Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7939
Peer-review started: August 8, 2017
First decision: September 6, 2017
Revised: September 22, 2017
Accepted: October 27, 2017
Article in press: October 27, 2017
Published online: November 28, 2017
Processing time: 111 Days and 15.7 Hours
We report a case of double domino liver transplantation in a 32-year-old woman who was diagnosed with familial amyloid polyneuropathy (FAP) and liver dysfunction. A two-stage surgical plan was designed, and one domino graft was implanted during each stage. During the first stage, an auxiliary domino liver transplantation was conducted using a domino graft from a 4-year-old female child with Wilson’s disease. After removing the right lobe of the FAP patient’s liver, the graft was rotated 90 degrees counterclockwise and placed along the right side of the inferior vena cava (IVC). The orifices of the left, middle, and right hepatic veins were reconstructed using an iliac vein patch and then anastomosed to the right side of the IVC. Thirty days later, a second domino liver graft was implanted. The second domino graft was from a 3-year-old female child with an ornithine carbamyl enzyme defect, and it replaced the residual native liver (left lobe). To balance the function and blood flow between the two grafts, a percutaneous transcatheter selective portal vein embolization was performed, and “the left portal vein” of the first graft was blocked 9 mo after the second transplantation. The liver function indices, blood ammonia, and 24-h urinary copper levels were normal at the end of a 3-year follow-up. These two domino donor grafts from donors with different metabolic disorders restored normal liver function. Our experience demonstrated a new approach for resolving metabolic disorders with domino grafts and utilizing explanted livers from children.
Core tip: We implanted two domino graft livers into a familial amyloid polyneuropathy patient. One domino graft liver was from a child with Wilson’s disease, and the other was from a child with ornithine carbamyl enzyme defect. The blood flows of the two grafts were balanced by a percutaneous transcatheter selective portal vein embolization. These two domino donor grafts from donors with different metabolic disorders restored normal liver function. Our experience demonstrated a new approach to resolving metabolic disorders with domino grafts and utilizing explanted livers from children.
- Citation: Zhu ZJ, Wei L, Qu W, Sun LY, Liu Y, Zeng ZG, Zhang L, He EH, Zhang HM, Jia JD, Zhang ZT. First case of cross-auxiliary double domino donor liver transplantation. World J Gastroenterol 2017; 23(44): 7939-7944
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7939
Liver transplantation has become a standard treatment for hereditary and metabolic liver diseases, such as familial amyloid polyneuropathy (FAP)[1,2]. Domino liver grafts from patients with some types of metabolic liver diseases, such as maple syrup urine disease[3] and methylmalonic acidemia[4], may function well in the recipient. Domino liver grafts are a good option for some recipients who might otherwise experience long wait times for liver transplantation, such as recipients with hepatocellular carcinoma (usually outside the Milan criteria)[5]. However, ethical concerns remain regarding the influence of the domino donor’s genetic disease on the recipient[5,6]. Sometimes, a domino transplantation is used only as a bridging therapy for fulminant liver failure[7]. The indications for auxiliary liver transplantation are also limited to potentially reversible fulminant hepatic failure[8-10] and liver-based metabolic disorders[11,12]. Thus, limitations exist for both domino donors and recipients and restrict the application of this technique. Additionally, explanted livers from small children with certain metabolic diseases are more difficult to use as domino grafts in adult patients because of their small sizes and metabolic problems.
In this report, we present a case of a cross-auxiliary double domino donor liver transplantation. The implantation of the double domino grafts from the children increased the total volume, and the two grafts compensated for each other’s metabolic defects and thus could be used for the complete replacement of the recipient’s liver. Based on this work, we believe that simultaneous double domino graft transplantation may also be conducted in most adult liver transplantation candidates. The reconstruction of the outflow tract in this case has previously been reported as an operative technique[13].
A 32-year-old woman was admitted into Beijing Friendship Hospital on September 9, 2013, with diagnoses of FAP and digestive tract hemorrhage. The patient had abdominal distension and decreased sensation in the lower limbs for 5 years. She received Chinese medicine treatments for nearly 2 years. Then, hematemesis and hematochezia began to occur intermittently. FAP was diagnosed in February 2013 at Peking Union Medical College Hospital using Congo red staining of the intestinal mucosa.
