our center, collected data on endoscopic ultrasound (EUS) from randomly-chosen patients who formed the training set, conducted univariate logistic regression analysis to summarize EUS features of CD, PIL and ITB, and created a diagnostic classification method. All cases found to have colorectal ulcers using EUS were obtained from the endoscopy database and formed the test set. We then removed the cases which were easily diagnosed, and the remaining cases formed the perplexing test set. We re-diagnosed the cases in the three sets using the classification method, determined EUS diagnostic accuracies, and adjusted the classification accordingly. Finally, the re-diagnosing and accuracy-calculating steps were repeated.
In total, 272 CD, 60 PIL and 39 ITB cases were diagnosed from 2008 to 2015 based on the in-patient database, and 200 CD, 30 PIL and 20 ITB cases were randomly chosen to form the training set. The EUS features were summarized as follows: CD: Thickened submucosa with a slightly high echo level and visible layer; PIL: Absent layer and diffuse hypoechoic mass; and ITB: Thickened mucosa with a high or slightly high echo level and visible layer. The test set consisted of 77 CD, 30 PIL, 23 ITB and 140 cases of other diseases obtained from the endoscopy database. Seventy-four cases were excluded to form the perplexing test set. After adjustment of the classification, EUS diagnostic accuracies for CD, PIL and ITB were 83.6% (209/250), 97.2% (243/250) and 85.6% (214/250) in the training set, were 89.3% (241/270), 97.8% (264/270) and 84.1% (227/270) in the test set, and were 86.7% (170/196), 98.0% (192/196) and 85.2% (167/196) in the perplexing set, respectively.
The EUS features of CD, PIL and ITB are different. The diagnostic classification method is reliable in the differential diagnosis of colorectal ulcerative diseases.
Core tip: A classification method was created for the differential diagnosis of Crohn’s disease (CD), primary intestinal lymphoma (PIL) and intestinal tuberculosis (ITB) by endoscopic ultrasound (EUS), and yielded good results. The classification was designed based on univariate logistic regression analysis of EUS features of CD, PIL and ITB. This classification method is useful for diagnosing these three diseases in daily EUS practice.
- Citation: Qiu EQ, Guo W, Cheng TM, Yao YL, Zhu W, Liu SD, Zhi FC. Diagnostic classification of endosonography for differentiating colorectal ulcerative diseases: A new statistical method. World J Gastroenterol 2017; 23(46): 8207-8216
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8207
Some gastrointestinal diseases, including Crohn’s disease (CD), primary intestinal lymphoma (PIL) and intestinal tuberculosis (ITB), can lead to colorectal ulcers, are difficult to differentiate[1-4], and usually require entirely different treatments. Their architecture on resection histology can be easily distinguished at low magnification[5-7]. Endoscopic ultrasound (EUS) can demonstrate bowel wall structural changes[8-10] and identify lesions under the mucosa[11-14], which are valuable signs in the above-mentioned diseases[15]. However, there are few reports available regarding the value of EUS in the differential diagnosis of these three diseases. We attempted to create an EUS diagnostic classification method for CD, PIL, ITB and other colorectal ulcerative diseases.
We searched our in-patient medical record database for patients who underwent EUS at our center from 2008 to 2015 and were confirmed to have CD, PIL or ITB, and found 272 cases of CD, 60 cases of PIL and 39 cases of ITB. We randomly chose 200 CD, 30 PIL and 20 ITB cases to form the training set, and summarized the EUS features. EUS images and written reports of these cases were obtained from the endoscopy database. The EUS data were recorded according to the following eight parameters: (1) Total bowel wall thickness (TWT, in mm); (2) Changes in layers (thickened, thinned or disappeared), including the mucosa (M), submucosa (SM), muscularis propria (MP) and serosa (S); (3) Echo level of lesions or changed layers, including Level 1 (echo level of normal SM), Level 2 (between Levels 1 and 3), Level 3 (echo level of liver), Level 4 (between Levels 3 and 5), Level 5 (echo level of normal MP), and Level 6 (echo level of fluid); (4) Echo homogeneity, including homogeneous and heterogeneous; in addition, an independent option of “diffuse lesion” was included; (5) Definition of layer borderlines, including clear, unclear and invisible; (6) Integrity of the S, including smooth, non-smooth and interrupted; (7) Special EUS bowel wall feature, including “cobblestone sign” (multiple thickened SM-like masses close to each other, with an intact M), vascular structures with a diameter > 2 mm in SM; and (8) Extra-luminal presentation, including nearby enlarged lymph nodes, abscesses, ascites, sinus and fistulae.
All data on these parameters were analyzed using univariate logistic regression analysis to calculate the odds ratio (OR) of each option for each disease. The tendency scores for each disease for each option were then set according to the following rules: (1) The score was +1 if: a: the option was pathological, OR > 1 and P < 0.05; or b: P ≥ 0.05, but the proportion was > 50%; (2) The score was -1 if: a: OR < 1 and P < 0.05; or b: OR was infinitesimal and P value was unavailable; and (3) The score was 0 when other situations were met.
