Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7863
Peer-review started: July 28, 2017
First decision: August 30, 2017
Revised: September 25, 2017
Accepted: September 28, 2017
Article in press: September 28, 2017
Published online: November 28, 2017
Processing time: 122 Days and 21.6 Hours
To investigate the usefulness of aspartate aminotransferase to platelet ratio index (APRI) in predicting hepatocellular carcinoma (HCC) risk in primary biliary cholangitis (PBC).
We identified PBC patients between 2000 and 2015 by searching the electronic medical database of a tertiary center. The hazard ratio (HR) of HCC with different risk factors was determined by Cox proportional hazards model.
One hundred and forty-four PBC patients were recruited. Patients were diagnosed at a median age of 57.8 years [interquartile range (IQR): 48.7-71.5 years), and 41 (28.5%) patients had cirrhosis at baseline. The median follow-up duration was 6.9 years (range: 1.0-26.3 years). Twelve patients developed HCC, with an incidence rate of 10.6 cases per 1000 patient-years. The overall 5-, 10- and 15-year cumulative incidences of HCC were 2.3% 95%CI: 0%-4.8%), 8.4% (95%CI: 1.8%-14.5%) and 21.6% (6.8%-34.1%), respectively. Older age (HR = 1.07), cirrhosis (HR = 4.38) and APRI at 1 year after treatment (APRI-r1) > 0.54 (HR = 3.94) were independent factors for HCC development. APRI-r1, when combined with treatment response, further stratified HCC risk (log rank P < 0.05). The area under receiver operating curve of APRI-r1 in predicting HCC was 0.77 (95%CI: 0.64-0.88).
APRI-r1 can be used to predict the development of HCC in PBC patients. Combination of APRI-r1 with treatment response can further stratify the HCC risk.
Core tip: Currently, no reliable predictive models exist for hepatocellular carcinoma (HCC) in primary biliary cholangitis (PBC). Our study showed that a higher aspartate aminotransferase to platelet ratio index (APRI) at 1 year after treatment (APRI-r1) was associated with a higher HCC risk. The performance of APRI-r1 in predicting HCC was satisfactory (area under the receiver operating curve: 0.77). Combination of APRI-r1 with treatment response further stratified HCC risk. Owing to its simplicity, non-invasiveness and cost-effectiveness, APRI can be used as a marker to streamline the HCC surveillance protocol in PBC patients.
- Citation: Cheung KS, Seto WK, Fung J, Mak LY, Lai CL, Yuen MF. Prediction of hepatocellular carcinoma development by aminotransferase to platelet ratio index in primary biliary cholangitis. World J Gastroenterol 2017; 23(44): 7863-7874
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7863
Primary biliary cholangitis (PBC) is an immune-mediated, chronic cholestatic liver disease due to the destruction of small-sized biliary ducts with progressive liver fibrosis[1]. The disease prevalence ranges from 19 to 402 per million[2] and 48 to 56 per million[3,4] in the western and Asian populations, respectively. If left untreated, patients will develop portal hypertension, cirrhosis and hepatocellular carcinoma (HCC) with resulting mortality[5].
Ursodeoxycholic acid (UDCA) is recommended in all PBC patients to delay histologic progression, reduce cirrhotic complications, and improve the long-term survival[6-9]. Recently, suboptimal treatment response to UDCA is recognized to be a risk factor for HCC development[10]. Various biochemical response criteria have been developed and validated, which include the Rotterdam criteria[11], Paris I criteria[12], Paris II criteria[13], Barcelona criteria[7], and Toronto criteria[14].
In addition, the HCC risk was significantly increased in patients with significant fibrosis and cirrhosis. Traditionally, liver biopsy is regarded as the gold standard diagnostic method, but it may not be desirable in daily clinical practice due to its associated invasiveness which may lead to various complications[15]. Aspartate aminotransferase (AST) to platelet ratio index (APRI) is a serum marker shown to be able to assess liver fibrosis and cirrhosis across a wide array of chronic hepatic diseases[16-22]. It has the advantage of being easily calculated from routine laboratory results. Studies suggested that it could predict HCC risk in chronic hepatitis B (CHB)[23] and C (CHC) infection[24] and had prognostic value in CHB patients who underwent surgery for early stage HCC[25].
Recently, APRI is also found to be a prognostic marker in PBC patients independent of UDCA response[26]. This is attributed to its ability to not only capture fibrosis/cirrhosis, but also to reflect other biologically significant pathways like hepatic necroinflammation or non-cirrhotic portal hypertension[26-28]. Both APRI at baseline and APRI at 1 year after treatment (APRI-r1) have been shown to predict adverse outcomes (liver transplantation and/or death) in PBC patients[26]. In addition, when combined with the treatment response criteria, APRI-r1 can further stratify the risk of adverse outcomes and improve the predictive performances[26,29].
However, whether APRI can predict HCC risk in PBC patients remains uncertain. We aimed to demonstrate the role of APRI, alone and in combination with treatment response, in predicting HCC development in PBC patients receiving UDCA.
PBC patients who followed up at the Clinic of Hepatology Unit of Queen Mary Hospital (QMH), a tertiary referral center, between January 2000 and October 2015 were recruited.
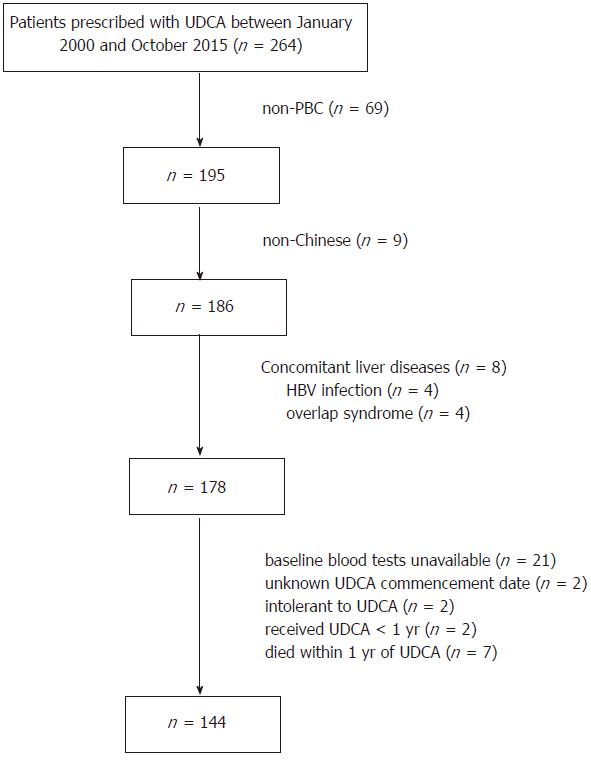
As all patients with PBC were prescribed with UDCA in QMH, we first identified patients receiving UDCA between 2000 and 2015 by searching the electronic medical database of QMH. Subsequently, we excluded non-PBC cases by reviewing the patient records, based on the criteria described in the subsequent section. Other exclusion criteria included non-Chinese ethnicity, cases with UDCA prescription for less than 1 year, overlap syndrome[30] and other coexisting hepatic diseases including CHB and CHC infection, steatohepatitis, alcoholic liver disease, and Wilson’s disease. Figure 1 illustrates the patient recruitment process.