The FAP patient was malnourished and exhibited symptoms of anemia. The laboratory test results were as follows: Blood group, A; HGB, 59 g/L; PT, 18.2 s; KPTT, 50.1 s; ALB, 28.4 g/L; and TBIL, 37.23 μmol/L. Transthyretin (TTR) protein was detected in the intestinal mucosa using immunohistochemical staining with an anti-TTR antibody. The Val30Met mutation in TTR was confirmed using TTR gene sequencing. The electrocardiogram results revealed a sinus rhythm and no microvoltage. Echocardiography revealed thickened cardiac walls (interventricular septum, 13 mm; posterior wall, 12 mm; left ventricle end-diastolic diameter, 42 mm; and left ventricular ejection fraction, 66%). Contrast-enhanced computed tomography (CT) of the liver revealed heterogeneous enhancement of the liver, an increase in liver volume, and multiple soft tissue nodules around the portal vein and in the retroperitoneal region. The patient was listed for liver transplantation, with diagnoses of FAP (with liver, digestive tract, and myocardial involvement) and liver dysfunction.
Hematemesis and hematochezia continued after plasma transfusion and mucosal protector and acid suppression therapies. These symptoms occurred because of the digestive tract mucosal injury caused by amyloid deposition and coagulation disorders. The patient’s condition deteriorated over time, with worsening intestinal dysfunction and anemia, which increased the patient’s need for transplantation. However, no deceased donor liver was readily available. The FAP patient’s mother, the only potential living donor, was not suitable for donation because she had severe hepatic steatosis, which was confirmed by ultrasound and pathological examinations. Two children were under evaluation for living donor liver transplantation at that time. One (donor 1) was a 4-year-old female child with type A blood group, who was diagnosed with Wilson’s disease. The other (donor 2) was a 3-year-old female child with type O blood group, who was diagnosed with an ornithine transcarbamylase deficiency (OTCD). Neither of these children was an optimal domino donor because of their metabolic liver defects. Moreover, auxiliary liver transplantation would not be a good choice for FAP patients because the deposition of mutated TTR may persist and result in heart failure after auxiliary liver transplantation. Therefore, we decided to implant both domino grafts and remove the FAP patient’s native liver. Thus, the two grafts could compensate for each other’s metabolic defects. However, it would have been difficult to perform all of the required operations at the same time (including two living donor liver transplantations and a double domino graft liver transplantation) because the number of liver transplant surgeons was insufficient. A two-stage surgical plan for the FAP patient was designed, and one domino graft was implanted after removing part of the liver in each stage. All of the procedures and potential risks were explained to the three patients or their parents, and written consents were obtained. These works were approved by the ethics committee of Beijing Friendship Hospital.
The donor of the first domino liver graft (donor 1) underwent liver transplantation because of central nervous system involvement and the failure of decoppering treatments due to a penicillamine allergy. Donor 1 received the left lateral liver lobe from her mother on September 16, 2013. The domino auxiliary liver transplantation of the FAP patient was performed at the same time.
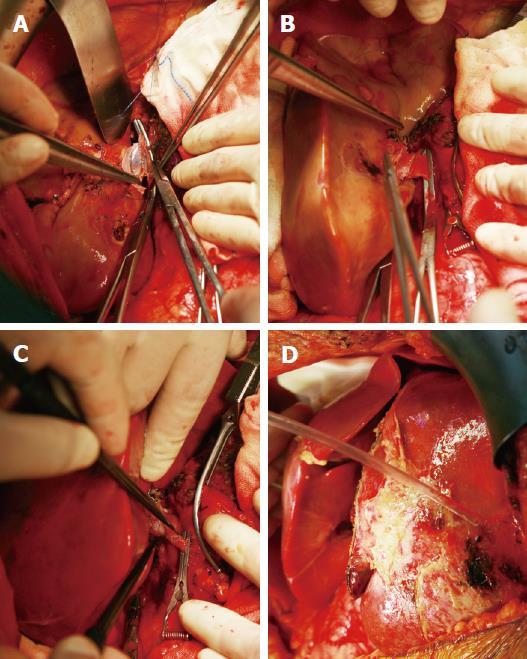
The auxiliary liver transplantation was conducted by removing the right lobe of the FAP patient’s liver (segments 5, 6, 7, 8 and the right part of segment 1) and implanting the whole donor liver (the domino liver from donor 1). The residual liver of the FAP patient included segments 2, 3, 4 and the left part of segment 1. The left and middle hepatic veins of the FAP patient were reserved. We retained the right branches of the portal vein, hepatic artery, and hepatic duct for as long as possible.
The domino donor liver from donor 1 was perfused with histidine-tryptophan-ketoglutarate (HTK) solution immediately after it was resected. Three separate orifices of the main branches of the hepatic vein were found in the domino graft, and they were reconstructed using a cold-preserved iliac venous patch graft from a deceased donor. For the convenience of vascular anastomosis, the caudate lobe was resected. The graft was rotated 90 degrees counterclockwise and placed along the right side of the inferior vena cava (IVC) in the FAP patient. The reconstructed hepatic vein of the graft was anastomosed to the open end of the FAP patient’s right hepatic vein, which was extended with an incision at the IVC. Next, the main portal vein was anastomosed to the right branch of the FAP patient’s portal vein. After graft reperfusion, the graft hepatic artery was anastomosed to the FAP patient’s right hepatic artery under a microscope. The common hepatic duct of the graft was anastomosed to the FAP patient’s right hepatic duct (Figure 1). The cold ischemia time of the domino graft from donor 1 was 796 min and the operative time of the first graft transplantation for the FAP patient was 610 min. The graft to recipient weight ratio (GRWR) calculated by the weight of first domino graft was 0.84%.
The immunosuppressive regimen consisted of tacrolimus, mycophenolate mofetil, and methylprednisolone. The FAP patient recovered well from the surgery. The coagulation indices, including PT, KPTT, and TT, returned to normal on day 3 after liver transplantation, and no hematemesis or hematochezia occurred thereafter. A 99mTc-EHIDA SPECT examination conducted on day 30 revealed that the proportions of the functional volumes of the domino graft and the residual left liver were 70.9% and 29.1%, respectively. However, the 24-h urinary copper levels continued to increase beyond the normal range, and there was a significant reduction in the serum copper protein level on day 14 after transplantation.
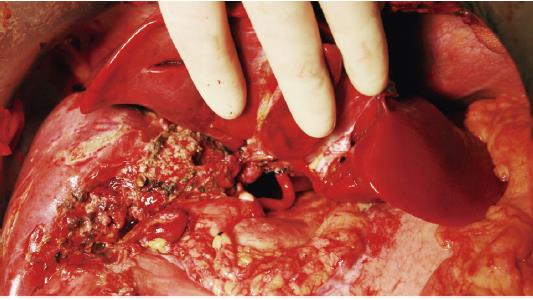
The donor of the second domino liver graft (donor 2) underwent a living donor liver transplantation for OTCD on October 16, 2013-one month after the first liver transplantation in the FAP patient. The second domino graft transplantation in the FAP patient was performed on the same day. The residual left lobe of the FAP patient’s liver was removed. The middle-left hepatic vein, left hepatic artery, left branch of the portal vein, and hepatic duct were reserved. During the back-table preparation, the second domino graft was perfused with HTK solution, and the orifices of the right hepatic vein and middle-left hepatic vein were reconstructed using an iliac venous patch in the same manner employed for the first graft. The caudate lobe of the second graft was also resected. The second graft was orthotopically positioned with its right lobe overlapping the first graft. The reconstructed hepatic vein of the graft was connected with the middle-left hepatic vein of the FAP patient. Then, the graft portal vein was connected to the left portal vein of the FAP patient. After reperfusion, the proper hepatic artery and the hepatic duct of the graft were anastomosed to the FAP patient’s left hepatic artery and left hepatic duct, respectively (Figures 2 and 3). The cold ischemia time of the domino graft from donor 2 was 469 min and the operative time of the second graft transplantation for the FAP patient was 504 min. The GRWR calculated by the weight of second domino graft was 0.92%.
The immunosuppressive therapy regimen was the same as that for the first transplantation and included tacrolimus, mycophenolate mofetil, and methylprednisolone (tapered gradually). There were no episodes of rejection or surgical complications during recovery or follow-up. The liver function indices (ALT, AST, and TBIL) recovered smoothly. The test results for Wilson’s disease and OTCD, including copper blue protein oxidase activity, blood copper, and blood ammonia, were negative. The hepatic arterial, portal venous, and hepatic venous blood flows were monitored using ultrasound and enhanced CT scans. Thirty days after the second domino transplantation, 99mTc-EHIDA SPECT revealed that the proportions of the functional volumes of the first and second grafts were 75.6% and 24.6%, respectively.
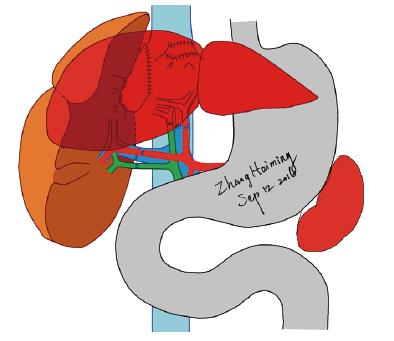
Two hundred fifty-eight days after the second transplantation, the FAP patient’s 24-h urinary copper excretion increased beyond the normal range. A contrast-enhanced CT scan revealed a markedly increased volume of the first graft, which received the greater part of the portal venous blood supply. To balance the function and blood flow between the two grafts, a percutaneous transcatheter selective portal vein embolization was performed, and the “left portal vein” of the first graft was blocked. Subsequently, the FAP patient’s 24-h urinary copper excretion returned to normal. The FAP patient was followed by our hospital for over 4 years (Figure 4). The latest test results indicated that the ALT, AST, TBIL, serum ammonia, and 24-h urinary copper excretion were normal. Sensation in the lower limbs improved slightly. No symptoms of cardiac problems emerged, and there was no change in echocardiography. No surgical complications were found in the two domino donors, and their grafts functioned well at the end of a 4-year follow-up.
Auxiliary liver transplantation with a living related partial graft or a domino liver graft was initially introduced as a temporary or permanent support for patients, as it avoided small-for-size syndrome[14]. A complete domino liver from a child donor can be used without reducing the liver graft size, which may reduce surgical complications, such as liver graft cross-sectional bleeding, hemorrhage, and bile leakage. We rotated the graft 90 degrees counterclockwise, which facilitated anastomosis and reduced the potential complications caused by the limitation of space. However, the risks of outflow tract obstruction and portal vein angulation were increased. Extending the orifice of the right hepatic vein[13] contributed to the outflow tract patency. Additionally, the trends and lengths of the portal veins of the graft and recipient should also be considered when the graft is positioned in the recipient. Resection of the caudate lobe may also be necessary to reduce the risk of portal vein angulation.
Domino liver grafts from small children can also be used in auxiliary liver transplantation. However, the metabolic deficiency of the domino liver graft limits the application of this approach. In this patient with FAP, we conducted a second domino liver transplantation instead of simply removing the remnant native liver. These two domino donor grafts, each from a donor with a different metabolic disorder, were used to restore full liver function. The metabolic disorder of a domino graft can be resolved in this manner.
Our experience with double domino transplantation will contribute to the improved utilization of explanted livers from children with metabolic disorders and expand the donor pool. Exchanging parts of livers between two patients with complementary metabolic liver diseases would also be practical when the body sizes and blood groups of the patients are suitable. “No donation liver transplantations” would represent a new mode of liver transplantation.
The main characteristics of familial amyloid polyneuropathy are pain, paresthesia, muscular weakness, autonomic dysfunction, and abnormalities caused by kidney and heart involvements.
Amyloid deposition can be found in many visceral organs.
Familial Mediterranean fever, familial polyneuropathy, senile amyloidosis, amyloidosis of central nervous system, and localized amyloidosis.
The mutation of the transthyretin gene can be found by genetic examinations.
Thickened cardiac walls can be found by echocardiography after heart involvement.
Depositions of amyloid can be found in the tissue sections after Congo red staining.
Liver transplantation is the only curable treatment.
Ando Y, Ueda M. Novel methods for detecting amyloidogenic proteins in transthyretin related amyloidosis. Front Biosci 2008;13: 5548-5558.
Domino donor liver transplantation: When a patient receives a liver transplantation, the explanted ill liver sometimes can be transplanted to another patient. The second transplantation is domino donor liver transplantation. Cold ischemia time: the time interval between liver graft explanting and implanting, during which liver graft is preserved in cold storage solution. Graft to recipient weight ratio (GRWR): graft weight/patient’s body weight, which is used to assess whether the graft is enough for a patient.
Two domino donor grafts, each from a donor with a different metabolic disorder, can be used to restore full liver function in cross-auxiliary double domino donor liver transplantation.
This case report shows that cross-auxiliary double domino donor liver transplantation is practicable. However, details of this technique should be further discussed.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aseni P, Kollmann D, Ozdemir F, Montasser F S- Editor: Chen K L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Holmgren G, Ericzon BG, Groth CG, Steen L, Suhr O, Andersen O, Wallin BG, Seymour A, Richardson S, Hawkins PN. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993;341:1113-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 407] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, Andersen O, Karlberg I, Nordén G, Nakazato M. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet. 1991;40:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA. Domino liver transplantation in maple syrup urine disease. Liver Transpl. 2006;12:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Khanna A, Gish R, Winter SC, Nyhan WL, Barshop BA. Successful Domino Liver Transplantation from a Patient with Methylmalonic Acidemia. JIMD Rep. 2016;25:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Popescu I, Dima SO. Domino liver transplantation: how far can we push the paradigm? Liver Transpl. 2012;18:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Franchello A, Paraluppi G, Romagnoli R, Petrarulo M, Vitale C, Pacitti A, Amoroso A, Marangella M, Salizzoni M. Severe course of primary hyperoxaluria and renal failure after domino hepatic transplantation. Am J Transplant. 2005;5:2324-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Casas-Melley AT, Thomas PG, Krueger LJ, Falkenstein KP, Flynn LM, Conley SB, Dunn SP. Domino as a bridge to definitive liver transplantation in a neonate. Pediatr Transplant. 2002;6:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660-665; discussion 665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 122] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Rela M, Kaliamoorthy I, Reddy MS. Current status of auxiliary partial orthotopic liver transplantation for acute liver failure. Liver Transpl. 2016;22:1265-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Azoulay D, Samuel D, Ichai P, Castaing D, Saliba F, Adam R, Savier E, Danaoui M, Smail A, Delvart V. Auxiliary partial orthotopic versus standard orthotopic whole liver transplantation for acute liver failure: a reappraisal from a single center by a case-control study. Ann Surg. 2001;234:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Kasahara M, Takada Y, Egawa H, Fujimoto Y, Ogura Y, Ogawa K, Kozaki K, Haga H, Ueda M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation: Kyoto University experience. Am J Transplant. 2005;5:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Trotter JF, Milliner D. Auxiliary liver transplant is an ineffective treatment of primary hyperoxaluria. Am J Transplant. 2014;14:241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Qu W, Zhu ZJ, Wei L, Sun LY, Liu Y, Zeng ZG. Reconstruction of the Outflow Tract in Cross-Auxiliary Double-Domino Donor Liver Transplantation. Transplant Proc. 2016;48:2738-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Inomata Y, Kiuchi T, Kim I, Uemoto S, Egawa H, Asonuma K, Fujita S, Hayashi M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation as an aid for small-for-size grafts in larger recipients. Transplantation. 1999;67:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |