Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8169
Peer-review started: September 20, 2017
First decision: October 10, 2017
Revised: October 24, 2017
Accepted: November 14, 2017
Article in press: November 14, 2017
Published online: December 14, 2017
Processing time: 83 Days and 19 Hours
To explore the pharmacokinetics and pharmacodynamics of Shengjiang decoction (SJD) in rats with acute pancreatitis (AP) for protecting against multiple organ injury.
An AP model was established by retrograde perfusion of 3.5% sodium taurocholate into the biliopancreatic duct, and a control group (CG) received 0.9% sodium chloride instead. Twelve male Sprague-Dawley rats were randomly divided into a CG treated with SJD (CG + SJD) and a model group treated with SJD (MG + SJD), both of which were orally administered with SJD (5 g/kg) 2 h after surgery. Blood samples were collected via the tail vein at 10, 20, and 40 min and 1, 2, 3, 4, 6, 8, and 12 h after a single dose of SJD to detect its main components using high-performance liquid chromatography-tandem mass spectrometry. The pharmacokinetic parameters were compared. In the pharmacodynamic experiment, 18 male Sprague-Dawley rats were randomly divided into a CG, an AP model group (MG), and an SJD treated AP group (SJDG). Serum amylase, lipase, and inflammatory cytokines were measured, and heart, lung, liver, spleen, pancreas, kidney, and intestine tissues were collected for pathological examination.
The MG + SJD displayed significantly shorter mean residence time (MRT) and higher clearance (CL) for emodin and aloe-emodin; significantly shorter time of maximum concentration and T1/2 and a lower area under curve (AUC) for aloe-emodin; a significantly higher AUC and lower CL for rhein; and longer MRT and lower CL for chrysophanol than the CG + SJD. In the pharmacodynamic experiment, the amylase, interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α levels in the MG were higher than those in the CG (P < 0.05). After the herbal decoction treatment, the SJDG had higher IL-10 and lower TNF-α levels than the MG (P < 0.05). The MG had the highest pathological scores, and the pathological scores of the lung, pancreas, kidney, and intestine in the SJDG were significantly lower than those in the MG (P < 0.05).
AP may have varying effects on the pharmacokinetics of the major SJD components in rats. SJD might alleviate pathological injuries of the lung, pancreas, kidney, and intestine in rats with AP via regulating pro- and anti- inflammatory responses, which might guide the clinical application of SJD for AP treatment.
Core tip: Shengjiang decoction (SJD) has been identified to be effective in treating acute pancreatitis (AP) in both in vivo and in vitro tests. We report the metabolic processes of major components of SJD in vivo and the pharmacodynamic mechanism of SJD in relieving AP. This study demonstrated that AP may have varying effects on the pharmacokinetics of the major SJD components in rats. Rhein and bisdemethoxycurcumin may be potential active components for the treatment of AP based on the hypothesis of tissue pharmacology of herbal recipe. SJD may attenuate AP by regulating inflammatory responses to protect against multiple organ injury.
- Citation: Zhu L, Li JY, Zhang YM, Kang HX, Chen H, Su H, Li J, Tang WF. Pharmacokinetics and pharmacodynamics of Shengjiang decoction in rats with acute pancreatitis for protecting against multiple organ injury. World J Gastroenterol 2017; 23(46): 8169-8181
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8169.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8169
Acute pancreatitis (AP) is an acute inflammatory disorder of the pancreas and the surrounding tissues caused by pancreatic digestive enzymes due to various aetiological factors[1]. The overall mortality of AP is currently approximately 2%[2], but it approaches 30%-56% among patients with severe acute pancreatitis (SAP), which is characterized by persistent systemic inflammatory response syndrome (SIRS) and persistent organ failure[3-5]. Mortality associated with AP has decreased over time due to the widespread application of Chinese medicines, including traditional Chinese decoctions and acupuncture[6-9].
Shengjiang decoction (SJD), a classical traditional Chinese herbal formula, was recorded in Shanghan Wenyi Tiaobian by Li-Shan Yang, a well-known heat disease specialist in the Qing Dynasty. Composed of Rhei Radix et Rhizoma B. (rhubarb root and rhizome), Curcumae Longae Rhizoma L. (turmeric), Bombyx Batryticatus L. (stiff silkworm), and Cicadae Periostracum F. (cicada slough), SJD has been a prestigious prescription for the treatment of various heat diseases or syndromes for hundreds of years. Rhei Radix et Rhizoma B. is recorded in Shennong’s Classic of Materia Medica and is reported to expel pathogens by purgation, clearing heat-fire, and removing stasis. It has also been declared to be effective in reducing injuries of the gastrointestinal tract, lung, and liver induced by sepsis via reducing oxidative stress and inflammation, ameliorating microcirculatory disturbances, and maintaining the immune balance[10,11]. Curcumae Longae Rhizoma L. promotes Qi and activates blood circulation. Moreover, curcumin, demethoxycurcumin, and bidemethoxycurcumin, which are extracted from Curcumae Longae Rhizoma L., have been shown to regulate anti-inflammatory responses and prevent systemic complications in AP associated with cytokine damage[12,13]. Both Bombyx Batryticatus L. and Cicadae Periostracum F. dispel wind and have an anti-inflammatory effect.
Notably, an increasing number of clinical and experimental studies have reported that SJD is effective for the treatment of AP, especially in individuals with fever or severe infections, which could be differentiated as heat syndrome. SJD, in combination with conventional Western medicine, markedly reduced the APACHE II score, multiple organ dysfunction syndrome (MODS) severity score, and intra-abdominal pressure in patients with SIRS/MODS compared to Western medical treatment alone[14]. SJD can significantly reduce the inflammatory response and improve the clinical symptoms and prognosis[15] in early sepsis patients[16]. Moreover, it also plays a protective role against gastric mucosal damage by weakening aggressive factors and strengthening protective factors[17] in SIRS via regulation of pro- and anti-inflammatory responses[18]. SJD can also protect against nonalcoholic fatty liver disease associated with metabolic syndrome[19]. Our previous study has demonstrated that SJD ameliorates inflammation of the systemic microenvironment, reduces apoptosis of pancreatic acinar cells, and promotes repair of the pancreas in rats with AP[20].
Indisputably, SJD is an effective prescription for the treatment of AP, but the exact active components are not clear. Little is known about the in vivo metabolic process of SJD. Full elucidation of the pharmacokinetic and pharmacodynamic mechanisms of SJD associated with the amelioration of AP is urgently needed. Therefore, this study aimed to explore the pharmacokinetics, pharmacodynamics, and pancreatic distribution of the main components of SJD in rats with AP to provide pharmacokinetic and pharmacodynamic evidence for its clinical application for the treatment of AP.
Male clean-grade, healthy Sprague-Dawley rats (body weight: 300 ± 20 g; age: 75 ± 5 d) were used in the study [Certification No. 0000589-SCXK (Chuan) 2013-24]. The rats were housed, fed, and handled according to the University Guidelines and Animal Ethics Committee Guidelines of the Animal Facility of the West China Hospital (protocol number: 2017052A, Chengdu, China). Animals were maintained in air-conditioned animal quarters under the following conditions: temperature, 22 ± 2 °C; relative humidity, 65% ± 10%; free access to water; and fed laboratory rodent chow (Chengdu, China). The animal were acclimatized to the facilities for one week and fasted for 12 h prior to the experiment.
Spray dried particles of SJD ingredients including Rhei Radix et Rhizoma B. (batch No. 16110150), Curcumae Longae Rhizoma L. (batch No. 16080008 ), Bombyx Batryticatus L. (batch No. 16100147), and Cicadae Periostracum F. (batch No. 16080020) were all purchased from the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Chengdu, China) and authenticated by Professor WM Wang (Department of Herbal Pharmacy, West China Hospital, Sichuan University, China) according to the Chinese Pharmacopoeia (The Pharmacopoeia Commission of PRC, 2010). Voucher specimens were deposited at our laboratory. The spray dried particles were mixed and reconstituted with sterile distilled water (proportions: 4:3:2:1; concentration: 0.5 g/mL).
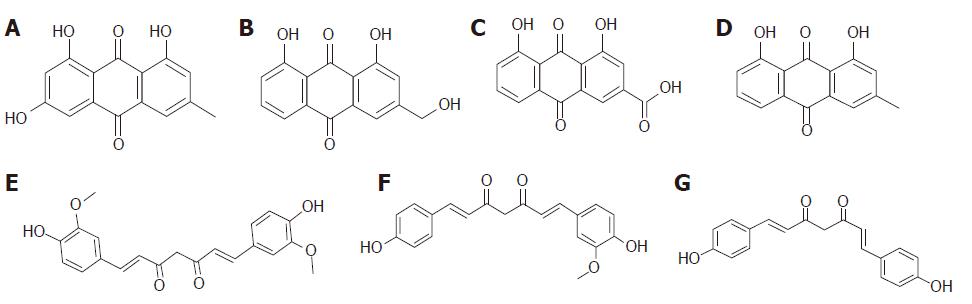
The components of SJD were determined by HPLC, with an ultimate XB-C18 column (5 μm, 50 mm × 4.6 mm) with methanol-water (92:8, v/v) at a flow rate of 0.5 mL/min with the column temperature set at 40 °C. The liquid chromatography mass spectrometry (LC/MS) system was operated under the multiple reaction monitoring mode using electrospray ionization in the negative ion mode. The ion pairs were emodin 269.0 → 241.2 (m/z), aloe-emodin 269.0 → 239.9 (m/z), rhein 283.2 → 238.8 (m/z), chrysophanol 253.2 → 224.7 (m/z), curcumin 366.6 → 216.9 (m/z), demethoxycurcumin 336.7 → 216.9 (m/z), bidemethoxycurcumin 306.7 → 186.8 (m/z), and inner standard ibuprofen 205.1 → 160.9 (m/z).
In the pharmacokinetic experiment, male Sprague-Dawley rats were randomly divided into a control group (CG) that received SJD (CG + SJD) (n = 6) and a model group that received SJD (MG + SJD) (n = 6). The AP model was established by retrograde perfusion of 3.5% sodium taurocholate (Sigma, St. Louis, MO, United States) into the biliopancreatic duct (1 mL/kg body weight) at a rate of 6 mL/h with a micro-infusion pump after intraperitoneal injection with 10% chloral hydrate (3 mL/kg body weight) for anesthesia, while the CG received 0.9% sodium chloride instead of sodium taurocholate. Both groups were orally administered with SJD (5 g/kg) 2 h after the operation.
In the pharmacodynamic experiment, male Sprague-Dawley rats were randomly divided into a CG (n = 6), an AP model group (MG) (n = 6), and an SJD treated AP group (SJDG) (n = 6). Model induction was identical to the procedure used in the pharmacokinetic experiment, and the SJDG was orally administered with SJD (5 g/kg) 2 h after the operation.
In the pharmacokinetic experiment, after administration of a single dose of SJD, a 0.5 mL blood sample was collected via the tail vein at 10, 20, and 40 min and 1, 2, 3, 4, 6, 8, and 12 h. After centrifugation at 3000 r/min for 7 min, the serum samples were stored at -80 °C for detection by HPLC-MS/MS. The rats were sacrificed 12 h after administration of SJD; pancreatic tissue samples were homogenized and the supernatants were obtained after centrifugation at 3000 r/min for 7 min and stored at -80 °C for detection.
In the pharmacodynamic experiment, the rats were sacrificed 12 h after administration of SJD. Blood samples (5 mL) were collected to obtain serum samples to measure amylase and lipase, using an Automatic Biochemical Analyzer (AU5400, SIEMENS, Munich, Germany), and to measure interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α levels using a Milliplex MAP Rat Cytokine/Chemokine magnetic bead immunoassay kit (Millipore Corporation, Billerica, MA, United States). Heart, lung, liver, spleen, pancreas, kidney, and intestine tissue samples were collected for pathological examination.
The concentrations of the main components of SJD in serum were measured by HPLC-MS/MS. Analyst 1.4.2 software for HPLC-MS/MS was used for data collection, peak integration, and calibration. The concentrations of the quality control and unknown samples were measured by interpolation from the calibration curves. Drug and statistics software programmed by the Chinese Pharmacological Society (DAS 2.0) was used to process the serum concentration data and for compartment model fitting; then, all pharmacokinetic parameters were determined. The following pharmacokinetic parameters were calculated: maximum concentration, time of maximum concentration (Tmax), area under the curve (AUC) (0-t), half-life (T1/2), mean residence time (MRT), and clearance (CL).
All statistical analyses were performed with PEMS3.1 statistical software for windows. Quantitative data are expressed as the mean ± standard deviation when normally distributed. Comparisons of the pharmacokinetic parameters between the CG + SJD and MG + SJD were performed by Student’s t-test. One-way repeated-measures ANOVA followed by multiple pair-wise comparisons using the Student-Newman-Keuls test was used to detect differences among the CG, MG, and SJDG. The level of statistical significance was set at P < 0.05.
In the study, seven components, emodin, aloe-emodin, rhein, chrysophanol, curcumin, demethoxycurcumin, and bisdemethoxycurcumin, were detected in the serum and pancreas in the CG + SJD and the MG + SJD by HPLC-MS/MS. The structural formula and HPLC chromatogram of each component are shown in Figures 1 and 2, respectively.
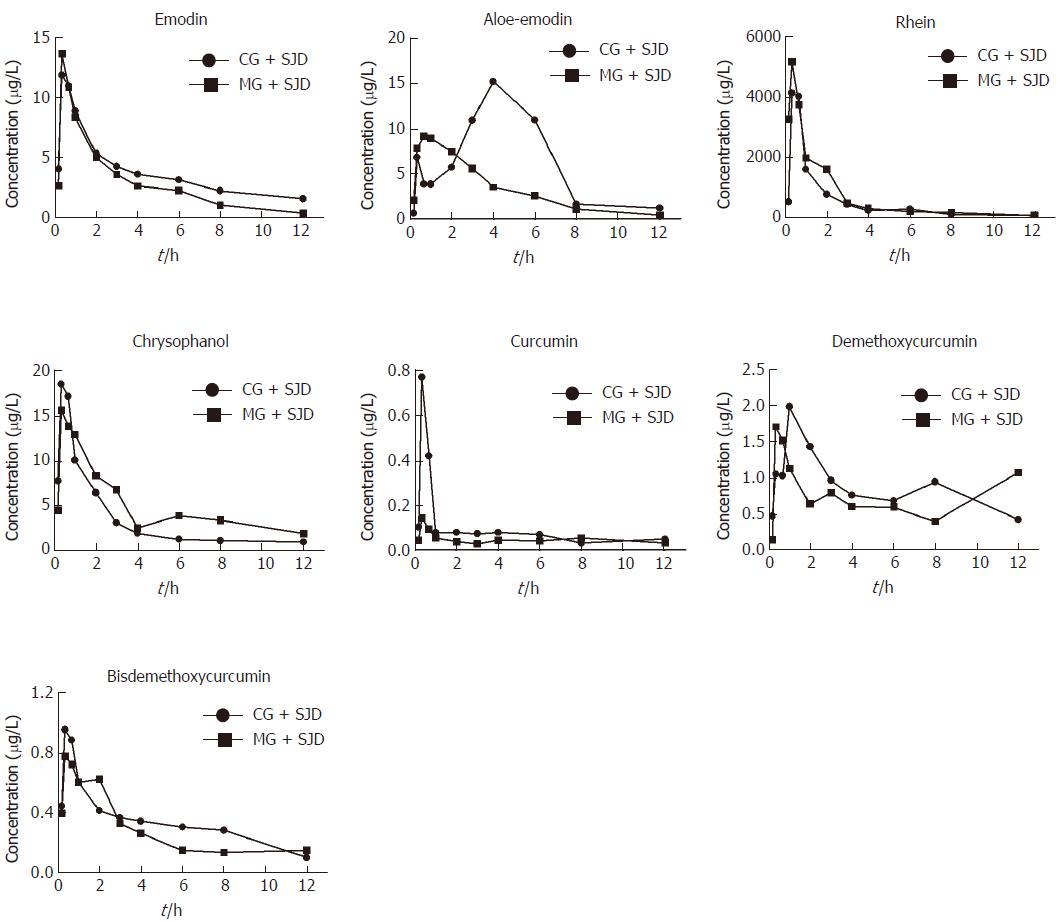
Both emodin and aloe-emodin displayed significantly shorter MRT and higher CL in the MG + SJD than in the CG + SJD (P < 0.05, Table 1), while aloe-emodin had significantly shorter Tmax and T1/2 and a lower AUC in the MG + SJD (P < 0.05, Table 1). The AUC of rhein was significantly higher and the CL was lower in the MG + SJD than in the CG + SJD (P < 0.05, Table 1). The MRT of chrysophanol was longer and the CL of chrysophanol was lower in the MG + SJD than in the CG + SJD (P < 0.05, Table 1). The pharmacokinetic parameters of curcumin, demethoxycurcumin, and bisdemethoxycurcumin were not significantly different between the two groups (P > 0.05, Table 1).
| Component | Group | Tmax (h) | T1/2 (h) | MRT (0-t) (h) | CL (L/h/kg) | Cmax (μg/L) | AUC (0-t)( μg/L/h) |
| Emodin | CG + SJD | 0.56 ± 0.27 | 0.85 ± 1.09 | 4.13 ± 0.41 | 396 ± 147 | 16.23 ± 4.11 | 44.78 ± 18.87 |
| MG + SJD | 0.50 ± 0.28 | 0.26 ± 0.12 | 3.08 ± 0.48a | 619 ± 193a | 16.47 ± 7.35 | 34.34 ± 14.78 | |
| ALEM | CG + SJD | 3.89 ± 2.12 | 4.15 ± 2.08 | 4.77 ± 0.40 | 261 ± 97 | 17.88 ± 5.90 | 80.61 ± 23.75 |
| MG + SJD | 0.83 ± 0.62a | 0.46 ± 0.49a | 3.18 ± 0.45a | 564 ± 209a | 13.77 ± 3.00 | 38.51 ± 16.63a | |
| Rhein | CG + SJD | 0.44 ± 0.18 | 0.71 ± 0.65 | 2.32 ± 0.40 | 3.07 ± 0.39 | 4388 ± 957 | 6136.71 ± 816.0 |
| MG + SJD | 0.39 ± 0.14 | 0.82 ± 0.57 | 2.19 ± 0.21 | 2.41 ± 0.35a | 5206 ± 1703 | 8025.27 ± 1074.26a | |
| CHRY | CG + SJD | 0.50 ± 0.19 | 1.02 ± 1.63 | 2.93 ± 0.57 | 411 ± 118 | 22.46 ± 7.78 | 39.12 ± 5.9 |
| MG + SJD | 0.56 ± 0.27 | 0.96 ± 0.66 | 4.04 ± 0.63a | 271 ± 76a | 17.44 ± 3.71 | 58.90 ± 23.74 | |
| Curcumin | CG + SJD | 0.44 ± 0.18 | 0.71 ± 0.27 | 4.60 ± 1.26 | 15957 ± 8161 | 0.93 ± 1.42 | 1.01 ± 0.86 |
| MG + SJD | 0.52 ± 0.08 | 0.69 ± 0.43 | 5.38 ± 0.71 | 25172 ± 11861 | 0.18 ± 0.04 | 0.56 ± 0.23 | |
| DEME | CG + SJD | 0.89 ± 0.27 | 0.60 ± 0.49 | 4.90 ± 0.58 | 1518 ± 944 | 2.08 ± 0.37 | 10.62 ± 3.41 |
| MG + SJD | 0.50 ± 0.19 | 0.36 ± 0.45 | 5.83 ± 0.91 | 1064 ± 717 | 1.99 ± 0.91 | 8.60 ± 1.90 | |
| BISD | CG + SJD | 0.50 ± 0.28 | 3.18 ± 0.30 | 4.44 ± 0.46 | 4117 ± 677 | 1.03 ± 0.27 | 3.97 ± 0.52 |
| MG + SJD | 0.61 ± 0.55 | 2.99 ± 0.14 | 3.93 ± 1.34 | 5159 ± 1829 | 0.83 ± 0.09 | 3.28 ± 0.47 |
According to the estimated concentration-time curve (Figure 3) and the pharmacokinetic values in Table 1, the concentration of emodin absorbed into the rat serum was similar between the two groups. However, the serum concentration of rhein was considerably higher in the MG + SJD during the first 12 h, which was in sharp contrast to curcumin and bisdemethoxycurcumin, which displayed lower serum concentrations in the MG + SJD than in the CG + SJD during the first 12 h. Both aloe-emodin and demethoxycurcumin showed a greater increase in serum concentrations during the early phase in the MG + SJD than in the CG + SJD, which was opposite to the trend of chrysophanol.
Regarding the distribution in the pancreas, although the concentration of rhein in the MG + SJD was significantly lower than that in the CG + SJD (P < 0.05, Table 2), it was notable among all components. Bisdemethoxycurcumin showed a drastically higher pancreatic distribution in the MG + SJD (P < 0.05, Table 2). No significant difference was observed between the two groups for the remaining components.
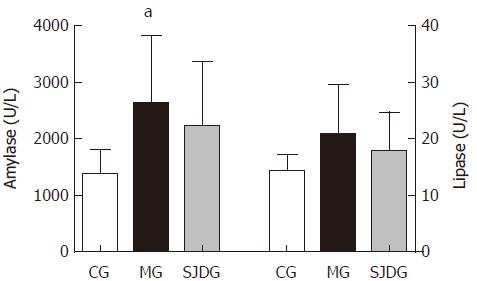
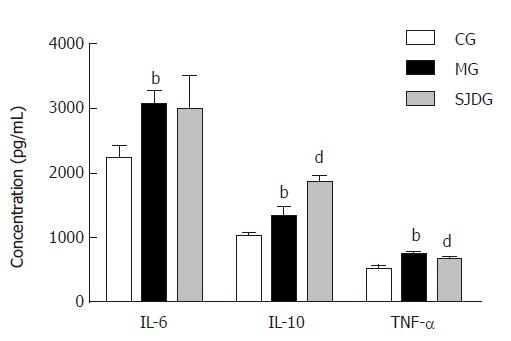
The serum amylase level was higher in the MG than in the CG (P < 0.05, Figure 4), which showed that the AP model was established successfully, while the lipase level in the two groups showed no significant difference. The IL-6, IL-10, and TNF-α levels in the MG were significantly higher than those in the CG (P < 0.05, Figure 5). Compared to the MG, the SJDG exhibited a significantly higher IL-10 level and a lower TNF-α level (P < 0.05, Figure 5).
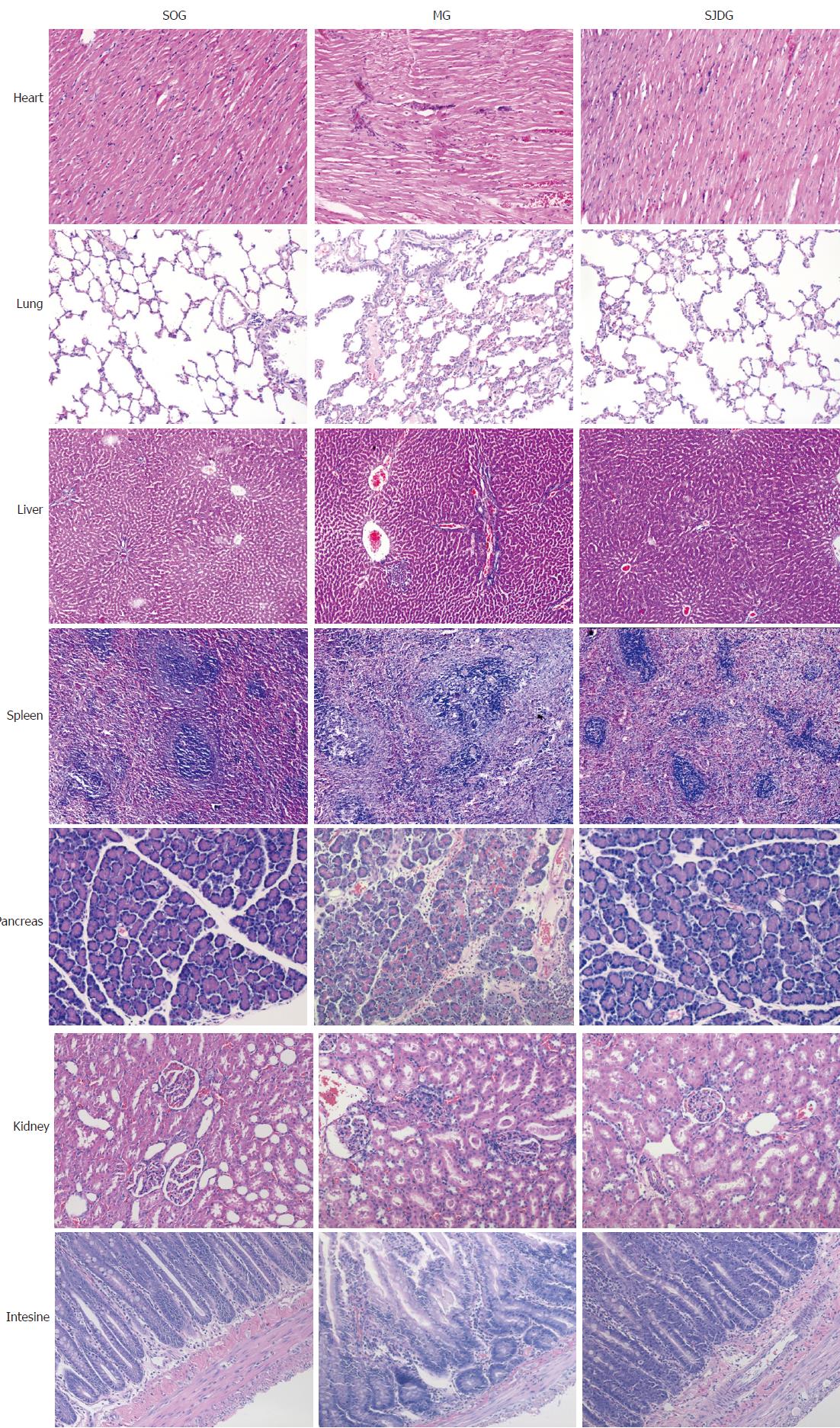
The CG showed no significant signs of edema, hemorrhage, inflammatory cell infiltration, or necrosis in the heart, lung, liver, spleen, pancreas, kidney, and intestine tissues. However, the MG exhibited a characteristic feature of pancreatitis. Pancreatic tissues showed interstitial congestion, edema, inflammatory cell infiltration, focal or confluent necrosis and hemorrhage. The pathological images indicated obvious edema in the alveolar space and lung interstitium, broadened alveolar wall, inflammatory cell infiltration, telangiectasia, congestion, focal or flake hemorrhage and necrosis. The kidney samples exhibited edema and inflammatory cell infiltration in the renal interstitium, blurry boundaries in renal tubule epithelial cells, and stenosis or atresia in the lumens. The intestinal mucosa showed inflammatory cell infiltration in various mucosal layers, broadened intervillous lacunae, decreased beaker cells, and atrophic mucosa. Organic damage was also found in the heart, liver, and spleen of rats in the MG.
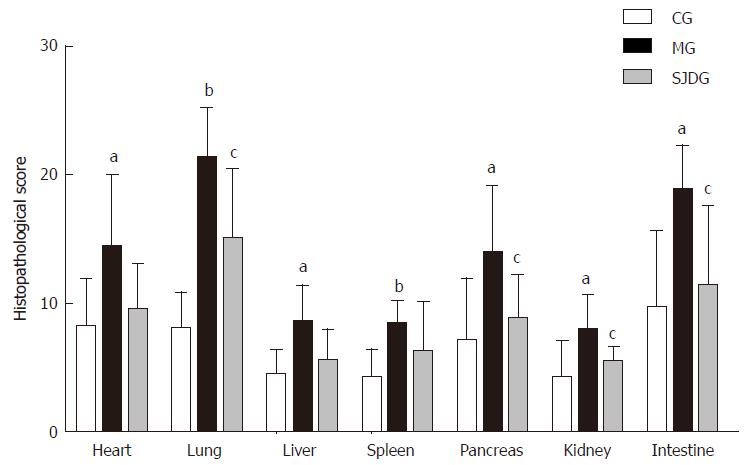
The MG had higher pathological scores than the CG for each organ tested (P < 0.05, Figure 6). After treatment with SJD, the pathological scores of the lung, pancreas, kidney, and intestine in the SJDG were significantly lower than those in the MG (P < 0.05, Figure 6). The pathological images of the seven organs are shown in Figure 7.
We performed pharmacokinetic research to study the effect of AP on the metabolic processes of SJD in vivo and found that AP had varying effects on the pharmacokinetics of different components of SJD. In addition to the pharmacodynamic study, we further assessed the therapeutic properties and mechanisms of SJD involved in attenuating AP through the regulation of inflammatory responses to protect against multiple organ injury.
Our previous study has established a quantitative method to determine 10 major components from Chinese Herbal Dachengqi Decoction (DCQD) simultaneously in rats and dogs[21,22]. Using the established method of HPLC-MS/MS, the main absorbed components including emodin, aloe-emodin, rhein, chrysophanol, curcumin, demethoxycurcumin, and bisdemethoxycurcumin in rats after administration of SJD were detected. The study confirmed that the seven major components of SJD could be absorbed into rat serum and pancreas through oral administration.
The pharmacokinetic parameters showed that AP had varying effects on different components of SJD. This study indicated that AP could promote the pharmacokinetic processes of emodin and aloe-emodin in rats, which is consistent with our previous studies[23,24], thus demonstrating that AP could lower the blood concentration and accelerate the process of elimination of aloe-emodin.
However, the clearly higher AUC and lower CL values of rhein in the MG + SJD indicate that rhein could be better absorbed in rats with AP. This finding is in sharp contrast with the results regarding rhein from the DCQD study, which presented a lower AUC and higher pancreatic distribution in the AP group[23]. One possible explanation may be that concentrations of absorbed components may be associated with the effects of the Chinese herbal formula and the complex interactions among different components[25,26]. Interestingly, although the concentration of rhein in the pancreas in the MG + SJD was lower than that in the CG + SJD, it was highest among other components in the pancreas (Table 2). This is consistent with the results of the DQCD study, which provides evidence that rhein may be the potential active component of pancreas-targeted treatment in rats with AP[27-29].
Furthermore, this study found that AP could slow the pharmacokinetic process of chrysophanol. In addition, AP had almost no influence on the pharmacokinetics of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Nevertheless, due to its higher concentration in the pancreas than in the CG + SJD, bisdemethoxycurcumin may be another potential active component of SJD for the treatment of AP.
Other studies have also revealed the effectiveness of rhein and bisdemethoxycurcumin in anti-inflammation in AP. Liu et al[30] demonstrated that rhein glucoside, rhein isomer methylation, and emodin glucuronide conjugation were the main anti-AP components in Da-Huang-Fu-Zi-Tang. It has been reported that rhein attenuates inflammation via inhibition of NF-κB and NALP3 inflammasome pathways in vivo and in vitro[31]. In order to reach sufficient therapeutic accumulation in the pancreas to inhibit both the local and systemic complications with AP, the inflammatory compound rhein has been tailored as dual pancreas- and lung-targeting therapy mediated by a phenolic propanediamine moiety[32].
Bisdemethoxycurcumin has been demonstrated to exhibit anti-oxidative and anti-inflammatory activities such as inhibiting NO production and COX-2 and iNOS expression and suppressing LPS-induced IκB-α phosphorylation[33,34], promote apoptosis through a GRP78-dependent pathway and mitochondrial dysfunctions, and potentiate the antitumor effect of gemcitabine in human pancreatic cancer cells[35].
Cytokines play major roles in the pathogenesis of AP, including underlying systemic inflammatory responses, tissue damage, and organ dysfunction. Due to the release of pancreatin and activation of mononuclear macrophages, the excess neutrophilic leukocytes may produce or release substantial inflammatory mediators that form a network to cause inflammatory “cascade effects”, which manifest clinically as SIRS[36] in AP. When SIRS is persistent, there is an increased risk of developing multiple organ failure[37]. TNF-α, an early onset pro-inflammatory cytokine, directly injures the cells of multiple organs, causes ischemia, hemorrhage, necrosis, inflammation, and edema, and even triggers the synthesis of a wide range of other pro-inflammatory mediators such as IL-6 and IL-1[38]. IL-6, which produces extensive pro-inflammatory effects that cause tissue damage, has been identified as an early biomarker of severe organ failure and mortality[39,40]. Moreover, IL-6 may induce the release of TNF-α in a positive feedback pattern, leading to a vicious cycle[41]. Notably, the elevated IL-10 level, which may inhibit and reduce the synthesis and release of pro-inflammatory cytokines and colony stimulating factor, signifies a pro-inflammatory state in SAP that is associated with an early anti-inflammatory response[42]. In this study, the MG showed higher TNF-α, IL-6, and IL-10 levels than the CG, indicating that AP could result in an inflammatory imbalance at the very beginning of the disease.
The imbalance of inflammatory mediators in circulation and target tissues may aggravate the progression of AP[43]. Moreover, the time courses of serum pro-inflammatory cytokine levels and anti-inflammatory cytokine levels differ, resulting in immune dysregulation, which leads to multisystem organ failure, mortality, and secondary infection[44]. Thus, varying degrees of pathological injuries in the heart, lung, liver, spleen, pancreas, kidney, and intestine were observed microscopically, which implies that SAP exhibits systemic involvement.
These pathological injuries may also explain the differences in pharmacokinetics between the CG + SJD and MG + SJD. Hypoperfusion of organs is common during shock and peripheral circulatory failure in SAP. When gastrointestinal disorders such as gastrointestinal mucosa ischemia and enteroplegia are present, they will have a detrimental impact on the absorption of the decoction[45]. Undermined by the initial local inflammatory reaction and microcirculation disturbance, the distortion of the blood-pancreas barrier may accelerate or inhibit the absorption of certain components into the pancreas[46]. Additionally, the initiation of organ failure in the hepatic and renal systems (such as the decreased serum albumin concentration and glomerular filtration rate), where most drugs are metabolized and eliminated, may also contribute to the changes in the pharmacokinetic parameters of SJD in rats with AP[47,48]. Moreover, other factors that may influence the pharmacokinetics of different components of Chinese herb prescriptions are associated with physical and chemical properties of the components, including the molecular size, lipid solubility, charge, and protein binding rate as well as methods of application[49].
Notably, after treatment with SJD, the SJDG displayed a lower TNF-α level and a higher IL-10 level than the MG, indicating that SJD could regulate the balance of pro- and anti- inflammatory responses in AP. By decreasing pro-inflammatory cytokines and elevating anti-inflammatory cytokines, SJD could reduce inflammatory reactions, thus ameliorating the severity of AP induced by inflammatory responses. Moreover, SJD was effective in relieving injuries of the lung, kidney, intestine, and pancreas, which provided basic evidence of the effectiveness of SJD for its clinical application in the treatment of AP.
Apart from the pharmacokinetic and pharmacodynamic parameters as well as the distribution of the major components of SJD, the distribution of the effective components to other target tissues should be tested to provide more systematic and comprehensive evidence for the Chinese decoction, thus allowing more effective clinical application. Furthermore, the specific molecular mechanism of how the potential active components alleviate the disease needs to be investigated to optimize herbal formulations and therapies.
In conclusion, AP may have varying effects on the pharmacokinetics of the major components of SJD in rats. AP could accelerate the pharmacokinetic process of emodin and aloe-emodin and slow that of rhein and chrysophanol. Rhein and bisdemethoxycurcumin may be potential active components for the treatment of AP. SJD may attenuate AP by regulating inflammatory responses to protect against multiple organ injury.
Acute pancreatitis (AP) is one of the most common gastrointestinal disorders associated with a mortality rate up to 30%-56% among severe cases with systemic inflammatory response syndrome. Shengjiang decoction (SJD) is an effective prescription for the treatment of AP, but the exact active components are not clear. Little is known about the in vivo metabolic process of SJD. Therefore, full elucidation of the pharmacokinetic and pharmacodynamic mechanisms of SJD associated with the amelioration of AP is urgently needed.
This study aimed to explore the pharmacokinetics, pharmacodynamics, and pancreatic distribution of the main components of SJD in rats with AP to provide pharmacokinetic and pharmacodynamic evidence for its clinical application for the treatment of AP in the future.
This study aimed to explore the pharmacokinetics and pharmacodynamics of SJD in rats with AP for protecting against multiple organ injury.
The AP model was established by retrograde perfusion of 3.5% sodium taurocholate into the biliopancreatic duct, which was widely accepted and used in the induction of AP in rats.
The concentrations of the main components of SJD in serum were measured by HPLC-MS/MS, which is a simple, rapid, accurate, and sensitive way to detect the components in serum and tissues of SJD. Analyst 1.4.2 software for HPLC-MS/MS was used for data collection.
All statistical analyses were performed with PEMS3.1 statistical software for windows. Quantitative data are expressed as the mean ± standard deviation when normally distributed. Comparisons of the pharmacokinetic parameters were performed by Student’s t-test. One-way repeated-measures ANOVA followed by multiple pair-wise comparisons using the Student-Newman-Keuls test was used to detect differences of the pharmacodynamic parameters.
In the pharmacokinetic experiment, the MG + SJD displayed significantly shorter mean residence time (MRT) and higher clearance (CL) for emodin and aloe-emodin; significantly shorter Tmax and T1/2 and a lower area under curve (AUC) for aloe-emodin; an apparently higher AUC and lower CL for rhein; and longer MRT and lower CL for chrysophanol than the CG + SJD. In the pharmacodynamic experiment, the amylase, IL-6, IL-10, and TNF-α levels in MG were higher than those in the CG (P < 0.05). After the herbal decoction treatment, the SJDG had higher IL-10 and lower TNF-α levels than the MG (P < 0.05). The MG had the highest pathological scores, and the pathological scores of the lung, pancreas, kidney, and intestine in the SJDG were significantly lower than those in the MG (P < 0.05). The results revealed metabolic process of the major components of SJD absorbed in serum and pancreas as well as the possible mechanism of SJD in alleviating AP.
What remains to be solved is that the distribution of the effective components to other target tissues to provide more systematic and comprehensive evidence for the clinical application of Chinese decoction. Furthermore, the specific molecular mechanism of how the potential active components alleviate the disease needs to be investigated to optimize herbal formulations and therapies.
In the study, we found that AP may have varying effects on the pharmacokinetics of the major SJD components in rats, rhein and bisdemethoxycurcumin may be potential active components for the treatment of AP, and SJD might alleviate pathological injuries of the lung, pancreas, kidney, and intestine in rats with AP via regulating pro- and anti- inflammatory responses. The conclusions are based on our previous theory of ‘tissue pharmacology of recipe’, and are in accordance with the clinical and experiment results available that SJD is an effective way to alleviate AP.
We report the metabolic processes of major components of SJD in vivo and the pharmacodynamic mechanism of SJD in relieving AP. The study indicated that diseased condition of the body as well as formula composition may have certain effect on the metabolic process of different components in decoctions. As AP involves systemic inflammatory responses, based on the findings above, the mechanism for SJD to attenuate AP may be through the regulation of inflammatory responses to protect against multiple organ injury.
As we have found the potential components of SJD in alleviating AP, further investigation about the interaction of these components is urgently needed to provide evidence for optimizing and simplifying the formula. Moreover, more in-depth studies about the molecular mechanism of SJD in alleviating AP should be explored to have a deeper and more comprehensive understanding of SJD in treating AP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chiu KW, Dambrauskas Z, Liao KF S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Ince AT, Baysal B. Pathophysiology, classification and available guidelines of acute pancreatitis. Turk J Gastroenterol. 2014;25:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1344] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 3. | Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 471] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 553] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 5. | Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, Windsor JA; Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 6. | Chen W, Yang X, Huang L, Xue P, Wan M, Guo J, Zhu L, Jin T, Huang Z, Chen G. Qing-Yi decoction in participants with severe acute pancreatitis: a randomized controlled trial. Chin Med. 2015;10:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Ji CH, Tang CW, Feng WM, Bao Y, Yao LQ. A Chinese Herbal Decoction, Huoxue Qingyi Decoction, Promotes Rehabilitation of Patients with Severe Acute Pancreatitis: A Retrospective Study. Evid Based Complement Alternat Med. 2016;2016:3456510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Zhu SF, Guo H, Zhang RR, Zhang Y, Li J, Zhao XL, Chen TR, Wan MH, Chen GY, Tang WF. Effect of electroacupuncture on the inflammatory response in patients with acute pancreatitis: an exploratory study. Acupunct Med. 2015;33:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Wan MH, Yao J, Li J, Xia Q, Zhu L, Tang WF. The effectiveness of purgation and electroacupuncture in extrahepatic bile duct stone complicated with acute biliary pancreatitis: management of biliary stone pancreatitis through traditional Chinese medicine. Pancreas. 2011;40:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Lai F, Zhang Y, Xie DP, Mai ST, Weng YN, Du JD, Wu GP, Zheng JX, Han Y. A Systematic Review of Rhubarb (a Traditional Chinese Medicine) Used for the Treatment of Experimental Sepsis. Evid Based Complement Alternat Med. 2015;2015:131283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Chen J, Jiang D, Zhang P. Adjuvant treatment with crude rhubarb for patients with systemic inflammation reaction syndrome/sepsis: a meta-analysis of randomized controlled trials. J Crit Care. 2015;30:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Gulcubuk A, Altunatmaz K, Sonmez K, Haktanir-Yatkin D, Uzun H, Gurel A, Aydin S. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 14. | Sun K. Shengjiangsan combined with routine therapy for effect on severity score and serological nutrition indicators of patients with SIRS/MODS. Shandong University of TCM. 2013;. |
| 15. | Gu WY, Zhao L, Qian FH, Wu Y, Cao DF. Treatment of Acute Pancreatitis with Shengjiang Powder. Jilin Zhongyiyao. 2013;33:1232-1234. |
| 16. | Gao B, Li R, Zhu L, Zhang W, Qian Y. Study of Shengjiangsan on Major Chinese Medicine Clinical Symptoms in Patients with Early Sepsis. Liaoning Zhongyiyao Daxue Xuebao. 2009;11:100-102. [DOI] [Full Text] |
| 17. | Niu D, Hou F. Effects of Shengjiang powder on contents of 6-keto-PGF-1α, TXB2, TNF-α and EGF in rats with acute gastric mucosal damage. Wujing Yixue. 2005;16:417-419. |
| 18. | Qian YM, Zhu L, Gao B. Effect of serum IL-2, IL-4 and IL-6 intervention in patients with systemic inflammatory response syndrome. Zhongwai Yiliao. 2008;10:34-35. [DOI] [Full Text] |
| 19. | Han JH, Su HS. Clinical effect of Modified Decoction Combined with metformin in the treatment of nonalcoholic fatty liver disease with metabolic syndrome. Shanxi Zhongyi. 2013;989-991. |
| 20. | Li J, Tang WF. Effect of Sheng-jiang-san in regulating obesity-mediated systemic inflammatory microenviroment and mitigating multiple organ injury in obese rats with acute pancreatitis. Proceedings of The Twelfth National Conference of Chinese traditional and Western medicine experimental medical Specialized Committee. 2015;41-42. |
| 21. | Liu YL, Zhao XL, Li J, Wan MH, Chen GY, Chen WW, Tang WF. Effect of acute pancreatitis on the pharmacokinetics of Chinese herbal micron Liuhe Pill ointment in rats. Chin J Integr Med. 2015;21:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Tang WF, Yu Q, Xiang J. Effect of AP on the pharmacokinetics of Dachengqi Decoction in rats. The twentieth National Conference on digestive system diseases of Chinese traditional and Western Medicine and Compilation of papers on the progress in diagnosis and treatment of digestive diseases. 2008;178. |
| 24. | Gong HL, Tang WF, Yu Q, Xiang J, Xia Q, Chen GY, Huang X, Liang MZ. Effect of severe acute pancreatitis on pharmacokinetics of Da-Cheng-Qi Decoction components. World J Gastroenterol. 2009;15:5992-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Gong HL, Tang WF, Wang J, Chen GY, Huang X. Effect of formula compatibility on the pharmacokinetics of components from Dachengqi Decoction [See Text] in rats. Chin J Integr Med. 2012;18:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zhang W, Di LQ, Li JS, Shan JJ, Kang A, Qian S, Chen LT. The effects of Glycyrrhizae uralenis and its major bioactive components on pharmacokinetics of daphnetin in Cortex daphnes in rats. J Ethnopharmacol. 2014;154:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Li JY, Zhu L, Xiang J, Zhao JL, Li J, Guo H. Targeted tissue distribution of Da-Cheng-Qi decoction in rat with acute. Zhongguo Shiyan Fangyixue Zazhi. 2016;22:40-45. |
| 28. | Zhao J, Tang W, Wang J, Xiang J, Gong H, Chen G. Pharmacokinetic and pharmacodynamic studies of four major phytochemical components of Da-Cheng-Qi decoction to treat acute pancreatitis. J Pharmacol Sci. 2013;122:118-127. [PubMed] |
| 29. | Zhao XL, Xiang J, Wan MH, Yu Q, Chen WW, Chen GY, Tang WF. Effect of acute pancreatitis on the pharmacokinetics of Chinese herbal ointment Liu-He-Dan in anaesthetized rats. J Ethnopharmacol. 2013;145:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Liu X, Wang XL, Wu L, Li H, Qin KM, Cai H, Pei K, Liu T, Cai BC. Investigation on the spectrum-effect relationships of Da-Huang-Fu-Zi-Tang in rats by UHPLC-ESI-Q-TOF-MS method. J Ethnopharmacol. 2014;154:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, Ma Z. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Li J, Zhang J, Fu Y, Sun X, Gong T, Jiang J, Zhang Z. Dual pancreas- and lung-targeting therapy for local and systemic complications of acute pancreatitis mediated by a phenolic propanediamine moiety. J Control Release. 2015;212:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Guo LY, Cai XF, Lee JJ, Kang SS, Shin EM, Zhou HY, Jung JW, Kim YS. Comparison of suppressive effects of demethoxycurcumin and bisdemethoxycurcumin on expressions of inflammatory mediators in vitro and in vivo. Arch Pharm Res. 2008;31:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Kim AN, Jeon WK, Lee JJ, Kim BC. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic Biol Med. 2010;49:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Yang HP, Fan SJ, An Y, Wang X, Pan Y, Xiaokaiti Y, Duan JH, Li X, Tie L, Ye M. Bisdemethoxycurcumin exerts pro-apoptotic effects in human pancreatic adenocarcinoma cells through mitochondrial dysfunction and a GRP78-dependent pathway. Oncotarget. 2016;7:83641-83656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Mansfield C. Pathophysiology of acute pancreatitis: Potential application from experimental models and human medicine to dogs. J Vet Intern Med. 2012;26:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, Banks PA. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Akinosoglou K, Gogos C. Immune-modulating therapy in acute pancreatitis: fact or fiction. World J Gastroenterol. 2014;20:15200-15215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Phillip V, Steiner JM, Algül H. Early phase of acute pancreatitis: Assessment and management. World J Gastrointest Pathophysiol. 2014;5:158-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (2)] |
| 40. | Sathyanarayan G, Garg PK, Prasad H, Tandon RK. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J Gastroenterol Hepatol. 2007;22:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Han T, Li X, Cai D, Zhong Y, Chen LG, Geng S, Yin S. Effect of glutamine on apoptosis of intestinal epithelial cells of severe acute pancreatitis rats receiving nutritional support in different ways. Int J Clin Exp Pathol. 2013;6:503-509. [PubMed] |
| 42. | Laveda R, Martinez J, Munoz C, Penalva JC, Saez J, Belda G, Navarro S, Feu F, Mas A, Palazon JM. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol. 2005;11:5309-5313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H, Lecron JC. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014;14:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Shen Y, Deng X, Xu N, Li Y, Miao B, Cui N. Relationship between the degree of severe acute pancreatitis and patient immunity. Surg Today. 2015;45:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Landahl P, Ansari D, Andersson R. Severe Acute Pancreatitis: Gut Barrier Failure, Systemic Inflammatory Response, Acute Lung Injury, and the Role of the Mesenteric Lymph. Surg Infect (Larchmt). 2015;16:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Tomkötter L, Erbes J, Trepte C, Hinsch A, Dupree A, Bockhorn M, Mann O, Izbicki JR, Bachmann K. The Effects of Pancreatic Microcirculatory Disturbances on Histopathologic Tissue Damage and the Outcome in Severe Acute Pancreatitis. Pancreas. 2016;45:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Petejova N, Martinek A. Acute kidney injury following acute pancreatitis: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Ou ZB, Miao CM, Ye MX, Xing DP, He K, Li PZ, Zhu RT, Gong JP. Investigation for role of tissue factor and blood coagulation system in severe acute pancreatitis and associated liver injury. Biomed Pharmacother. 2017;85:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Xu H, Jiang J, Xia ZL, Wang ML. Permeability of cefpiramide through blood- pancreatic barrier in the mice with sever acute pancreatitis. Zhongguo Yaowu Jingjie. 2014;11:645-648. |