Ethics approval was issued by the Institutional Review Board, The University of Hong Kong and West Cluster of Hospital Authority, Hong Kong.
A diagnosis of PBC was made if two out of the following three criteria were fulfilled: (1) cholestatic liver function pattern with raised alkaline phosphatase (ALP) ≥ 1.5 times the upper limit of normal (ULN); (2) presence of anti-mitochondrial antibody (AMA); and (3) liver biopsy showing the histology of “nonsuppurative destructive cholangitis with destruction of interlobular biliary ducts”[5]. For liver biopsy cases, histologic staging was reported in accordance with Ludwig et al[31].
The Paris II criteria were proposed by Corpechot et al[13] for predicting adverse events in PBC patients with early-stage disease. Early PBC can be defined either histologically (Ludwig’s stages I and II) or biochemically (normal levels of albumin and bilirubin).
Patients had regular follow-up every 3 to 6 mo to monitor the platelet count, liver biochemistry, prothrombin time (PT) and alpha-fetoprotein level. Patients were recommended for ultrasonography (USG) of the liver every 6 mo for HCC[32].
A diagnosis of HCC was made by histology and/or imaging features [i.e. arterial enhancement and venous wash-out on triphasic computed tomography (CT) scan or magnetic resonance imaging (MRI)].
A diagnosis of cirrhosis was made by any one of the following: (1) imaging (USG, CT or MRI) showing small liver with surface nodularity, or signs of portal hypertension (including splenomegaly, ascites and varices); (2) fibrosis score > 16.9 kPa on transient elastography[33]; and (3) clinical features including thrombocytopenia, prolonged prothrombin time, ascites, varices, hepatic encephalopathy and hepatorenal syndrome.
APRI was calculated by the following formula proposed by Wai et al[16]: [(AST value/ULN) / platelet count (109/L)] x 100.
For the initial analysis, we used the Rotterdam criteria (abnormal levels of bilirubin or albumin) to define suboptimal treatment response. This is because the Rotterdam criteria were shown to have better predictive performances than other treatment response criteria in predicting requirement for liver transplantation and death in Chinese PBC patients[34,35]. Analyses by using other treatment response criteria were also performed. Table 1 illustrates the description of other prognostic models[26]. Combination of APRI-r1 with treatment response could further stratify PBC patients into low-risk (APRI-r1 ≤ 0.54 with treatment response), intermediate-risk (APRI-r1 ≤ 0.54 with suboptimal treatment response, or APRI-r1 > 0.54 with treatment response) and high-risk (APRI-r1 > 0.54 with suboptimal treatment response) groups of developing adverse events in terms of liver transplantation or death.
| Time of evaluation | Definition of suboptimal treatment response | |
| Rotterdam | 1 yr | Abnormal bilirubin and/or albumin |
| Paris I | 1 yr | ALP ≥ 3 × ULN or AST ≥ 2 × ULN or bilirubin > 1 mg/dL |
| Paris II | 1 yr | ALP > 1.5 × ULN or AST > 1.5 × ULN or bilirubin > 1 mg/dL |
| Barcelona | 1 yr | ALP > 1 × ULN and decrease in ALP < 40% |
| Toronto | 2 yr | ALP > 1.67 × ULN |
| APRI | Baseline | AST/ULN of AST/platelet (× 109) × 100 |
| APRI-r1 | 1 yr | AST/ULN of AST/platelet (× 109) × 100 |
Statistical analyses were performed using R version 3.2.3 (R Foundation for Statistical Computing) statistical software. We expressed continuous variables in terms of median and interquartile range (IQR). The correlation between continuous variables was assessed by Spearman’s bivariate correlation. We used Mann-Whitney U-test to assess the difference in continuous variables of two groups. We used χ2 test or Fisher’s exact test for the comparison of categorical variables. The hazard ratio (HR) of HCC with different variables was derived from the Cox proportional hazards model. Patients not meeting the clinical endpoint (HCC) were censored at latest follow-up or death. The follow-up duration was calculated from the date of diagnosis to the censored date. Missing data in the Cox model were handled by multiple imputation, wherein 50 complete datasets were constructed by imputing the missing values[36]. The development of HCC was analyzed by the Kaplan-Meier method, and statistical significance was determined by the log-rank test. By plotting “sensitivity” against “1-specificity”, the receiver operating curve was generated. The performances of different models were expressed by area under the receiver operating curve (AUROC), with the 95% CI being deduced from bootstrapping by sampling with replacement from the original dataset and repeating the process by 1000 times. A two-sided P-value of < 0.05 was used to define statistical significance.
One hundred and forty-four PBC patients were recruited, and 127 were female (88.2%). Table 2 shows the patient characteristics, laboratory and histology results. Patients were diagnosed at a median age of 57.8 years (IQR: 48.7 to 71.5 years). The median follow-up duration was 6.9 years (range: 1.0 to 26.3 years), making a total of 1136 patient-years. Twelve patients developed HCC, with an incidence rate of 10.6 cases per 1000 patient-years. Ten patients underwent liver transplantations, and there were 40 deaths (21 were liver-related and 19 were non-liver-related).
| Variable | Whole cohort,n = 144 | Patients with HCC,n = 12 | Patients without HCC,n = 132 | P value |
| Age, yr | 57.8 (48.7-71.5) | 68.1 (56.2-74.6) | 57.0 (48.2-70.7) | 0.278 |
| Female sex | 127 (88.2) | 9 (75.0) | 118 (89.4) | 0.153 |
| Duration of follow-up, yr | 6.9 (3.5-10.4) | 8.9 (5.2-11.4) | 6.8 (3.5-10.1) | 0.499 |
| Ursodeoxycholic acid, mg | 750 (750-750) | 750 (750-750) | 750 (750-750) | 0.576 |
| Suboptimal treatment response, | 61 (42.4) | 9 (75.0) | 52 (39.4) | 0.0173 |
| Rotterdam criteria | ||||
| Diabetes | 29 (20.1) | 6 (50.0) | 23 (17.4) | 0.0163 |
| Smoking1 | 13 (9.5) | 4 (33.3) | 9 (6.8) | 0.0113 |
| Alcohol1 | 17 (13.7) | 2 (16.7) | 15 (11.4) | 0.623 |
| Cirrhosis | 41 (28.5) | 8 (66.7) | 33 (25.0) | 0.0053 |
| Histological stage 3-42 | 23 (44.2) | 3 (50.0) | 20 (43.5) | 1.00 |
| Platelet, x 109/L1 | 216 (152-262) | 133 (95-150) | 229 (175-266) | < 0.0013 |
| Creatinine, μmol/L1 | 69 (60-82) | 73 (60-79) | 68 (60 - 82) | 0.0473 |
| Albumin, g/L1 | 40 (36-42) | 24 (14-30) | 40 (36-42) | 0.087 |
| Bilirubin, μmol/L1 | 14 (10-26) | 30 (19-55) | 14 (10-26) | < 0.0013 |
| ALP (U/L) | 284 (196-484) | 343 (227-362) | 273 (196-496) | 0.991 |
| ALT, U/L1 | 74 (54-130) | 85 (64-109) | 74 (53-133) | 0.565 |
| AST, U/L1 | 68 (51-115) | 76 (56-109) | 68 (51-115) | 0.741 |
| GGT, U/L1 | 517 (256-771) | 626 (353-843) | 490 (224-760) | 0.285 |
| PT, s | 11.3 (10.5-11.7) | 11.8 (11.7-12.5) | 11.2 (10.5-11.7) | 0.0073 |
| AMA positivity | 119 (82.6) | 8 (66.7) | 111 (84.1) | 0.223 |
| Globulin, mg/dL1 | 41 (37-46) | 40 (37-44) | 41 (37-46) | 0.337 |
| IgM, mg/dL1 | 363 (250-502) | 446 (282-579) | 359 (250-478) | 0.563 |
| Mayo risk score1 | 4.7 (3.8-5.5) | 5.1 (4.8-6.6) | 4.6 (3.8-5.4) | 0.0223 |
| MELD score | 6 (6-8) | 8 (6-9) | 6 (6-7) | 0.097 |
| CP score1 | 5 (5-6) | 6 (5-6) | 5 (5-6) | 0.125 |
| CP class B/C1 | 29 (20.1) | 2 (16.7) | 25 (19.2) | 1.00 |
| APRI | 1.00 (0.60-1.84) | 2.02 (1.05-3.34) | 0.97 (0.59-1.72) | 0.053 |
| APRI > 0.541 | 102 (78.5) | 9 (90.0) | 93 (77.5) | 0.689 |
| APRI-r11 | 0.22 (0.13-0.43) | 0.54 (0.31-0.70) | 0.20 (0.13-0.38) | 0.0023 |
| APRI-r1> 0.541 | 27 (19.6) | 6 (50.0) | 21 (16.7) | 0.0133 |
Cirrhosis was noted in 41 patients (28.5%) before treatment commencement, while the median APRI and APRI-r1 levels of the cohort were 1.00 (0.60 to 1.84) and 0.22 (0.13 to 0.43), respectively. A significantly higher proportion of patients who developed HCC had baseline cirrhosis compared with the non-HCC group (66.7% vs 25.0%, P = 0.005). The HCC group also had a higher median APRI-r1 level (0.54 vs 0.20, P = 0.002), with a larger proportion having APRI-r1 > 0.54 (50.0% vs 16.7%, P = 0.013). The difference in median APRI levels between the two groups was of borderline significance (2.02 vs 0.97, P = 0.050), while no significant difference existed for the proportions of patients with APRI > 0.54 (90.0% vs 77.5%, P = 0.689). For other prognostic scores, the HCC group had a higher median Mayo risk score (5.1 vs 4.6, P = 0.022), while no significant differences existed for the model for end-stage liver disease (MELD) or Child-Pugh (CP) scores between the two groups.
Patients were prescribed with UDCA at a median dose of 750 mg. The number of patients who had suboptimal treatment response was as follows: 61 (42.4%; Rotterdam criteria), 52 (36.1%; Paris I criteria), 48 (33.3%; Barcelona criteria) and 50 (38.8%; Toronto criteria). None of our patients received fibric acid derivatives.
Liver biopsies were performed in 62 patients. Out of the 52 patients with histology reports available, 21 were regarded as having early-stage PBC. If only the biochemical criteria were considered, 52 patients had early-stage disease. None of these patients developed HCC, and therefore analysis could not be performed using the Paris II criteria.
APRI had positive correlations with levels of AST (r = 0.86, P < 0.001), ALT (r = 0.68, P < 0.001), bilirubin (r = 0.43, P < 0.001), ALP (r = 0.31, P < 0.001) and gamma-glutamyl transferase (GGT) (r = 0.31, P < 0.001). It had negative correlations with platelet counts (r = -0.43, P < 0.001) and albumin levels (r = -0.27, P = 0.002). The correlation between APRI and PT was of borderline significance (r = 0.17, P = 0.052). For the correlations with other prognostic models, there were positive correlations between APRI and Mayo risk score (r = 0.32, P < 0.001) and CP score (r = 0.43, P < 0.001), but not for the MELD score (r = 0.12, P = 0.664).
Table 3 shows the association between HCC development and various factors. On univariate analysis, significant factors for HCC development included older age, male sex, presence of cirrhosis, hypoalbuminemia and suboptimal treatment response (defined by the Rotterdam criteria). On multivariate analysis, only older age (HR = 1.07; 95%CI: 1.02-1.12), cirrhosis (HR = 4.38; 95%CI: 1.06-18.14) and APRI-r1 > 0.54 (HR = 3.94; 95%CI: 1.04-14.94) were independent risk factors (Table 4). Suboptimal treatment response was not a significant independent risk factor irrespective of which treatment response criteria being used.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | 1.06 | 1.01-1.11 | 0.0162 | 1.07 | 1.02-1.12 | 0.0042 |
| Male sex | 5.22 | 1.27-21.44 | 0.0222 | 3.67 | 0.69-19.56 | 0.128 |
| Diabetes mellitus | 3.01 | 0.96-9.44 | 0.058 | |||
| Cirrhosis | 8.02 | 2.35-27.29 | < 0.0012 | 4.38 | 1.06-18.14 | 0.0412 |
| APRI > 0.54 | 3.43 | 0.43-27.19 | 0.243 | |||
| APRI-r1 > 0.54 | 5.10 | 1.64-15.86 | 0.0052 | 3.94 | 1.04-14.94 | 0.0432 |
| Creatinine, μmol/L | 1.02 | 0.99-1.05 | 0.222 | |||
| Albumin, g/L | 0.85 | 0.75-0.96 | 0.0072 | |||
| Bilirubin, µmol/L | 1.01 | 0.98-1.03 | 0.514 | |||
| ALP, U/L | 0.997 | 0.994-1.00 | 0.104 | |||
| ALT, U/L | 0.996 | 0.987-1.00 | 0.331 | |||
| AST, U/L | 0.996 | 0.975-1.01 | 0.467 | |||
| GGT, U/L | 1.00 | 0.999-1.001 | 0.975 | |||
| PT, s | 1.40 | 0.99-1.98 | 0.0602 | |||
| AMA positivity | 0.52 | 0.16-1.75 | 0.292 | |||
| Globulin, mg/dL | 0.99 | 0.90-1.08 | 0.804 | |||
| IgM, mg/dL | 1.00 | 0.997-1.002 | 0.830 | |||
| Suboptimal treatment response, Rotterdam criteria1 | 5.95 | 1.59-22.26 | 0.0082 | 2.18 | 0.45-10.58 | 0.334 |
| Criteria | HR | 95%CI | P value |
| Rotterdam | |||
| Age | 1.07 | 1.02-1.12 | 0.0041 |
| Male sex | 3.67 | 0.69-19.56 | 0.128 |
| Cirrhosis | 4.38 | 1.06-18.14 | 0.0411 |
| APRI-r1 > 0.54 | 3.94 | 1.04-14.94 | 0.0431 |
| Suboptimal treatment response | 2.18 | 0.45-10.58 | 0.334 |
| Paris I | |||
| Age | 1.07 | 1.02-1.12 | 0.0031 |
| Male sex | 3.04 | 0.54-17.12 | 0.207 |
| Albumin | 0.94 | 0.80-1.09 | 0.386 |
| Cirrhosis | 4.37 | 1.07-17.75 | 0.0391 |
| APRI-r1 > 0.54 | 3.92 | 1.06-14.54 | 0.0411 |
| Suboptimal treatment response | 1.7 | 0.41-7.03 | 0.466 |
| Barcelona | |||
| Age | 1.07 | 1.02-1.12 | 0.0051 |
| Male sex | 3.26 | 0.56-18.96 | 0.188 |
| Albumin | 0.93 | 0.80-1.07 | 0.307 |
| Cirrhosis | 4.44 | 1.06-18.56 | 0.0411 |
| APRI-r1 > 0.54 | 4.47 | 1.26-15.93 | 0.0211 |
| Suboptimal treatment response | 1.22 | 0.33-4.49 | 0.768 |
| Toronto | |||
| Age | 1.07 | 1.02-1.13 | 0.0031 |
| Male sex | 3.22 | 0.56-18.50 | 0.19 |
| Albumin | 0.94 | 0.80-1.09 | 0.425 |
| Cirrhosis | 4.56 | 1.09-19.17 | 0.0381 |
| APRI-r1 > 0.54 | 4.16 | 1.10-15.69 | 0.0361 |
| Suboptimal treatment response | 1.46 | 0.31-6.89 | 0.631 |
Patients were further stratified into low-, intermediate- and high-risk groups. High-risk patients (APRI-r1 > 0.54 with suboptimal biochemical response) had the highest risk of developing HCC, with consistent results for the Rotterdam, Paris I and Barcelona criteria, while the difference was of borderline significance using the Toronto criteria (Table 5).
| Criteria | Univariate analysis | Multivariate analysis1 | |||||||
| HR | 95%CI | P value | P trend | HR | 95%CI | P value | P trend | ||
| Rotterdam | |||||||||
| Low-risk | Ref | - | - | Ref | - | - | |||
| Intermediate-risk | 2.81 | 0.56-14.01 | 0.208 | < 0.0012 | 1.54 | 0.25-9.63 | 0.644 | 0.0062 | |
| High-risk | 10.29 | 2.55-41.48 | 0.0012 | 7.95 | 1.56-40.45 | 0.0122 | |||
| Paris I | |||||||||
| Low-risk | Ref | - | - | Ref | - | - | |||
| Intermediate-risk | 2.81 | 0.63-12.60 | 0.177 | 0.0032 | 2.34 | 0.40-13.60 | 0.345 | 0.0132 | |
| High-risk | 8.38 | 1.99-35.21 | 0.004 | 7.28 | 1.45-36.71 | 0.0162 | |||
| Barcelona | |||||||||
| Low-risk | Ref | - | - | Ref | - | - | |||
| Intermediate-risk | 1.28 | 0.29-5.72 | 0.75 | 0.0022 | 0.53 | 0.08-3.34 | 0.496 | 0.0382 | |
| High-risk | 10.66 | 2.85-39.89 | < 0.0012 | 5.54 | 1.29-23.71 | 0.0212 | |||
| Toronto | |||||||||
| Low-risk | Ref | - | - | Ref | - | - | |||
| Intermediate-risk | 3.25 | 0.81-13.06 | 0.097 | 0.052 | 4.4 | 0.97-19.90 | 0.055 | 0.061 | |
| High-risk | 4.22 | 0.85-20.97 | 0.079 | 4.77 | 0.78-29.24 | 0.091 | |||
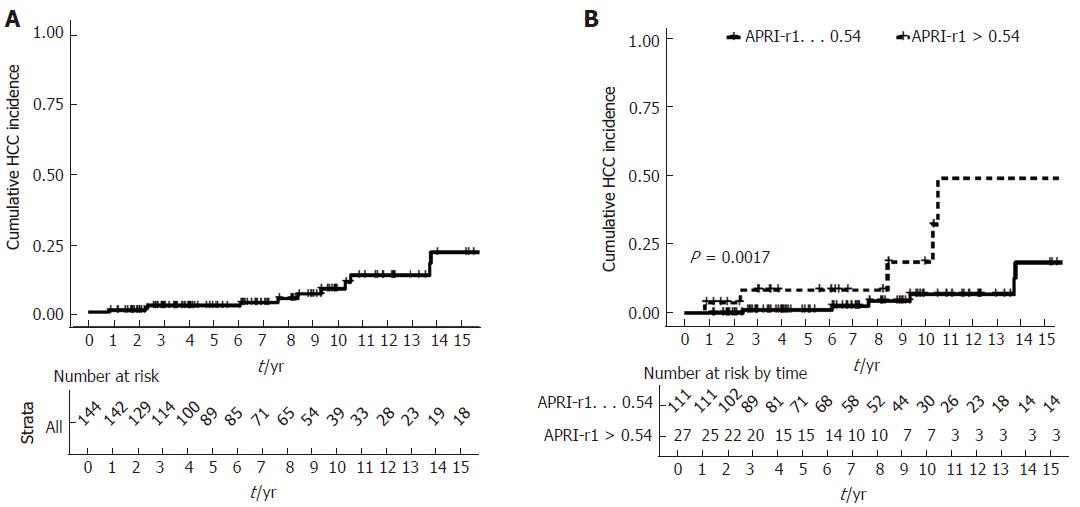
The 5-, 10- and 15-year cumulative incidences of HCC were 2.3% (95%CI: 0%-4.8%), 8.4% (95%CI: 1.8%-14.5%) and 21.6% (6.8%-34.1%), respectively (Figure 2A).
Cumulative incidence of HCC was significantly higher for patients with APRI-r1 > 0.54 (log rank P = 0.002; Figure 2B). Among patients with APRI-r1 ≤0.54, the 5-, 10- and 15-year cumulative incidences of HCC were 2.5%(95%CI: 0%-3.0%), 6.7%(95%CI: 0%-13.1%) and 18.3% (95%CI: 0.4%-33.0%), respectively. Among patients with APRI > 0.54, the 5-, 10- and 15-year cumulative incidences of HCC development were 8.3% (95%CI: 0%-18.7%), 18.5% (95%CI: 0%-37.2%) and 49.0% (95%CI: 0%-75.2%).
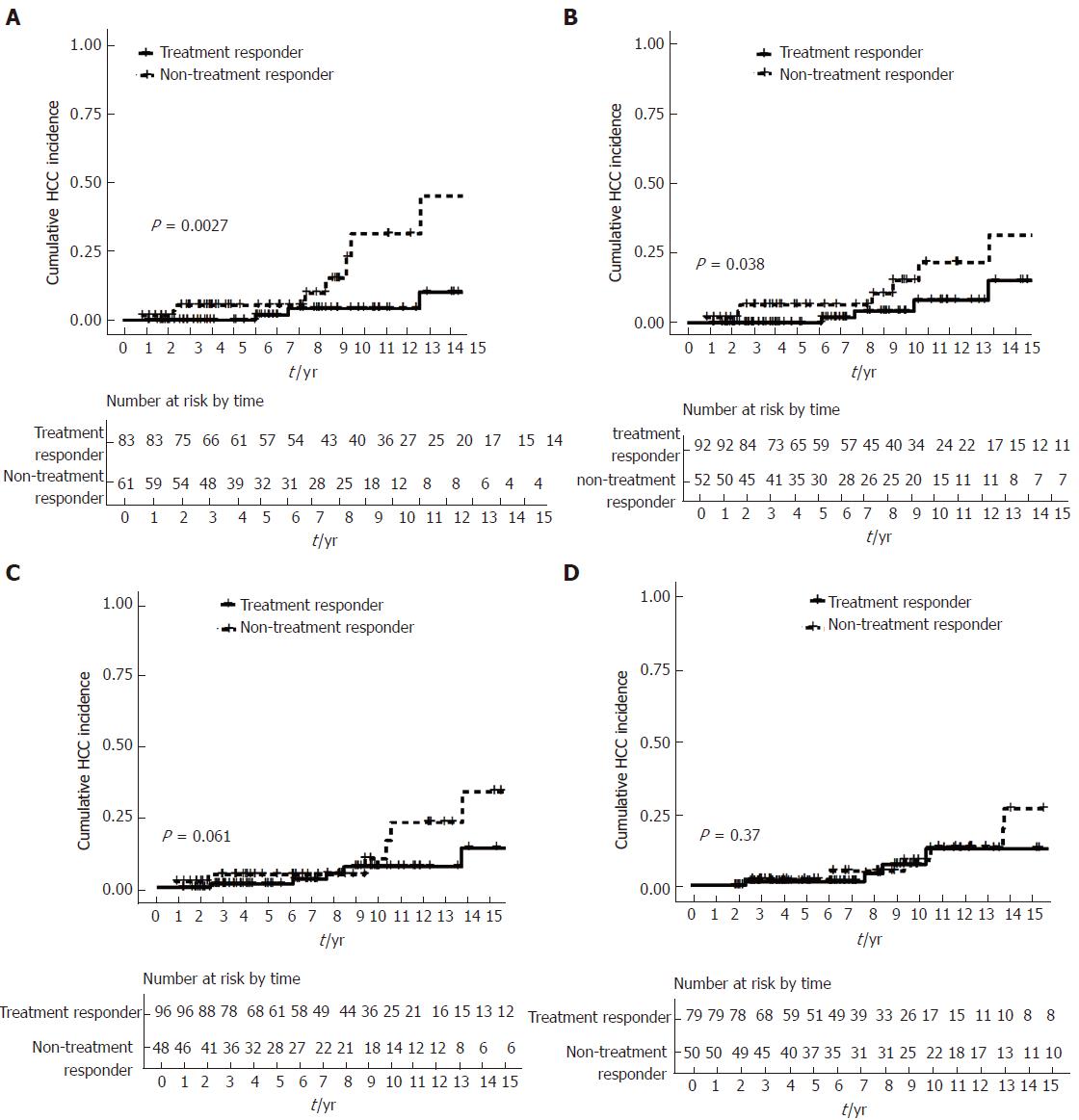
Cumulative incidence of HCC was significantly higher for patients who had suboptimal biochemical response by using the Rotterdam (log rank P = 0.003) and Paris I criteria (log rank P = 0.038). The difference was of borderline significance by using the Barcelona criteria (log rank P = 0.061), while there was no significant difference by using the Toronto criteria (log rank P = 0.370) (Figure 3A-D).
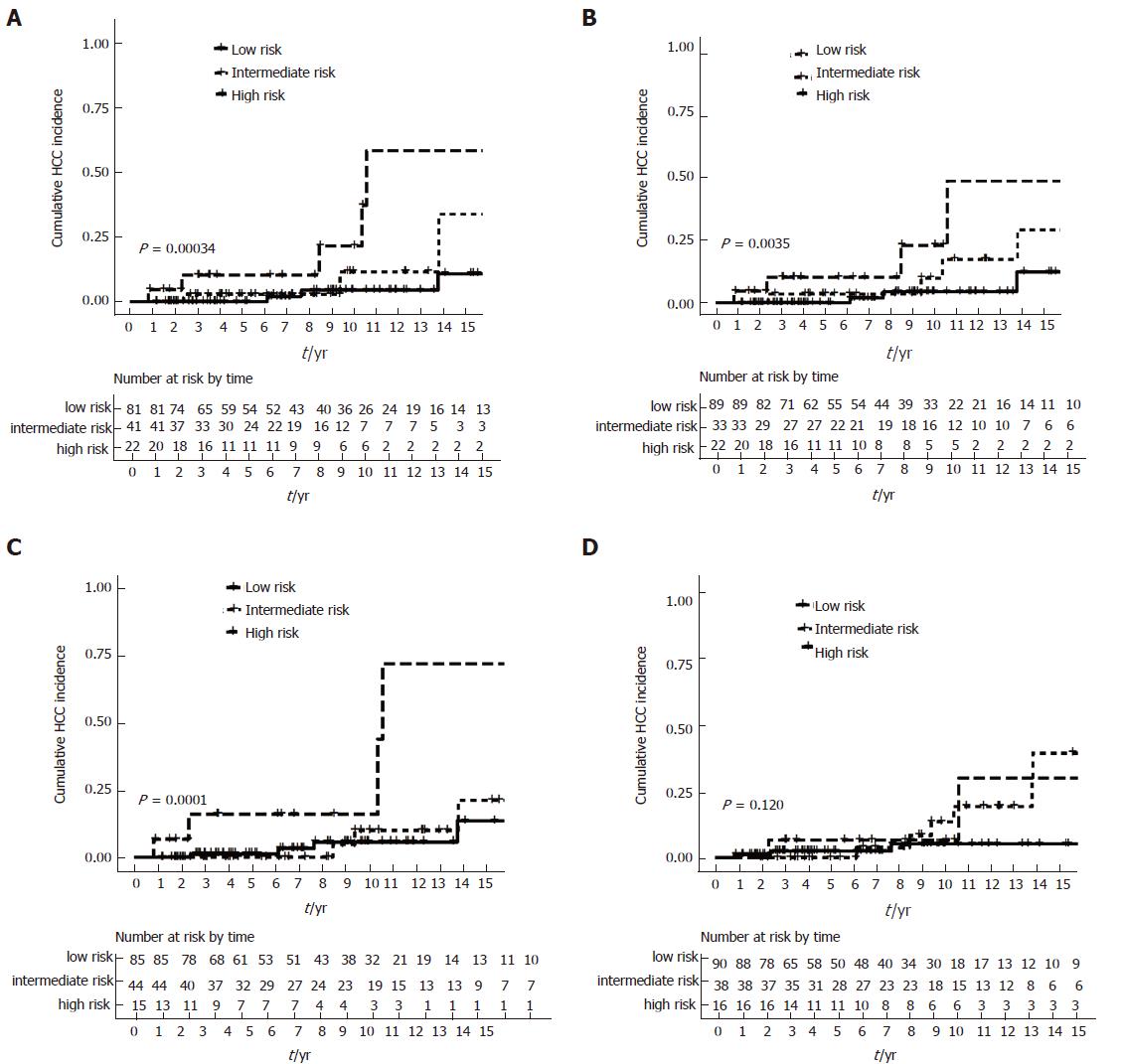
Using the combination of APRI-r1 and biochemical response to define low-, intermediate- and high-risk groups further stratified HCC risk (all log rank P < 0.05), with the exception for the Toronto criteria (log rank P = 0.120) (Figure 4A-D). When APRI-r1 was combined with treatment response as defined by the Rotterdam criteria, the 5-, 10- and 15-year cumulative incidences of HCC were 0%, 4.3% (95%CI: 0%-10.0%) and 10.3% (95%CI: 0%-22.0%), respectively among the low-risk group. For the intermediate-risk group, the 5-year, 10- and 15-year cumulative incidences of HCC were 2.7% (95%CI: 0%-7.8%), 11.5% (95%CI: 0%-27.2%) and 33.7% (95%CI: 0%-63.5%), respectively. The high-risk group was at the highest risk, with the 5-, 10- and 15-year cumulative incidences of HCC being 10.2% (95%CI: 0%-22.7%), 21.4% (95%CI: 0%-41.9%) and 58.1% (95%CI: 0%-84.0%), respectively.
Table 6 shows the predictive performances of various prognostic models. APRI-r1 had the best performance in predicting HCC development (AUROC = 0.77, 95%CI: 0.64-0.88), although the 95%CI overlapped with that of Mayo risk score (AUROC = 0.70, 95%CI: 0.54-0.84), cirrhosis (AUROC = 0.71, 95%CI: 0.56-0.86) and thrombocytopenia (< 150 × 109/L) (AUROC = 0.75, 95%CI: 0.58-0.90). APRI, MELD CP scores and hyperbilirubinemia (> μmol/L) did not have satisfactory performances, as the AUROCs were less than 0.70, with the 95%CI crossing 0.50.
| Categorical variable | Rotterdam | Paris I | Barcelona | Toronto | Cirrhosis | Thrombocytopenia, < 150 × 109/L | Hyperbilirubinemia,> 17 mmol/L |
| AUROC | 0.68 | 0.67 | 0.64 | 0.64 | 0.71 | 0.75 | 0.64 |
| (95%CI) | (0.52-0.80) | (0.52-0.81) | (0.48-0.78) | (0.47-0.78) | (0.56-0.86) | (0.58-0.90) | (0.49-0.77) |
| Sensitivity | 75.00% | 66.60% | 58.30% | 63.60% | 66.70% | 70.00% | 66.70% |
| Specificity | 60.60% | 66.60% | 68.90% | 63.60% | 75.00% | 79.00% | 60.80% |
| PPV | 14.80% | 15.40% | 14.60% | 14.00% | 19.50% | 21.20% | 13.60% |
| NPV | 96.40% | 95.70% | 94.80% | 94.90% | 96.10% | 97.00% | 84.90% |
| Continuous variable | APRI | APRI-r1 | Mayo risk score | MELD score | CP score | ||
| AUROC | 0.68 | 0.77 | 0.7 | 0.63 | 0.62 | ||
| (95%CI) | (0.49-0.87) | (0.64-0.88) | (0.54-0.84) | (0.43-0.79) | (0.47-0.76) | ||
| Sensitivity | - | - | - | - | - | ||
| Specificity | - | - | - | - | - | ||
| PPV | - | - | - | - | - | ||
| NPV | - | - | - | - | - | ||
The performances of various treatment response criteria were also unsatisfactory (all AUROCs less than 0.70), with the Rotterdam and Paris I criteria showing comparable AUROCs (around 0.68), while the 95%CI of the AUROCs of the Barcelona and Toronto criteria crossed 0.50. The Rotterdam criteria had the highest sensitivity and negative predictive value.
In the current study, a total of 144 Chinese PBC patients with UDCA use were recruited. A study with the same cohort of patients assessed by different prognostic models for prediction of long-term transplant-free survival was recently published[34]. Our patients were diagnosed at a slightly older age than that reported in the West (median: 57.8 vs 54.5 years)[37]. The female preponderance (88% of our cohort) and the treatment response (33%-42%) were consistent with those reported in the West[5,12].
APRI is widely used in the assessment of fibrosis/cirrhosis in various kinds of hepatic diseases (CHB and CHC infection, alcoholic liver disease and non-alcoholic fatty liver disease)[16-22]. A recent study proposed that APRI could be used to predict adverse outcomes in PBC patients[26]. However, its potential role in HCC prediction in PBC patients is still not clear. A meta-analysis reports an 18.8-fold increase of HCC risk in PBC patients compared with the general population[38], but it suggests that HCC may not be as common in PBC patients compared with other liver diseases[39]. Therefore, a non-invasive test that is simple and cost-effective will be of significant clinical importance in the management of PBC patients.
Our study showed that APRI correlated with adverse liver function (in terms of both laboratory parameters and also traditional PBC prognostic models - Mayo risk score and CP score). In addition, a higher APRI-r1 level was associated with a higher HCC risk. Although being regarded as a fibrosis/cirrhosis marker, APRI-r1 retained the association with HCC development despite the inclusion of cirrhosis into the multivariate analysis (adjusted HR = 3.94). This is likely because APRI has an additional role of reflecting other pathological pathways including liver inflammation and non-cirrhotic portal hypertension[26-28].
Suboptimal treatment response was not an independent risk factor, in contrary to that reported by Trivedi et al[10]. There are a few possible reasons to account for this. First, as disease stage is known to affect the treatment response[11,12], the effect of treatment response on HCC risk would be attenuated by including APRI and cirrhosis into the multivariate analysis. Second, our study might not be adequately powered to detect this effect given the limited number of events. However, by combining both factors (APRI-r1 and treatment response), the HCC risk of individuals could be further stratified into low-risk, intermediate-risk and high-risk.
Hyperbilirubinemia was recognized to be a risk factor for liver transplantation and death in PBC patients in previous studies[37,40,41], but was not a significant independent risk factor for HCC development in the current study. This is likely related to the fact that hyperbilirubinemia in patients with PBC can also be due to cholestasis, while APRI is more specifically related to fibrosis/cirrhosis.
Our study also determined the predictive performances of APRI and APRI-r1 in addition to the traditional prognostic models and treatment response criteria. We showed that APRI-r1 had a satisfactory performance, with an AUROC of 0.77. It appears that APRI-r1 outperformed other prognostic models, although the result should be interpreted with caution as there was overlapping of the 95%CIs of some models (e.g., Mayo risk score) due to the relatively small sample size of our cohort.
Cases were identified by searching the electronic database system of the hospital with subsequent review of the clinical records. This ensures the accurate and complete capture of all PBC cases. Another noticeable strength of the study is the long follow-up duration (up to 26 years), since a long lag time is usually required from PBC diagnosis to HCC development. Moreover, the inclusion of a homogenous group of Chinese patients and exclusion of concomitant liver diseases removed the confounding factors of ethnicity and hepatitis due to other liver diseases.
A few limitations are present in the current study. First, a relatively small sample size may render the study underpowered to confirm a significant association of some factors with HCC development (e.g., smoking and alcohol), although previous study also failed to show an association with these factors[42]. Second, since this study was carried out in a tertiary center, selection bias may account for the higher HCC incidence rate in our cohort (10.6 cases per 1000 person-years). On the contrary, the study by Trivedi et al[10], which was a multi-center study involving 4565 patients, reported an incidence rate of only 3.4 cases per 1000 person-years. Third, our study recruited only Asian subjects, and therefore the applicability of this finding to other ethnicities remains to be investigated. Fourth, as this is only a single-center study with a relatively small sample size, validation studies from other centers are necessary. Lastly, while APRI was shown to be of both predictive and prognostic values in chronic viral hepatitis patients[23-25], studies on the usefulness of this marker in other chronic liver diseases are still lacking. Consistent findings are expected as fibrosis/cirrhosis is the major risk factor for HCC development, although further studies on other chronic liver diseases are warranted.
In conclusion, our study confirmed the usefulness of APRI-r1 in predicting HCC development in PBC patients receiving UDCA. The combination of APRI-r1 with treatment response allowed further stratification of HCC risk. Owing to its non-invasiveness and cost-effectiveness, APRI can be used as a marker to streamline the HCC surveillance protocol in PBC patients.
No reliable predictive models exist for hepatocellular carcinoma (HCC) in primary biliary cholangitis (PBC). Aspartate aminotransferase (AST) to platelet ratio index (APRI) not only captures fibrosis/cirrhosis, but also reflects other biologically significant pathways like hepatic necroinflammation or non-cirrhotic portal hypertension. The usefulness of APRI in predicting HCC in PBC remains unknown.
A predictive marker for HCC development in PBC patients will help disease prognostication and streamline the follow-up and HCC surveillance strategy.
To investigate the usefulness of APRI in predicting HCC in PBC.