The tendency scores formed the EUS diagnostic classification as follows: (1) All scores of each matched option were summed for each disease to obtain three tendency scores for CD, PIL and ITB, respectively; (2) When the parameters “layers changed” and “layer borders” both met the option “disappeared”, only one point was added or subtracted; (3) The highest scoring disease was considered as the new EUS diagnosis; if the highest score was < 2 or was non-unique, the diagnosis was “other diseases”; and (4) When a sign unique to one disease (special sign) was detected, this disease was considered as the diagnosis directly, without including the score.
We assessed the cases which formed the test set to evaluate the accuracy of EUS in differentiating colorectal ulcerative diseases. The search option “endoscopic findings” and key word “ulcer” were used to identify all cases of ulcers diagnosed by EUS at our center from 2008 to 2015. All EUS images of these cases were obtained from the endoscopy database. The EUS images (without written report) for each case were placed in the patient file, and then copied to two blinded researchers by another researcher.
The cases were deleted before being copied when they met the following conditions: (1) Appearance in the training set; (2) Having an obvious visible epithelial or subepithelial tumor in the images; and (3) Having images that did not provide enough information on the eight parameters mentioned above.
Two endosonographers re-evaluated the EUS images in each case and recorded the data according to the eight parameters. If the data for one case recorded by the two endosonographers were inconsistent, the difference was resolved through discussion. The new EUS diagnoses in the test set were then established using the classification method.
We consulted the clinical and out-patient databases, and the endoscopy database, to determine the final diagnosis of each case in the test set. Cases were excluded if the final diagnosis was not successfully obtained or the clinical data were incomplete. The diagnoses of all patients were confirmed by one of the following four methods: Endoscopic biopsy pathology; Surgical pathology; Experimental treatment; or Other clinical methods (imaging modalities, special signs, laboratory examinations). Endoscopic biopsy specimens were obtained by forceps, endoscopic mucosal resection, endoscopic submucosal dissection and EUS-guided fine needle aspiration. Experimental treatment referred to: (1) CD: Infliximab, mesalazine or glucocorticoid treatment for at least 6 mo; (2) ITB: Quadruple anti-TB therapy for at least 2 mo; and (3) Other enteritis: Anti-infection (infective enteritis), immunosuppressant (autoimmune diseases) and tailored treatments (ischemic, drug and radiation enteritis). After the experimental treatment, final diagnoses were established if the symptoms were relieved, and colorectal ulcers were healed and did not reappear within 6 mo.
The EUS and actual diagnoses in all cases were compared. The overall EUS diagnostic accuracy, sensitivity and specificity were calculated. We excluded the cases easily diagnosed in the test set and calculated the EUS diagnostic accuracy in the remaining cases (perplexing test set). Finally, the classification was adjusted and the diagnostic accuracies were recalculated. All processes are shown in Figure 1.
All data were analyzed using SPSS (Statistical Product and Service Solutions 13.0.0.246, International Business Machines Corporation, Armonk, NY, United States). Measurement data (age, TWT) are presented as the mean ± SD. Multiple comparisons of groups were analyzed using the LSD-t test for TWT. Enumeration data (case number) are presented as a proportion, and comparisons of groups were analyzed using univariate logistic regression analysis. P < 0.05 was considered statistically significant.
The data on sex and age obtained from all cases are shown in Table 1.
The data on mean TWT in the three diseases are shown in Table 2. TWT in the PIL group was greater than that in the other two groups (P < 0.05). The case numbers and proportions of each option in each group are shown in Table 3.
| TWT, mm | P value | ||||
| Range | Mean ± SD | ||||
| Diseases | CD | 2.7-19.4 | 8.48 ± 2.90 | (CD and PIL) | < 0.001 |
| PIL | 3.7-29.6 | 13.49 ± 6.38 | (PIL and ITB) | 0.002 | |
| ITB | 3.2-22.0 | 10.19 ± 6.14 | (ITB and CD) | 0.080 | |
| F | 23.389 | ||||
| P | < 0.001 | ||||
| CD | PIL | ITB | |
| Thickened layers | |||
| None | 6 (3.0) | 0 (0) | 2 (10.0) |
| M | 21 (10.5) | 5 (16.7) | 14 (70.0) |
| SM | 160 (80.0) | 2 (6.7) | 0 (0) |
| M + SM | 4 (2.0) | 0 (0) | 1 (5.0) |
| SM + MP | 3 (1.5) | 0 (0) | 0 (0) |
| All | 2 (1.0) | 0 (0) | 0 (0) |
| Layers disappeared1 | 4 (2.0) | 23 (76.7) | 3 (15.0) |
| Thinned layers | |||
| SM | 0 (0) | 0 (0) | 6 (30.0) |
| M/SM border | |||
| Clear | 85 (42.5) | 3 (10.0) | 8 (40.0) |
| Unclear | 111 (55.5) | 4 (13.3) | 9 (45.0) |
| Invisible | 4 (4.0) | 23 (76.7) | 3 (15.0) |
| SM/MP border | |||
| Clear | 157 (78.5) | 3 (10.0) | 12 (60.0) |
| Unclear | 41 (21.5) | 4 (13.3) | 5 (25.0) |
| Invisible | 2 (1.0) | 23 (76.7) | 3 (15.0) |
| Echo level of main lesion or changed layer | |||
| 1 (hyperechoic) | 14 (7.0) | 0 (0) | 4 (20.0) |
| 2 | 166 (83.0) | 0 (0) | 12 (60.0) |
| 3 (medium) | 3 (1.5) | 3 (10.0) | 1 (5.0) |
| 4 | 11 (5.5) | 5 (16.7) | 1 (5.0) |
| 5 (hypoechoic) | 6 (3.0) | 22 (73.3) | 2 (10.0) |
| Echo homogeneity2 | |||
| Homogeneous | 87 (43.5) | 23 (76.7) | 5 (25) |
| Heterogeneous | 106 (53) | 7 (23.3) | 13 (65) |
| Diffuse lesion | 3 (1.5) | 26 (86.7) | 0 (0) |
| Serosal integrity | |||
| Smooth | 185 (92.5) | 14 (46.7) | 15 (75) |
| Non-smooth | 9 (4.5) | 2 (6.6) | 4 (20) |
| Interrupted | 6 (3) | 14 (46.7) | 1 (5) |
The frequencies and proportions of special bowel wall signs and extra-luminal EUS images are shown in Table 4.
| CD | PIL | ITB | |
| Special bowel wall signs | |||
| Cobblestone | 18 (9.0) | 0 (0) | 0 (0) |
| Vasculature in SM | 19 (9.5) | 0 (0) | 0 (0) |
| ≤ 2 mm | 11 (5.5) | 0 (0) | 0 (0) |
| > 2 mm | 8 (4.0) | 0 (0) | 0 (0) |
| Extra-luminal presentations | |||
| Abscesses | 2 (1.0) | 0 (0) | 0 (0) |
| Sinus or fistulae | 7 (3.5) | 0 (0) | 0 (0) |
| Ascites | 7 (3.5) | 2 (6.7) | 2 (10.0) |
| Lymph nodes | 36 (18.0) | 19 (63.3) | 5 (25.0) |
| Single | 22 (11.0) | 3 (10.0) | 4 (20.0) |
| Multiple | 14 (7.0) | 16 (53.3) | 1 (5.0) |
| Merged | 2 (1.0) | 4 (13.3) | 0 (0) |
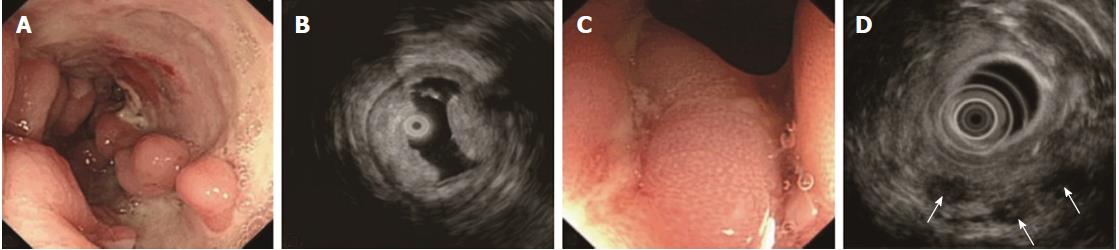
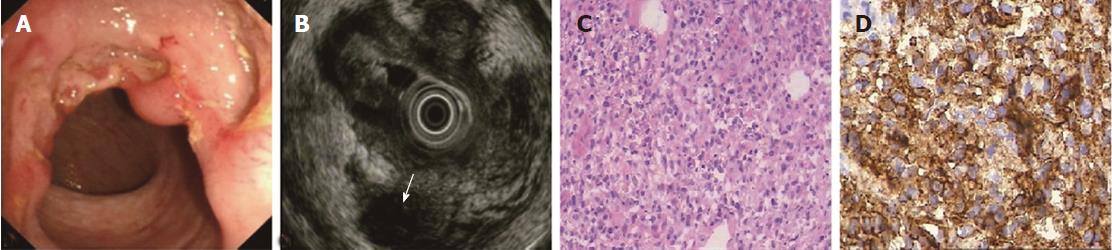
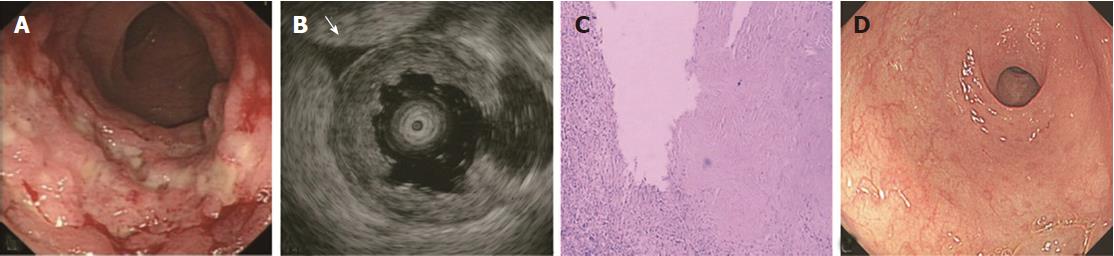
The ORs and P values from univariate logistic regression analysis, and the corresponding scores of each option set according to the above-mentioned rules, are listed in Table 5. An option was not shown in the table if all P values were unavailable or were > 0.05. The options scoring +1 and -1 are summarized in Table 6. Classical EUS patterns of the three diseases are shown in Figures 2-4.
| Parameters | CD | PIL | ITB | Score | |||||
| OR | P value | OR | P value | OR | P value | CD | PIL | ITB | |
| Layer changed (discrete variable) | |||||||||
| M normal | 67.81 | < 0.001 | 0.02 | < 0.001 | 0.04 | < 0.001 | 0 | -1 | -1 |
| M thickened | 0.23 | < 0.001 | 0.85 | 0.75 | 18.56 | < 0.001 | -1 | 0 | 1 |
| SM normal | 0.22 | < 0.001 | 0.82 | 0.708 | 24.75 | < 0.001 | -1 | 0 | 0 |
| SM thickened | 92.38 | < 0.001 | 0.02 | < 0.001 | 0.02 | < 0.001 | 1 | -1 | -1 |
| MP normal | 29.87 | < 0.001 | 0.01 | < 0.001 | 0.85 | 0.804 | 0 | -1 | 0 |
| S normal | 53.08 | < 0.001 | 0.01 | < 0.001 | 0.75 | 0.668 | 0 | -1 | 0 |
| Layer disappeared | 0.01 | < 0.001 | 141.29 | < 0.001 | 1.45 | 0.576 | -1 | 1 | 0 |
| Layer borders (discrete variable) | |||||||||
| M/SM clear | 2.62 | 0.009 | 0.15 | 0.003 | 1.08 | 0.878 | 0 | -1 | 0 |
| M/SM unclear | 3.55 | < 0.001 | 0.13 | < 0.001 | 0.82 | 0.668 | 1 | -1 | 0 |
| M/SM invisible | 0.02 | < 0.001 | 99.98 | < 0.001 | 1.33 | 0.668 | -1 | 1 | 0 |
| SM/MP clear | 8.52 | < 0.001 | 0.03 | < 0.001 | 0.66 | 0.379 | 0 | -1 | 0 |
| SM/MP invisible | 0.01 | < 0.001 | 141.29 | < 0.001 | 1.45 | 0.576 | -1 | 1 | 0 |
| Echo level (discrete variable) | |||||||||
| 1 (hyperechoic) | 0.87 | 0.807 | In | N/A | 3.86 | 0.03 | 0 | -1 | 1 |
| 2 | 15.46 | < 0.001 | In | N/A | 0.58 | 0.253 | 1 | -1 | 1 |
| 3 (medium) | 0.18 | 0.026 | 6 | 0.023 | 1.96 | 0.541 | -1 | 1 | 0 |
| 4 | 0.11 | 1.217 | 3.47 | 0.03 | 0.7 | 0.74 | 0 | 1 | 0 |
| 5 (hypoechoic) | 0.03 | < 0.001 | 72.87 | < 0.001 | 0.8 | 0.775 | -1 | 1 | 0 |
| Echo homogeneity (discrete variable) | |||||||||
| Homogeneous | 0.6 | 0.115 | 4.57 | < 0.001 | 0.36 | 0.058 | 0 | 1 | 0 |
| Heterogeneous | 1.69 | 0.102 | 0.26 | 0.003 | 1.92 | 0.179 | 1 | -1 | 1 |
| Diffuse lesion | 0.01 | < 0.001 | 470.17 | < 0.001 | In | N/A | -1 | 1 | -1 |
| Serosa integrity (discrete variable) | |||||||||
| Smooth | 9.62 | < 0.001 | 0.08 | < 0.001 | 0.45 | 0.148 | 0 | -1 | 0 |
| Non-smooth | 0.35 | 0.055 | 1.14 | 0.87 | 4.98 | 0.012 | 0 | 0 | 1 |
| Interrupted | 0.07 | < 0.001 | 26.62 | < 0.001 | 0.55 | 0.573 | -1 | 1 | 0 |
| Lymph nodes (ordinal variable) | |||||||||
| Multiple | 0.13 | < 0.001 | 17.36 | < 0.001 | 0.39 | 0.369 | 0 | 1 | 0 |
| Emerged | 0.12 | 0.015 | 16.77 | 0.003 | In | N/A | -1 | 1 | -1 |
| CD | PIL | ITB | |
| +1 | SM thickened M/SM unclear Echo level 2 Heterogeneous Lesion echo | Layer disappeared Echo level 3-5 Homogeneous and diffuse Lesion echo Interrupted S Multiple and emerged lymph nodes | M thickened Echo level 1and 2 Heterogeneous Echo Non-smooth S |
| -1 | M thickened SM normal Layer disappeared Echo level 3 and 5 Diffuse lesion echo Interrupted S Lymph nodes emerged | M, MP, S normal SM thickened Visible layer borders Echo level 1 and 2 Heterogeneous echo level Smooth S | M normal SM thickened Diffuse lesion Echo Lymph nodes emerged |
Using the classification method, we obtained the concordance between EUS and final diagnoses. The diagnostic accuracies for CD, PIL and ITB were 83.6% (209/250), 95.6% (239/250) and 91.2% (228/250), sensitivities were 79.5% (159/200), 73.3% (22/30) and 70.0% (14/20) and specificities were 100.0% (50/50), 98.6% (217/220) and 93.0% (214/230), respectively.
We collected EUS data on 752 cases from the endoscopy database, and 482 of these cases were excluded according to the exclusion criteria described in the Materials and Methods. The remaining 270 cases consisted of 77 CD, 30 PIL, 23 ITB and 140 patients with other diseases, including 30 cases of ulcers after endoscopic surgery, 29 cases of ulcerative colitis, 22 cases of colorectal cancer, 16 cases of nonspecific enteritis, 12 cases of infective colitis, 9 cases of radiation-induced bowel injury, 7 cases of ischemic enteritis, 6 cases of solitary ulcer, 3 cases of Bechet’s disease, and 6 cases of multiple myeloma, abdominal-type allergic purpura, eosinophilic gastroenteritis, congenital megacolon, inflammatory granuloma after trauma, and indeterminate colitis, respectively. Using the classification methods, we yielded an accuracy for CD, PIL and ITB of 88.9% (240/270), 88.9% (240/270) and 83.7% (226/270), a sensitivity of 77.9% (60/77), 60.0% (18/30) and 78.3% (18/23), and a specificity of 93.2% (180/193), 92.5% (222/240) and 84.2% (208/247), respectively. After excluding a total of 74 cases of ulcers after surgery, infective and nonspecific colitis, radiation-induced bowel injury, and ischemic enteritis, we yielded accuracies for CD, PIL and ITB of 86.2% (169/196), 86.7% (170/196) and 84.7% (166/196), unchanged sensitivities, and specificities of 91.6% (109/119), 91.6% (152/166) and 85.5% (148/173), respectively.
The adjustments were as follows: (1) The cases diagnosed as PIL previously were re-diagnosed as cancer (other diseases) when the echo of the lesion was heterogeneous; and (2) One case was diagnosed as PIL when a diffuse lesion echo was detected, without including any other factors. The accuracies increased after adjustment in the three sets. In the training set, the accuracy, sensitivity and specificity of PIL changed to 97.2% (243/250), 90.0% (27/30) and 98.2% (216/220), respectively, with no change in the CD and ITB groups. In the test set, the accuracies of CD, PIL and ITB improved to 89.3% (241/270), 97.8% (264/270) and 84.1% (227/270), sensitivities to 77.9% (60/77), 90.0% (27/30) and 78.3% (18/23), and specificities to 93.8% (181/193), 98.8% (237/240) and 84.6% (209/247), respectively. After excluding cases which were easily diagnosed, the accuracies of CD, PIL and ITB changed to 86.7% (170/196), 98.0% (192/196) and 85.2% (167/196), sensitivities were the same, and specificities changed to 92.4% (110/119), 99.4% (165/166) and 86.1% (149/173), respectively.
EUS can detect lesions which cannot be identified by white light endoscopy[16-20], and allows observation of the bowel wall structure[21]. EUS is also used for the treatment of complications and follow-up studies[22-27]. This study investigated the differential diagnosis of CD, PIL and ITB using EUS. We created an EUS diagnostic classification method by reviewing and summarizing previous articles on pathology at low magnification and EUS data.
The EUS features of CD included thickened SM, visible layer borders, and the echo level of the SM ranging between hyperechoic and medium echoic[15,26]. Significantly thickened SM was closely associated with severe edema and lymphangiectasis[28], which lowered the echo level of SM by increasing the sonolucency, and is always observed in resected CD bowels at low magnification. Invisible stratification is hardly seen, unless the illness is extremely severe. This sign was detected in only 4 cases in our study. We also found that the lesion was involved in the S and interrupted in 6 severe CD cases, which seemed impossible if CD was not complicated by malignancy. We suggest that severe fibrosis occurred in the bowels, making the S deformed, causing echo artifacts. Some studies have reported that vessels in the SM with a diameter > 2 mm were a specific EUS sign of CD[29-32]. This was not detected in the PIL and ITB cases in the training set; thus, the other specific signs of CD, such as fistulae, in this study were considered independent differentiation factors accordingly. In the test set, fistulae were found in only 2 cases. One was due to necrosis of lymphoma, and the other was due to anastomotic leakage.
The diagnostic sensitivity (77.9%) was not high. In total, 17 CD cases were misdiagnosed, 12 cases due to thickened M and 5 cases due to the collapse stratification or low-level echo caused by severe inflammation or infection. Five of these twelve cases with small TWT (< 5 mm) were in the early stage, resulting in incorrect recognition of thickened layers. The other 7 misdiagnosed cases were caused by two reasons which are commonly seen at the start of the disease - difficulty in scanning ulcers on the ileocecal valve and disturbance due to pseudopolyps.
The EUS features of PIL included invisible layer borders, thus layer thickening was impossible to identify; the lesions were diffusely hypoechoic. The lymphoma cells derived from M or SM were always densely packed[33,34] and tight with rare stromal cells, making the lesion echo diffusely hypoechoic and homogeneous. We found that several cases just had thickened M (5/30 = 16.7%) or SM (2/30 = 6.7%) with visible layer borders, which were quite different from the majority. These PIL cases were in the early stage, and the stratification had not yet been destroyed.
In the present study, low sensitivity (60.0%) was observed before adjustment and these early cases were excluded. If a diffusely hypoechoic lesion was the only diagnostic consideration, 9 misdiagnosed PIL cases would have the correct diagnoses, increasing the sensitivity to 90% without a decrease in specificity. On the other hand, echo level in PIL is lower and more homogeneous than in cancer, which is a useful clue for differential diagnosis. We re-diagnosed several previously diagnosed PIL cases as cancer, further improving the specificity with little change in accuracy in the CD and ITB groups. We also followed these two principles (adjustments) in daily practice when faced with the same conditions.
The EUS features of ITB included M thickening, the echo level of M being hyperechoic or a little higher than medium level, and visible layer borders[35,36]. ITB is mainly caused by TB bacilli in the swallowed sputum, which likely invade the ileocecum where lymph tissues are located[37]. Thus, the M bears the brunt of invasion and subsequent inflammation, and then becomes thickened. In contrast, the SM does not thicken. Some studies have shown that SM is thin or sometimes interrupted due to inflammation and scarring[38,39]. This could be a differentiation factor for CD and other forms of enteritis. However, we observed this sign in only 30% of ITB cases. Furthermore, we also found thinned SM in 3 cases with other diseases (radiation-induced bowel injury, solitary ulcer, ulcers after surgery). Similar to CD, the layer borders of the bowel in ITB were visible, except in the very few cases with severe inflammation. In addition, similar to CD, the S was interrupted in fewer ITB cases, possibly for the same reason. Several cases with non-smooth S were complicated by TB peritonitis.
A sensitivity of 78.3% showed that almost one-fourth of ITB cases were misdiagnosed using the classification method. This may be due to the small number of cases, which provided poor reliability of the summarized EUS features of ITB. Moreover, the possibility of confusion between ITB and common enteritis exists with this method which lacks specificity[39]. In total, 15 cases of nonspecific enteritis were misdiagnosed as ITB. Our confidence in diagnosing ITB using EUS in daily practice does not match the high diagnostic accuracy of ITB observed in this study. High diagnostic accuracies for CD and PIL greatly increased the specificity of ITB due to the few cases of ITB (approximately 12% of total cases).
In this study, we created a classification method based on univariate logistic regression analysis and the algorithm reported by Lee et al[40] and Mao et al[41]. In general, when P < 0.05 is an option, the score increases if OR is > 1 and decreases if OR is < 1; when P ≥ 0.05, the score did not change. When a pathological option was not statistically significant (P ≥ 0.05) but the proportion was greater than 50% and OR > 1 in a disease (i.e. echo level 2 in ITB), one point should be added because this option is a clue in distinguishing a pathological state from a normal state, and still showed the tendency to the disease, although it was not powerful enough to differentiate the three diseases. In contrast, the score was zero even when a non-pathological option met the score-raising condition, because the option could not be used to identify whether the state was pathological.
The reasons for some of the rules used in the classification are as follows: (1) The cause of an infinitesimal OR and an unavailable P value was a zero case number, which is a strong clue for ruling out a disease; thus, the score was -1 when this condition was met; (2) The borders disappear with the layer disappearance; therefore, when the parameters “layers changed” and “layer borders” both met the option “disappeared”, only one point should be added or subtracted; (3) Special signs are the specific symbols of a disease. In general, special signs belong to different diseases and would not be seen in an independent case, such as in the three sets. If this occurs, such as an ITB case with a fistula, our classification mode would not be applicable. However, this situation is rare in clinical practice; and (4) The highest score of < 2 suggests that the EUS pattern is not found in the three diseases. A non-unique highest score indicates a difficult case; thus, the diagnosis would be “other diseases”.
There were limitations to this study. There was an attempt to eliminate interference from the original written reports when dealing with the test set. The images were analyzed without written reports; therefore, it was difficult to determine the location of the lesion and to match the EUS images to white light endoscopic images. However, this problem can be solved easily in clinical practice, and the diagnostic accuracy may be even higher in the real situation.
Some gastrointestinal diseases, including Crohn’s disease (CD), primary intestinal lymphoma (PIL) and intestinal tuberculosis (ITB), can lead to colorectal ulcers, are difficult to differentiate, and usually require entirely different treatments. Their architecture on resection histology can be easily distinguished at low magnification. Endoscopic ultrasound (EUS) can demonstrate bowel wall structural changes, and identify lesions under the mucosa
There are few reports available regarding the value of EUS in the differential diagnosis of these three diseases. The authors attempted to explore the EUS diagnostic accuracy of these diseases and to create a new reliable diagnostic method.
The authors attempted to create an EUS diagnostic classification method which can be used for accurately differentiating CD, PIL, ITB and other colorectal ulcerative diseases.
The authors searched the in-patient medical record database for confirmed cases of CD, PIL and ITB from 2008 to 2015 at our center, and collected data on EUS from randomly-chosen patients who formed the training set. All cases found to have colorectal ulcers using EUS were obtained from the endoscopy database and formed the test set. The authors then removed the cases which were easily diagnosed, and the remaining cases formed the perplexing test set. The authors conducted univariate logistic regression analysis on the training set to summarize EUS features of CD, PIL and ITB, and created a diagnostic classification method, rediagnosed the cases in the training set, test set and perplexing set using the classification method, and determined EUS diagnostic accuracies. The authors analyzed the origin of the problems, which were reflected from the diagnostic accuracy, adjusted the classification then repeated the rediagnosing and accuracy-calculating steps, obtaining a result which was closer to the facts.
The EUS features of CD, PIL and ITB are different. The diagnostic classification method, as a new statistical method, is reliable in the differential diagnosis of colorectal ulcerative diseases. But, the case numbers of PIL and ITB were too small.
EUS is good for differentiating CD, PIL and ITB; An EUS classification system for differentiating CD, PIL and ITB; A new statistical method and an original scoring system.
The authors will increase the number of ITB and PIL to obtain a higher reliability of the classification method, and will perform a multicenter study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chamberlain MC, Higuchi K, Okada S S- Editor: Ma Y J L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Kiesslich R, Neurath MF. Advanced endoscopy imaging in inflammatory bowel diseases. Gastrointest Endosc. 2017;85:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Tontini GE, Wiedbrauck F, Cavallaro F, Koulaouzidis A, Marino R, Pastorelli L, Spina L, McAlindon ME, Leoni P, Vitagliano P. Small-bowel capsule endoscopy with panoramic view: results of the first multicenter, observational study (with videos). Gastrointest Endosc. 2017;85:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Sato S, Yao K, Yao T, Schlemper RJ, Matsui T, Sakurai T, Iwashita A. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc. 2004;59:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Kucharski M, Karczewski J, Mańkowska-Wierzbicka D, Karmelita-Katulska K, Kaczmarek E, Iwanik K, Rzymski P, Grzymisławski M, Linke K, Dobrowolska A. Usefulness of Endoscopic Indices in Determination of Disease Activity in Patients with Crohn’s Disease. Gastroenterol Res Pract. 2016;2016:7896478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Ellrichmann M, Wietzke-Braun P, Dhar S, Nikolaus S, Arlt A, Bethge J, Kuehbacher T, Wintermeyer L, Balschun K, Klapper W. Endoscopic ultrasound of the colon for the differentiation of Crohn’s disease and ulcerative colitis in comparison with healthy controls. Aliment Pharmacol Ther. 2014;39:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Wu D, Li JN, Qian JM. Endoscopic Diagnosis and Treatment of Precancerous Colorectal Lesions in Patients with Inflammatory Bowel Disease: How Does the Latest SCENIC International Consensus Intersect with Our Clinical Practice? J Transl Int Med. 2017;5:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Miu YL, Ouyang Q, Zhou ZF, Pu P, Chen DY. Histological pathologic study on Crohn’s disease and intestinal tuberculosis (in Chinese). Linchuang Neike Zazhi. 2002;2:109-111. |
| 8. | Malmstrøm ML, Săftoiu A, Vilmann P, Klausen TW, Gögenur I. Endoscopic ultrasound for staging of colonic cancer proximal to the rectum: A systematic review and meta-analysis. Endosc Ultrasound. 2016;5:307-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ustundag Y, Fusaroli P. Are rigid probes sufficient to provide reliable data for rectal cancer staging? Endosc Ultrasound. 2015;4:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ge N, Sun S. Endoscopic ultrasound: An all in one technique vibrates virtually around the whole internal medical field. J Transl Int Med. 2014;2:104-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Okasha HH, Amin M, Ezzat R, El-Nady M, Nagy A. Small bowel intussusception induced by a jejunal gastrointestinal stromal cell tumor diagnosed by endoscopic ultrasound. Endosc Ultrasound. 2016;5:346-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Bhutani MS, Annangi S, Koduru P, Aggarwal A, Suzuki R. Diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound: Biopsy is not needed! Endosc Ultrasound. 2016;5:335-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Leighton D, Oudjhane K, Ben Mohammed H. The sternoclavicular joint in trauma: retrosternal dislocation versus epiphyseal fracture. Pediatr Radiol. 1989;20:126-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Makino T, Kanmura S, Sasaki F, Nasu Y, Funakawa K, Tanaka A, Arima S, Nakazawa J, Taguchi H, Hashimoto S. Preoperative classification of submucosal fibrosis in colorectal laterally spreading tumors by endoscopic ultrasonography. Endosc Int Open. 2015;3:E363-E367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Gast P. Endorectal ultrasound in infectious colitis may predict development of chronic colitis. Endoscopy. 1999;31:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Seicean A, Mosteanu O, Seicean R. Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy. World J Gastroenterol. 2017;23:25-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Roshdy MA, Marzouk AS. The subgenus Persicargas (Ixodoidea: Argasidae: Argas). 36. structure and postembryonic development of the neurohemal organ in A. (P.) arboreus. Z Parasitenkd. 1982;66:345-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Mukae M, Kobayashi K, Sada M, Yokoyama K, Koizumi W, Saegusa M. Diagnostic performance of EUS for evaluating the invasion depth of early colorectal cancers. Gastrointest Endosc. 2015;81:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Choudhary NS, Puri R, Lipi L, Saraf N. Eosinophilic gastroenteritis mimicking as a malignant gastric ulcer with lymphadenopathy as shown by computed tomography and endoscopic ultrasound. Endosc Ultrasound. 2015;4:78-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Arya N, Sahai AV, Paquin SC. Credentialing for endoscopic ultrasound: A proposal for Canadian guidelines. Endosc Ultrasound. 2016;5:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Ratone JP, Bertrand J, Godat S, Bernard JP, Heyries L. Transrectal drainage of pelvic collections: Experience of a single center. Endosc Ultrasound. 2016;5:108-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Luz LP, Cote GA, Al-Haddad MA, McHenry L, LeBlanc JK, Sherman S, Moreira DM, El Hajj II, McGreevy K, DeWitt J. Utility of EUS following endoscopic polypectomy of high-risk rectosigmoid lesions. Endosc Ultrasound. 2015;4:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Sirtori C. [The biology of gastric cancer]. Minerva Med. 1970;61:3575-3577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Spradlin NM, Wise PE, Herline AJ, Muldoon RL, Rosen M, Schwartz DA. A randomized prospective trial of endoscopic ultrasound to guide combination medical and surgical treatment for Crohn’s perianal fistulas. Am J Gastroenterol. 2008;103:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Lew RJ, Ginsberg GG. The role of endoscopic ultrasound in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wiese DM, Beaulieu D, Slaughter JC, Horst S, Wagnon J, Duley C, Annis K, Nohl A, Herline A, Muldoon R. Use of Endoscopic Ultrasound to Guide Adalimumab Treatment in Perianal Crohn’s Disease Results in Faster Fistula Healing. Inflamm Bowel Dis. 2015;21:1594-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Hu PJ, Qian JM, Wu KC, Ran ZH. Consensus about diagnosis and treatment of inflammatory bowel disease in China. Neike Lilun Shijian. 2013;1:61-75. |
| 29. | Dağli U, Over H, Tezel A, Ulker A, Temuçin G. Transrectal ultrasound in the diagnosis and management of inflammatory bowel disease. Endoscopy. 1999;31:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Gast P, Belaïche J. Rectal endosonography in inflammatory bowel disease: differential diagnosis and prediction of remission. Endoscopy. 1999;31:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, Pittilo RM, Rowles PM, Hudson M, Lewis AA. Granulomatous vasculitis in Crohn’s disease. Gastroenterology. 1991;100:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Cangir A, Vietti TJ, Gehan EA, Burgert EO Jr, Thomas P, Tefft M, Nesbit ME, Kissane J, Pritchard D. Ewing’s sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing’s sarcoma studies. Cancer. 1990;66:887-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | El-Zahabi LM, Jamali FR, El-Hajj II, Naja M, Salem Z, Shamseddine A, El-Saghir NS, Zaatari G, Geara F, Soweid AM. The value of EUS in predicting the response of gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication. Gastrointest Endosc. 2007;65:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Schizas D, Ntanasis-Stathopoulos I, Tsilimigras DI, Sioulas AD, Moris D, Spartalis E, Scotiniotis I, Papanikolaou IS. The Role of Endoscopic Ultrasound in the Diagnosis and Management of Primary Gastric Lymphoma. Gastroenterol Res Pract. 2017;2017:2397430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Rana SS, Bhasin DK, Rao C, Srinivasan R, Singh K. Tuberculosis presenting as Dysphagia: clinical, endoscopic, radiological and endosonographic features. Endosc Ultrasound. 2013;2:92-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Han XM, Yang JM, Xu LH, Nie LM, Zhao ZS. Endoscopic ultrasonography in esophageal tuberculosis. Endoscopy. 2008;40:701-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Rathi P, Gambhire P. Abdominal Tuberculosis. J Assoc Physicians India. 2016;64:38-47. [PubMed] |
| 38. | Shimizu S, Tada M, Kawai K. Endoscopic ultrasonography in inflammatory bowel diseases. Gastrointest Endosc Clin N Am. 1995;5:851-859. [PubMed] |
| 39. | Sharma V, Rana SS, Chhabra P, Sharma R, Gupta N, Bhasin DK. Primary esophageal tuberculosis mimicking esophageal cancer with vascular involvement. Endosc Ultrasound. 2016;5:61-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, Kim KJ, Lee GH, Jung HY, Hong WS. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn’s disease. Endoscopy. 2006;38:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Mao R, Liao WD, He Y, Ouyang CH, Zhu ZH, Yu C, Long SH, Chen YJ, Li ZP, Wu XP. Computed tomographic enterography adds value to colonoscopy in differentiating Crohn’s disease from intestinal tuberculosis: a potential diagnostic algorithm. Endoscopy. 2015;47:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |