Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8027
Peer-review started: June 22, 2017
First decision: July 13, 2017
Revised: July 28, 2017
Accepted: September 6, 2017
Article in press: September 6, 2017
Published online: December 7, 2017
Processing time: 166 Days and 12.1 Hours
To analyze the homogeneity of pathologic response to preoperative chemotherapy (PRPC) after chemotherapy in patients with multiple liver metastases (LM).
From September 2011 to August 2014, patients with at least two LM undergoing preoperative chemotherapy prior to resection were included in this retrospective, single-center study. The endpoints were PRPC homogeneity (according to both the Rubbia-Brandt and MD Anderson classifications), the impact of PRPC on the MDT decision, factors associated with homogeneous PRPC and overall survival of patients with vs. without homogeneous PRPC.
seventy-three patients with a total of 88 liver resections (including 15 two-stage procedures) were included in the study. The homogeneous PRPC rate was 55% according to the Rubbia-Brandt classification and 53% according to the MD Anderson classification. The MDT decision was modified by the PRPC in only 2.7% of patients (n = 2).
The PRPC was homogeneous in only one half of patients and had very little influence on the MDT decision.
Core tip: Pathologic response to preoperative chemotherapy (PRPC) is correlated with survival after resection of liver metastases. This study analyzed the homogeneity of PRPC after chemotherapy in patients with multiple liver metastases. The study underlines that homogeneous PRPC rate was low (55% according to the Rubbia-Brandt classification and 53% according to the MD Anderson classification) and has little impact on the multidisciplinary team meeting decision (modified by the PRPC in only 2.7% of patients).
- Citation: Sabbagh C, Chatelain D, Attencourt C, Joly JP, Chauffert B, Cosse C, Regimbeau JM. Impact of homogeneous pathologic response to preoperative chemotherapy in patients with multiple colorectal liver metastases. World J Gastroenterol 2017; 23(45): 8027-8034
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8027
One half of patients with colorectal cancer develop liver metastases (LM) with a 5-year overall survival rate of 50%[1,2]. The curative management of LM includes surgical resection and chemotherapy (combined with targeted therapies, in some cases)[3-5].
Three main classifications of pathologic response to preoperative chemotherapy (PRPC) have been described (Rubbia-Brandt classification[6], MD Anderson classification[7] and the Sebagh classification)[8]. Two of these classifications, the Rubbia-Brandt classification and the MD Anderson classification, are used in routine clinical practice in our institution. The Rubbia-Brandt classification[6] is based on whether or not chemotherapy induces fibrosis in the metastasis, whereas the MD Anderson classification reflects the proportion of metastatic tumor cells that remain viable after chemotherapy[7]. A complete PRPC is defined as the absence of tumor cells at the liver site in both classifications[7]. However, the two classifications differ markedly in patients with multiple LM. The Rubbia-Brandt classification is based on the worst nodule, whereas the MD Anderson classification is based on the mean PRPC of all the nodules. Furthermore, the best category (in terms of survival) in the Rubbia-Brandt classification includes both complete tumor regression (tumor regression grade 1, TRG1) and a major response (TRG2), whereas the best category in the MD Anderson classification consists solely of a complete response[9-11].
PRPC scores appear to be correlated with survival after LM resection[6] but the really use of PRPC is a daily question. PRPC may be useful in three situations in which adjuvant chemotherapy may be required in patients with multiple LM, provided a homogeneous response is observed for all LM: (1) after neoadjuvant chemotherapy in a perioperative management setting; (2) between surgical stages in patients scheduled for two-stage hepatectomy for bilobar LM; and (3) in the case of recurrence. In these situations, PRPC could help to guide modification of the chemotherapy regimen when necessary.
For example, in the two-stage hepatectomy setting, Mentha et al. addressed this question by studying the difference in TRG grade between the two operative specimens from a given individual patient as a measure of chemotherapy resistance due to interruption of treatment or as a result of the immunosuppression that follows a surgical procedure[12]. However, Sebagh et al[8] did not assess the homogeneity of the PRPC in individual patients or whether a homogeneous PRPC had an impact on prognosis. Recently, Sebagh et al reported for the first time a 19.7% rate of PRPC heterogeneity.
The objective of the present study was therefore to analyze the homogeneity of PRPC after chemotherapy and to assess the impact of PRPC on the multidisciplinary team meeting (MDT) decision, on survival and on the management of two-stage procedures.
From September 2011 to August 2014, patients undergoing resection of at least two colorectal cancer LMs, who had received preoperative chemotherapy and for whom both PRPC classifications were available were included in the present study.
This was a retrospective, single-center study. Data were extracted from a single-center database. The study was initiated following systematic use of PRPC by our institution (according to the MD Anderson and Rubbia-Brandt classifications) in pathology reports and MDTs.
Patient-related: Age, gender, body mass index (BMI), and comorbidities. Tumor-related: Number of metastases, size of the metastases (after chemotherapy on pathological exam), site in the liver (central or peripheral), primary tumor stage (according to the TNM classification) and site (colon or rectum), and tumor markers (CEA, CA 19.9). Treatment-related: Type of chemotherapy, number of cycles, and association with targeted therapy. Related to surgery: The type of liver resection (minor or major). Related to pathologic response: A homogeneous PRPC (defined as the same classification for all metastases resected in a given patient, for example all metastases were classified as having a major regression when Rubbia-Brandt classification is considered and for example all metastases had a minor response to PRPC when the MD Anderson classification is considered). A heterogenous PRPC was determined if in a single specimen, one metastasis had a major regression and others have partial regression or no regression in the Rubbia-Brandt classification or if in a single specimen, one metastasis had a major PRPC and others have complete or minor PRPC in the MD Anderson classification. The homogeneity of PRPC was assessed separately for each classification (Rubbia-Brandt and MD Anderson), but the two classifications were not compared in terms of homogeneity of PRPC.
The primary endpoints were homogeneity of the PRPC according to the Rubbia-Brandt classification and the MD Anderson classification, and the impact of PRPC on the MDT decision.
The secondary endpoints were factors associated with homogeneous PRPC and the PRPC between the two surgical procedures for patients who underwent two-stage hepatectomy with preoperative chemotherapy.
Only operative specimens and no biopsies were examined. All specimens were examined independently by two pathologists (DC + AC). Tumors less than 2 cm in diameter were fully embedded, while an average of 5 slides were taken from tumors measuring more than 2 cm. All data were reported on a standardized pathology report form and included the Rubbia-Brandt and MD Anderson classifications for all LM[6,7]. The pathologists were blinded from each other for the analysis but in the event of disagreement between the two pathologists, a consensus was reached.
Rubbia-Brandt classification: The Rubbia-Brandt classification scores patients from TRG 1 to TRG 5 (Table 1)[6]. Patients were then categorized into three groups, defined as major regression (TRG1 or TRG2), partial regression (TRG3) or no regression (TRG4 or TRG5). Patients with multiple liver metastases and heterogeneous PRPC scores were categorized according to the poorest response.
| The Rubbia-Brandt classification | The MD Anderson classification |
| TRG1: Absence of residual cancer and large amount of fibrosis | Complete response: No residual cancer cells |
| TRG2: Rare residual cancer cells scattered throughout the fibrosis | Major response: 1%-49% of residual cancer cells |
| TRG3: More residual tumor cells but fibrosis predominates | Minor response: More than 50% of residual cancer cells |
| TRG4: Residual cancer cells predominate over fibrosis | |
| TRG5: No signs of regression. |
MD Anderson classification: The MD Anderson classification scores patients as having complete, major or minor PRPC (Table 1)[7]. Patients with multiple liver metastases and heterogeneous PRPC scores were categorized according to the mean response.
All operated patients were discussed at the MDT meeting before and after liver surgery. In the situation of liver metastases, at our institution, all patients had preoperative chemotherapy except for patients with small LM that could disappear with preoperative chemotherapy or patients with a limited number of metastases who had the resection of the primary tumour during the same procedure than liver resection. The decision to use a target agent was considered on a case-by-case basis. The MDT records were standardized and accessed with in-house software. The MDT decision (withdrawal from postoperative chemotherapy or modification of the chemotherapy regimen) was noted by the team leader (JPJ) in a register. The MDT records and decisions were analyzed retrospectively. The reason for modifying chemotherapy was always reported (disease progression, treatment response, or toxicity), thereby identifying all cases in which, the MDT decision was modified by either a complete PRPC or no PRPC.
Data are expressed as mean ± SD, median (range) or number (percentage). Logistic regression analysis was then performed to identify risk factors for homogeneous PRPC, with homogeneous PRPC as dependent variable. Only variables with a P-value < 0.2 in univariate analysis were included as independent variables in a multivariate model. Variables with a P value ≤ 0.1 in the multivariable model were considered to be risk factors. Statistical analyses were performed by a datamanager with SAS 9.2 statistical analysis software (SAS Institute Inc., Cary, NC, United States).
The present study was reviewed and approved by the Commission Nationale de l’information et des libertés (CNIL) with the number DRCI T135.
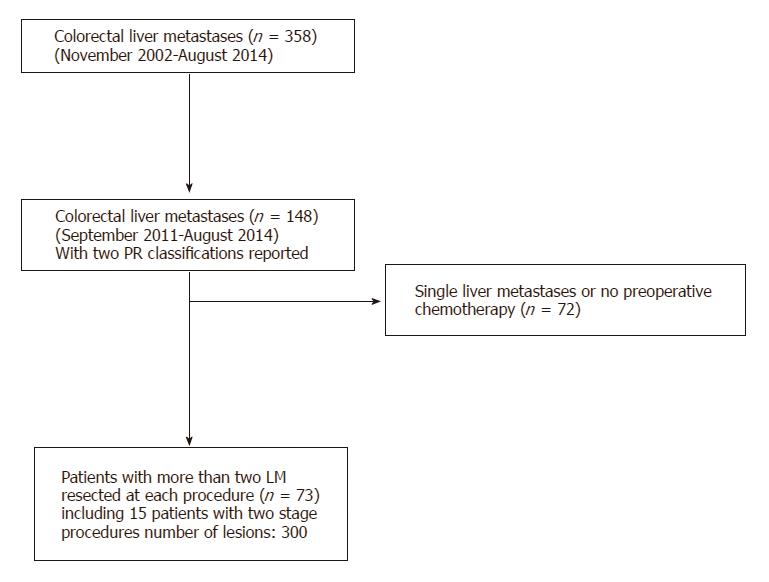
Seventy-three patients (with a total of 300 LM, including 15 two-stage procedures) met the inclusion criteria and were included in the study (Figure 1). The study population had a median age of 62.5 years (range: 40-80) and included 45 men (61%). The primary tumor was located in the colon in 66% of patients (n = 48). It was on the ascending colon in 14% (n = 14), on the transverse colon in 7% (n = 5), on the descending colon in 45% (n = 45) and in the rectum in 34% (n = 25). The primary tumour was resected in 98% (n = 72) of the cases with a mean delay between the primary tumour resection and the first liver resection of 15.2 mo (range: 2-60). The median number of LM was 3 (range: 1-14), and metastases were synchronous with the primary tumor in 75% of patients (n = 55). The rate of patients with BRAF mutation was 5% (n = 4). The rate of patients with KRAS mutation was 9.5% (n = 7). The sites of LM are detailed in Table 2. The chemotherapy regimen included targeted therapy in 45% of cases (n = 40). The median number of preoperative cycles was 12 (range: 4-38) and the median number of overall cycles was 17 (range: 4-42) (Table 2). Median follow-up was 17 mo (ext: 2-78).
| Variable | Study population |
| Demographic data | |
| Male gender | 45 (61) |
| Age, median (range), yr | 62.5 (40-80) |
| body mass index, mean ± SD, kg/m2 | 25.36 ± 4.42 |
| Tumor markers | |
| CEA level, mean ± SD (mg/L) | 17 ± 3.5 |
| Ca 19.9 level, mean ± SD (UI/L) | 23 ± 5.2 |
| Primary tumor site | |
| Ascending colon | 10 (14) |
| Transverse colon | 5 (7) |
| Descending colon | 33 (45) |
| Rectum | 25 (34) |
| Liver metastases | |
| Median (range) number of preoperative LM | 3 (1-14) |
| Synchronous LM | 55 (75) |
| Surgical procedure | |
| Right hepatectomy | 15 (16) |
| Left lobectomy | 4 (4) |
| Right lobectomy | 3 (3) |
| Posterior segmentectomy | 8 (11) |
| Wedge | 58 (66) |
| Two-stage hepatectomy | 15 (17) |
| Site of the 300 metastases (%) | |
| I | 2.5 |
| II | 10 |
| III | 17.5 |
| IV | 11 |
| V | 16 |
| VI | 20 |
| VII | 13 |
| VIII | 10 |
| Preoperative chemotherapy | |
| Regimen | |
| Folfox | 28 (32) |
| Folfiri/Folfox and bevacizumab | 28 (32) |
| Folfiri with or without cetuximab | 8 (9) |
| Campto or folfiri with or without cetuximab | 20 (23) |
| Folfirinox | 4 (4) |
| Median (range) number of preoperative cycles | 12 (4-38) |
| Pathology | |
| T stage | |
| 2 | 7 (9) |
| 3 | 56 (77) |
| 4 | 10 (14) |
| N stage | |
| 0 | 18 (24) |
| 1 | 37 (51) |
| 2 | 11 (15) |
| X | 7 (10) |
| Median (range) size of metastases, cm | 3.1 (0.2-5) |
| PRPC | |
| Rubbia-Brandt classification | |
| Major response | 13 (15) |
| Partial response | 12 (14) |
| Absence of response | 63 (71) |
| MD Anderson classification | |
| Complete response | 8 (9) |
| Major response | 26 (30) |
| Minor response | 54 (61) |
According to the Rubbia-Brandt classification, 15% of patients (n = 13) displayed major response, 14% (n = 12) displayed partial response and 71% (n = 63) had no response. The rate of concordance between the two pathologists for the Rubbia-Brandt classification was 98% (n = 86).
According to the MD Anderson classification, 9% of patients (n = 8) displayed a complete response, 30% (n = 26) displayed a major response and 61% (n = 54) displayed a minor response. The rate of concordance between the two pathologists for the MD Anderson classification was 96% (n = 85). A concordance was observed between the two classifications in 69% of cases (n = 61).
Homogeneity of PRPC: According to the Rubbia-Brandt classification, 55% of patients (n = 48) obtained a homogeneous PRPC. According to the MD Anderson classification, 53% of patients (n = 47) obtained a homogeneous PRPC.
Impact of PRPC on the MDT decision: The PRPC changed the MDT decision in only 2 cases (2.7%; withdrawal of chemotherapy in both cases). For both patients, the PRPC was classified as major in the Rubbia-Brandt classification or complete in the MD Anderson classification. The PRPC was homogeneous in both patients and according to both classifications. Both patients had severe (grade III) oxaliplatin-induced peripheral neuropathy. The absence of PRPC or the presence of heterogeneous PRPC did not change the MDT decision in any of the other cases.
Factors associated with a homogeneous PRPC: For the Rubbia-Brandt classification, only the use of bevacizumab [OR (95%CI): 3.5 (1.2- 10.5); P = 0.02] was associated with a homogeneous PRPC (Table 3).
| Variable | Homogeneity (Rubbia-Brandt) | Homogeneity (MD - Anderson) | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| OR [95%CI] | P value | OR [95%CI] | P value | OR [95%CI] | P value | OR [95%CI] | P value | |
| Age | 2.33 [0.89-6.07] | 0.82 | / | / | 1.5 [0.61-3.67] | 0.37 | / | / |
| Gender | 1 [0.48-2.09] | 0.99 | / | / | 1 [0.48-2.09] | 0.99 | / | / |
| Hypertension | 1.13 [0.43-2.92] | 0.81 | / | / | 1.13 [0.43-2.92] | 0.81 | / | / |
| Body mass index | 0.99 [0.98-1.02] | 0.95 | / | / | 1.01 [0.98-1.03] | 0.86 | / | / |
| Rectal cancer | 1.14 [0.56-2.34] | 0.72 | / | / | 1.73 [0.82-3.63] | 0.15 | / | / |
| Number of peroperative LM | 0.96 [0.86-1.07] | 0.45 | / | / | 0.99 [0.89-1.10] | 0.87 | / | / |
| Time interval between chemotherapy and surgery | 3 [0.31-28.84] | 0.34 | / | / | 1.5 [0.53-4.21] | 0.44 | / | / |
| Folfiri-based chemotherapy | 0.007 [0.09-0.6] | 0.90 | 0.8 [0.3-2.0] | 0.60 | ||||
| Metachronous liver metastases | 2.11 [0.96-4.67] | 0.14 | 2.8 [0.92-8.5] | 0.06 | 1.33 [0.63-2.82] | 0.45 | / | / |
| T stage | 1.26 [0.73-2.18] | 0.41 | / | / | 1.17 [0.68-2.01] | 0.58 | / | / |
| N0 stage | 0.8 [0.22-2.98] | 0.74 | / | / | 0.8 [0.22-2.98] | 0.74 | / | / |
| ASA score | 1.05 [0.88-1.25] | 0.62 | / | / | 1.05 [0.88-1.26] | 0.56 | / | / |
| MSI | 1.9 [0.2-18.3] | 0.90 | 1.5 [0.2-9.8] | 0.60 | ||||
| RAS status | 1.05 [0.0-99] | 0.90 | 4.5 [0.8-23.9] | 0.30 | ||||
| Braf mutation | 1.6 [0.0-120] | 0.90 | 3.3 [0.32-34.6] | 0.30 | ||||
| Use of bevacizumab | 3.20 [1.17-8.74] | 0.02 | 3.5 [1.2-10.5] | 0.02 | 1.33 [0.56-3.16] | 0.51 | / | / |
| Metastases in the left lobe of the liver | 0.67 [0.24-1.87] | 0.44 | / | / | 0.67 [0.24-1.87] | 0.44 | / | / |
| Number chemotherapy cycles | 1.79 [0.93- 3.44] | 0.12 | 1.06 [0.97-1.1] | 0.10 | 1.44 [0.76- 2.72] | 0.27 | / | / |
For the MD Anderson classification, no factor was associated with a homogeneous PRPC (Table 3).
PRPC in two-stage procedures: After the first stage of hepatectomy, a homogeneous PRPC was observed in 100% of cases (n = 15) with the Rubbia-Brandt classification and 73% of cases (n = 11) with the MD Anderson classification. After the second stage of hepatectomy, a homogeneous PRPC was observed in 53% of cases (n = 8) with the Rubbia-Brandt classification and 53% of cases (n = 8) with the MD Anderson classification.
A homogeneous PRPC was obtained in only 55% of cases according to the Rubbia-Brandt classification and in only 53% of cases according to the MD Anderson classification and PRPC had little impact on the MDT decision and patient survival. This study is the second to report these findings and to have identified factors associated with a homogeneous PRPC.
Recently, Sebagh et al[8] reported a heterogeneous PRPC in 19.7% of cases. In their study, the authors considered PRPC to be heterogeneous when at least 50% of metastases did not present the same PRPC. They also demonstrated the lack of impact of homogeneous PRPC on survival. In another study by the same group, the authors emphasized the limited impact of PRPC on survival according to the definition of heterogeneous PRPC. Thus, according to the MD Anderson classification, PRPC was not a prognostic factor when based on the mean value but tended towards significance when based on the median PRPC[8].
The high proportion of major or complete PRPC (i.e. similar to the rates reported in the literature) and the high quality of examination of our specimens support the robustness of the present study. Our findings therefore question the real value of PRPC in everyday practice. In our series, the PRPC influenced the MDT decision in only 2 cases. In everyday practice, the decision to prescribe adjuvant chemotherapy is based on laboratory data (decreased tumor marker levels), morphological data (RECIST score) and clinical data (postoperative performance status and tolerability of chemotherapy) and randomized controlled clinical trials and cohort studies have such a major impact on the decision to prescribe perioperative and adjuvant chemotherapy[5,13], that the potential impact of the PRPC in the MDT decision is negligible.
However, the proportion of patients with a homogeneous PRPC in our series was much lower than that published in the initial report by Rubbia-Brandt et al[6] (90%). Firstly, this disparity might be due to differences in chemotherapy regimens. In the study by Rubbia-Brandt et al[6] none of the patients received targeted therapies vs 61% of the patients in the present study. Secondly, the studies differed in terms of the number of slides prepared per metastasis. In the study by Rubbia-Brandt et al, specimens were prepared as 0.5-cm-thick slices. In the present study, a mean of 10 slides per metastasis were prepared, and metastases measuring less than two centimeters were fully embedded. One can argue that the retrospective design of the study is a limitation since no special analysis or additional slide for each metastasis was performed. Furthermore, no information on the distribution of the residual tumour cells in a single metastasis is available since there is no classification for that particular point.
Our findings concerning the proportion of patients with a homogeneous PRPC also question the conclusions reached by Mentha et al[12] on interval treatment in patients undergoing two-stage hepatectomy. Mentha et al[12] found that, when comparing the PRPC after the first and second stages, 10 out of 22 patients (45%) had a poorer PRPC at the second stage (compared with 23% in the present study). These authors suggested that this difference in PRPC might be due to interruption of treatment for 5-15 wk of chemotherapy[12]. Our results suggest another possible explanation for the difference in PRPC between the two stages of hepatectomy, as a heterogeneous PRPC was observed in one-half of our patients. The difference in PRPC classification therefore cannot be solely attributed to putative chemoresistance between the two stages of hepatectomy. Data on homogeneity also reflect the biological heterogeneity of liver metastases, derived from independent colonies with their own biological profile[14] and information on PRPC homogeneity is crucial regardless of the impact of PRPC on survival as it shows that the treatment strategy does not need to be adapted to the PRPC, which is variable from one metastasis to another.
Bevacizumab is known to be associated with an increased likelihood of complete PRPC. The present study is the first to report the association between bevacizumab and an increased likelihood of homogeneous PRPC[15]. One possible explanation is related to the mechanism of action of bevacizumab (necrosis and modification of vasculogenesis)[16]. The outcomes of this analysis should nevertheless interpret with caution, since there was a lot of tested variable of a limited number of patients and events[17]. An interesting extension of this work would be to perform the same analysis on patients who have received intra-arterial chemotherapy (which is known to influence the PRPC).
Although it has been clearly established that a complete PRPC is a major prognostic factor[6,7], it is a static variable (like age or the presence of metachronous vs synchronous metastases) in contrast with the dynamic nature of tumor markers and the RECIST score, and therefore constitutes another limitation to the practical value of PRPC.
The present study nevertheless presents a number of limitations, due to the heterogeneous characteristics of liver resections, preoperative chemotherapy, inclusion of patients receiving targeted therapy and the number of preoperative cycles. Moreover, as previously demonstrated in the series published by Rubbia-Brandt et al[6] and Kishi et al[7], complete PRPC (but not homogeneous PRPC) is a prognostic factor but the presence of two classifications is disturbing[9] and contributes to the poor understanding and correct use of PRPC. All these points could have a direct impact on the homogeneity of PRPC.
In conclusion, the PRPC was homogeneous in only half of patients with multiple LM and had little impact on the MDT decision. Routine use of PRPC to guide treatment may be questionable (due to differences between classifications and the heterogeneity of the PRPC for multiple LMs in the same patient). Further investigations are therefore necessary in order to improve the value of the PRPC.
Pathologic response to preoperative chemotherapy scores appears to be correlated with survival after liver metastases (LM) resection. Pathologic response to preoperative chemotherapy may be useful in situations in which adjuvant chemotherapy may be required in patients with multiple LM. However a little is know about the homogeneity rate of pathologic response to preoperative chemotherapy and on its use in daily practice.
Fifty percent of patients with colorectal cancer will develop LM with a 5-yr overall survival rate of 50%. The curative management of LM includes surgical resection and chemotherapy (combined with targeted therapies, in some cases.
The study underlines that homogeneous PRPC rate was low and has little impact on the multidisciplinary team meeting decision.
Doing a liver biopsy to know the pathological response to preoperative chemotherapy is useless. Pathological response to preoperative chemotherapy is not a crutial point in MDT discussions.
The authors review a cohort of 73 patients undergoing liver resection for colorectal LM after systemic chemotherapy in order to assess the impact of homogeneity of pathological response to chemotherapy on survival and routine management of patients. They conclude that pathological response to chemotherapy is not a powerful prognostic factor and do not influence treatment or management in patients with advanced resectable LM. Overall this is a concise and well written manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Benoist S, Morris DL, Link A, Ozawa T S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Gigot JF, Schulick RD, Choti MA, Aldrighetti L. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755-764, 764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744-752, 752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 3. | Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-657; discussion 657-658. [PubMed] |
| 4. | Fuks D, Cook MC, Bréhant O, Henegar A, Dumont F, Chatelain D, Yzet T, Mulieri G, Joly JP, Nguyen-Khac E. Colorectal carcinoma with potentially resectable metastases: factors associated with the failure of curative schedule. Gastroenterol Clin Biol. 2008;32:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1440] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 6. | Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Kishi Y, Zorzi D, Contreras CM, Maru DM, Kopetz S, Ribero D, Motta M, Ravarino N, Risio M, Curley SA. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Sebagh M, Allard MA, Bosselut N, Dao M, Vibert E, Lewin M, Lemoine A, Cherqui D, Adam R, Sa Cunha A. Evidence of intermetastatic heterogeneity for pathological response and genetic mutations within colorectal liver metastases following preoperative chemotherapy. Oncotarget. 2016;7:21591-21600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731-740; discussion 741-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Gruenberger T, Arnold D, Rubbia-Brandt L. Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol. 2012;21:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Sebagh M, Allard MA, Cunha AS, Ruiz A, Araujo R, Lemoine A, Paule B, Delvart V, Cherqui D, Vibert E. A proposed new method for assessing the pathological response to chemotherapy in resected colorectal liver metastases. Br J Cancer. 2014;111:470-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Mentha G, Terraz S, Morel P, Andres A, Giostra E, Roth A, Rubbia-Brandt L, Majno P. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res. 2015;75:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Pietrantonio F, Mazzaferro V, Miceli R, Cotsoglou C, Melotti F, Fanetti G, Perrone F, Biondani P, Muscarà C, Di Bartolomeo M. Pathological response after neoadjuvant bevacizumab- or cetuximab-based chemotherapy in resected colorectal cancer liver metastases. Med Oncol. 2015;32:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Lim C, Eveno C, Pocard M. [Microenvironment and colorectal liver metastases angiogenesis: surgical implications]. Bull Cancer. 2013;100:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [PubMed] |