The authors recruited all PBC patients who had follow-up in the Hepatology Clinic of Queen Mary Hospital (QMH) between January 2000 and October 2015. Patients were followed up every 3 to 6 mo with regular monitoring of platelet count, liver biochemistry, prothrombin time and alpha-fetoprotein level. In the analysis of the risk factors for adverse events, suboptimal response to UDCA was identified by using various treatment response criteria. APRI-r1 in combination with treatment response criteria enables further stratification of PBC patients into low-risk (biochemical response with APRI-r1 ≤ 0.54), intermediate-risk (suboptimal biochemical response with APRI-r1 ≤ 0.54, or biochemical response with APRI-r1 > 0.54) and high-risk (suboptimal biochemical response with APRI-r1 > 0.54) groups of developing adverse outcomes in terms of liver transplantation or death. The Cox proportional hazards model was used to determine the hazard ratio (HR) of HCC with different variables. The Kaplan-Meier method was used to analyze the development of HCC. The performances of various prognostic models were expressed in terms of area under the receiver operating curve (AUROC).
A total of 144 patients were identified. The median age at diagnosis was 57.8 years (interquartile range: 48.7-71.5 years), and 41 (28.5%) had baseline cirrhosis. The median follow-up duration was 6.9 years (range: 1.0-26.3 years), with a total of 1136 patient-years. Twelve patients developed HCC, with an incidence rate of 10.6 cases per 1000 patient-years. The overall 5-, 10- and 15-year probabilities of HCC development were 2.3% [95% confidence interval (CI): 0%-4.8%], 8.4% (95%CI: 1.8%-14.5%) and 21.6% (6.8%-34.1%), respectively. Independent risk factors for HCC development were older age (HR = 1.07), cirrhosis (HR = 4.38) and APRI at 1 year after treatment (APRI-r1) > 0.54 (HR = 3.94). APRI-r1 in combination with treatment response further stratified the risk of HCC development (log rank P < 0.05). The AUROC of APRI-r1 in predicting HCC was 0.77 (95%CI: 0.64-0.88).
APRI-r1 can be used as a predictive marker for HCC development in PBC patients. Combination of APRI-r1 with treatment response can further stratify the HCC risk.
Our study confirmed the usefulness of APRI-r1 in predicting HCC development in PBC patients receiving UDCA. The combination of APRI-r1 with treatment response allowed further stratification of HCC risk. Owing to its non-invasiveness and cost-effectiveness, APRI can be used as a marker to streamline the HCC surveillance protocol in PBC patients. Future multi-center studies with larger sample size are warranted to confirm our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abu El Eneen Khattab MA, Bilir C, Cao GW, Kreisel W, Tomizawa M S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 2. | Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 3. | Kim KA, Ki M, Choi HY, Kim BH, Jang ES, Jeong SH. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment Pharmacol Ther. 2016;43:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin Transl Gastroenterol. 2017;8:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 894] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 6. | Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 245] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 427] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:1313-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Trivedi PJ, Lammers WJ, van Buuren HR, Parés A, Floreani A, Janssen HL, Invernizzi P, Battezzati PM, Ponsioen CY, Corpechot C. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR; Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, Chazouillères O, Poupon R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 13. | Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 324] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, Heathcote EJ, Hirschfield GM. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1574] [Article Influence: 98.4] [Reference Citation Analysis (1)] |
| 16. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3234] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 17. | Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 786] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 20. | Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782-9.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 21. | Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006;101:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Hann HW, Wan S, Lai Y, Hann RS, Myers RE, Patel F, Zhang K, Ye Z, Wang C, Yang H. Aspartate aminotransferase to platelet ratio index as a prospective predictor of hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2015;30:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Ji F, Zhou R, Wang W, Bai D, He C, Cai Z, Shen Y, Wang S, Deng H, Li Z. High Post-treatment α-Fetoprotein Levels and Aspartate Aminotransferase-to-Platelet Ratio Index Predict Hepatocellular Carcinoma in Hepatitis C Virus Decompensated Cirrhotic Patients with Sustained Virological Response After Antiviral Therapy. J Interferon Cytokine Res. 2017;37:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, Huo TI, Lee PC, Kao WY, Lee SD. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Trivedi PJ, Bruns T, Cheung A, Li KK, Kittler C, Kumagi T, Shah H, Corbett C, Al-Harthy N, Acarsu U. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J Hepatol. 2014;60:1249-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Patanwala I, McMeekin P, Walters R, Mells G, Alexander G, Newton J, Shah H, Coltescu C, Hirschfield GM, Hudson M. A validated clinical tool for the prediction of varices in PBC: the Newcastle Varices in PBC Score. J Hepatol. 2013;59:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Huet PM, Vincent C, Deslaurier J, Coté J, Matsutami S, Boileau R, Huet-van Kerckvoorde J. Portal hypertension and primary biliary cirrhosis: effect of long-term ursodeoxycholic acid treatment. Gastroenterology. 2008;135:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Trivedi PJ, Corpechot C, Pares A, Hirschfield GM. Risk stratification in autoimmune cholestatic liver diseases: Opportunities for clinicians and trialists. Hepatology. 2016;63:644-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 479] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 31. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 528] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6561] [Article Influence: 468.6] [Reference Citation Analysis (1)] |
| 33. | Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, Poupon R. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Prognostic Factors for Transplant-Free Survival and Validation of Prognostic Models in Chinese Patients with Primary Biliary Cholangitis Receiving Ursodeoxycholic Acid. Clin Transl Gastroenterol. 2017;8:e100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Yang F, Yang Y, Wang Q, Wang Z, Miao Q, Xiao X, Wei Y, Bian Z, Sheng L, Chen X. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: the additional effect of anti-gp210. Aliment Pharmacol Ther. 2017;45:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 725] [Cited by in RCA: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 37. | Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.e5; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 38. | Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012;56:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Shibuya A, Tanaka K, Miyakawa H, Shibata M, Takatori M, Sekiyama K, Hashimoto N, Amaki S, Komatsu T, Morizane T. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, Griffiths L, Lim R, Trembling P, Williamson K. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 41. | Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015;149:1804-1812.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 314] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 42. | Cavazza A, Caballería L, Floreani A, Farinati F, Bruguera M, Caroli D, Parés A. Incidence, risk factors, and survival of hepatocellular carcinoma in primary biliary cirrhosis: comparative analysis from two centers. Hepatology. 2009;50:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |