Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7840
Peer-review started: May 16, 2017
First decision: July 27, 2017
Revised: August 8, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: November 28, 2017
Processing time: 198 Days and 23.5 Hours
To investigate the behavior of pulsatile pressure zones (PPZ’s) as noted on high resolution esophageal impedance manometry (HREIM), and determine their association with dysphagia.
Retrospective, single center case control design screening HREIM studies for cases (dysphagia) and controls (no dysphagia). Thoracic radiology studies were reviewed further in cases for (thoracic cardiovascular) thoracic cardiovascular (TCV) structures in esophageal proximity to compare with HREIM findings. Manometric data was collected for number, location, axial length, PPZ pressure and esophageal clearance function (impedance).
Among 317 screened patients, 56% cases and 64% controls had PPZ’s. Fifty cases had an available thoracic radiology comparison. The distribution of PPZ’s in these 50 cases and 59 controls was similar (average 1.4 PPZ/patient). Controls (mean 31.2 ± SD 12 years) were a significantly younger population than cases (mean 67.3 ± SD 14.9 years) with P < 0.0001. The upright posture PPZ pressure was higher in controls (15.7 ± 10.0 mmHg) than cases (10.8 ± 9.7 mmHg). Although statistically significant (P = 0.005), it was a weak predictor using logistic regression and ROC model (AUC = 0.65). Three dysphagia patients had partial compression from external TCV on radiology (1 aberrant subclavian artery, 2 dilated left atrium). The posture (supine vs upright) with more prominent PPZ’s impaired bolus clearance in 9 additional cases by > 20%.
Transmitted TCV pulsations observed in HREIM bear no significant impact on swallowing. However, in older adults with dysphagia, evidence of impaired bolus clearance on impedance should be evaluated for external TCV compression. These associations have never been explored previously in literature, and are novel.
Core tip: Transmitted pulsations from thoracic cardiovascular structures are frequently observed on HREIM, and usually bear no significant impact on swallowing. However, in older adults with dysphagia, evidence of impaired bolus clearance on impedance should be further reviewed using clinical data and functional esophageal swallow studies in order to assess the possibility of external compression from cardiovascular structures.
- Citation: Chaudhry NA, Zahid K, Keihanian S, Dai Y, Zhang Q. Transmitted cardiovascular pulsations on high resolution esophageal impedance manometry, and their significance in dysphagia. World J Gastroenterol 2017; 23(44): 7840-7848
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7840
The esophagus has a close correlation with several thoracic cardiovascular (TCV) landmarks along its course. Prominent arterial structures include ascending aorta, arch of the aorta, left atrium and descending aorta. Cases of TCV compression on the esophagus have been classically associated with congenital anomalies, e.g., dysphagia lusoria (aberrant subclavian artery causing esophageal compression)[1]. It has also been proposed that the esophagus can be potentially compressed between the left atrium and descending aorta[2] if it takes a left course on transition from thorax to abdomen. There are case reports of previously asymptomatic patients diagnosed with external cardiovascular compression on esophagus when they presented with pill induced esophagitis[3] or pill related dysphagia[4]. Initial clinical suspicion of TCV compression on esophagus is never paramount in dysphagia. However in older patients with an established history of atherosclerosis, cardiomegaly, or left atrial dilatation[5], there can be a suspicion due to secondary causes[6], e.g., atrial fibrillation, mitral valve regurgitation or stenosis. There are reports of Ortner’s syndrome (Cardiovocal syndrome) which is hoarseness caused by cardiopulmonary disease compressing the left recurrent laryngeal nerve[7]. This interplay of the physiologic dynamics resulting from the anatomical proximity of these structures needs to be studied further. High Resolution Esophageal Impedance Manometry (HREIM) can provide an interesting insight on the significance and diagnosis of these cases. Effect of TCV pulsations, or pulsatile pressure zones (PPZ’s), on the esophagus can be noted on HREIM[8]. They present as a steady pulsation that remains unaffected with the stage of swallowing, and have been reported in previous literature as a high pressure barrier[9]. Their characteristics and role in patients with or without dysphagia remain uninvestigated. Our aim with this study is to investigate the characteristics and significance of transmitted TCV pulsations on the esophagus as noted on HREIM, especially in terms of dysphagia.
This is a retrospective single center case control study using HREIM studies of 317 patients conducted at a tertiary care center with institutional IRB approval.
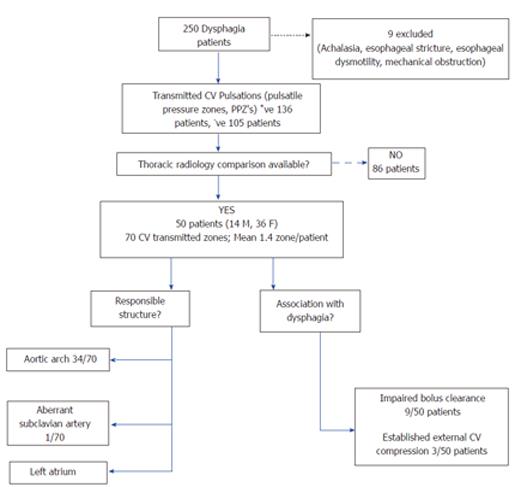
Studies of 250 patients undergoing HREIM for dysphagia from July 2012- January 2014 were screened. Nine patients were eliminated on basis of achalasia, stricture, poor esophageal motility or mechanical obstruction. Cases were selected based on findings of PPZ’s noted on HREIM, which were independent from the swallows, and hence representative of transmitted TCV pulsations on the esophagus. In these patients further electronic medical record was searched for available thoracic imaging, showing TCV structures likely responsible due to their anatomical proximity to esophagus. Fifty dysphagia patients with available radiology (CT/MR chest, esophagogram) were identified (Figure 1) and their data was used for statistical analysis.
HREIM studies of 67 patients without dysphagia (per chart review) were screened as controls. Eight were eliminated due to presence of artifacts on study and catheter malfunction. The remaining 59 were reviewed for presence or absence of PPZ. In subjects with PPZ’s data were collected regarding number and location of PPZ, axial length, PPZ pressure in upright posture, postural effect and impedance. Comparative thoracic radiology was not available for the controls hence only their HREIM data was included for analysis.
As per the institutional HREIM protocol (modified from Chicago protocol[10]), patients received 10 swallows each in the supine and then in the upright position using 5 mL GatoradeTM. Some patients had also received additional 10 mL swallows but these were excluded from the results analysis. A 4.2 mm outer diameter solid-state combined manometry and impedance recording catheter assembly incorporating 36 circumferential pressure sensors spaced at 1-cm intervals and 18 impedance segments spaced at 2-cm intervals were used (ManoScanTM Eso, Given Imaging Yogneam, Israel). The catheter was calibrated prior to each procedure and was inserted transnasally and positioned to record from the hypopharynx to the stomach with a goal of three intragastric sensor recording loci. Data were analyzed using ManoView ESO 3.0 analysis software (Given Imaging). Failed peristalsis was defined as no peristaltic contraction following a swallow. PPZ was included if pressure across it was > 5 mmHg. Pressure was determined using the isobar contour and measured in mmHg.
Data were collected from HREIM studies of selected patients regarding number of PPZ’s present, axial length, PPZ pressures in upright posture, postural effect, location along esophagus (upper, middle or lower third) and impedance. The esophagus was divided into upper, middle and lower thirds. For our study, we only recorded pressures in the upright posture since prior research has demonstrated decreased integrated relaxation pressure, distal contractile integers, and elimination of vascular artifacts in a sitting posture[11]. Since we were interested in measuring the impact of transmitted pulsations on dysphagia, we considered the physiological posture (upright) of swallowing more appropriate for pressure measurement. Even though some PPZ’s were observed with the patient both in upright and supine posture, there were instances when it appeared more prominent in one posture as compared to the other. This was considered as a dominant posture for the PPZ. For PPZ’s present only in one posture, this was documented as its dominant posture. Available radiology images were reviewed by the investigators for correlating TCV structures, and where possible it was also assessed if the patients had a noticeable left turn of esophagus on transition from thorax to abdomen.
The data was tabulated in descriptive format and statistical analysis was done using SAS 9.4 (Copyright© 2013, SAS Institute Inc., Cary, NC, United States). Characteristics of age, sex, anatomical location of PPZ (upper, middle or lower esophagus), PPZ pressure and axial length were analyzed using Wilcoxon rank sum analysis and logistic regression was performed for marginally significant variables. A P-value of < 0.05 was considered statistically significant for the purposes of our study. We performed logistic regression analysis using the disease status (dysphagia vs. no dysphagia) as the response variable and gender, location, axial length, pressure and posture as explanatory variables. A backward selection procedure at the 0.2 significance level identified that pressure was the only explanatory variable significantly associated with dysphagia. We decided to assess the strength of the association by using pressure as a predictor for dysphagia.
In 241 patients with dysphagia (cases), 136 (56.4%) had PPZ’s in either supine or upright posture on HREIM, in comparison to 38 (64.4%) in non-dysphagia group (controls). Since some patients had more than one independently behaving pulsatile zone, 70 PPZ’s were noted in these 50 patients (Average 1.4 PPZ per patient). This was similar in prevalence to the 38 controls with PPZ’s, 54 PPZ’s were observed with an average 1.4 per patient. Demographic and clinical characteristics are presented in Table 1. The prevalence of PPZ’s, was similar in both the cases and controls. The cases were markedly older (67.3 ± 14.9, median 70 years) than controls (31.2 ± 12.2, median 27 years) with P < 0.0001.
| Variable | Dysphagia (n = 50) | No dysphagia (n = 38) | P value | |
| Age, yr | mean ± SD | 67.3 ± 14.9 | 31.2 ± 12.2 | < 0.0001 |
| Median (min, max) | 70 (24, 89) | 27 (19, 61) | ||
| Gender | Male | 14 (28) | 18 (47) | 0.097 |
| Female | 36 (72) | 20 (53) | ||
| Number of pulsatile zones | Total (% prevalence overall) | 70 (56.4) | 54 (64.4) | - |
| Average per patient | 1.4 | 1.4 | - | |
| Location of pulsatile zone in esophagus | Upper third | 12 (17) | 7 (13) | 0.769 |
| Middle third | 24 (34) | 21 (39) | ||
| Lower third | 34 (49) | 26 (48) | ||
| Axial length of pulsatile zone (cm) | mean ± SD | 2.2 ± 1.4 | 2.4 ± 1.2 | 0.258 |
| Median (min, max) | 2 (0.5, 6) | 2 (0.5, 5) | ||
| Avg upper third (min, max) | 1 (0.5-2) | 1 (0.5-2) | ||
| Avg middle third (min, max) | 0.7 (0.5-2.5) | 2.2 (0.5-4) | ||
| Avg lower third (min, max) | 3.3 (0.5-6) | 2.3 (1.5-5) | ||
| PPZ | mean ± SD | 10.8 ± 9.7 | 15.7 ± 10.0 | < 0.01 |
| Pressure (mmHg) | Median (min, max) | 10 (0, 35) | 17 (0, 45) | |
| Avg upper third | 9.8 | 16.7 | ||
| Avg middle third | 5.9 | 13.3 | ||
| Avg lower third | 14.3 | 16.9 | ||
| Dominant posture for pulsatile zone | Supine | 21 (30) | 10 (18.5) | 0.282 |
| Upright | 10 (14.3) | 6 (11.1) | ||
| Both postures | 39 (55.7) | 38 (70.4) | ||
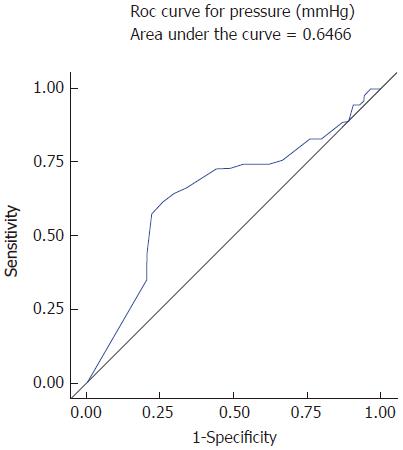
Average pressure of PPZ’s (upright postures) in cases was 10.8 ± 9.7 mmHg, which was significantly lower than controls 15.7 ± 10.0 mmHg (P < 0.01). However, since rising external pressure does not correlate with resolution of dysphagia physiologically, we evaluated its strength as a predictor (see methods). The prediction model had an associated receiver operating characteristic (ROC) curve (Figure 2) with the area under the ROC curve (AUC) of 0.65 (weak significance). Hence, even though low PPZ pressure was significantly associated with dysphagia, the quality of predicting dysphagia by pressure was low; AUC, sensitivity and specificity were quite low.
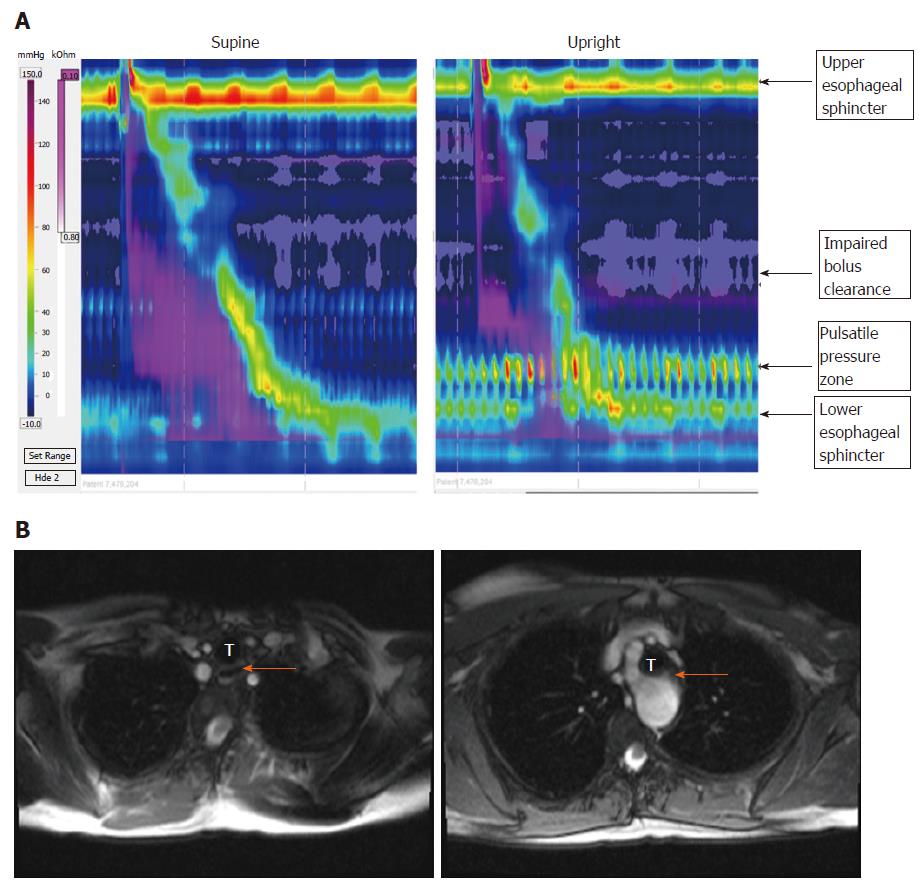
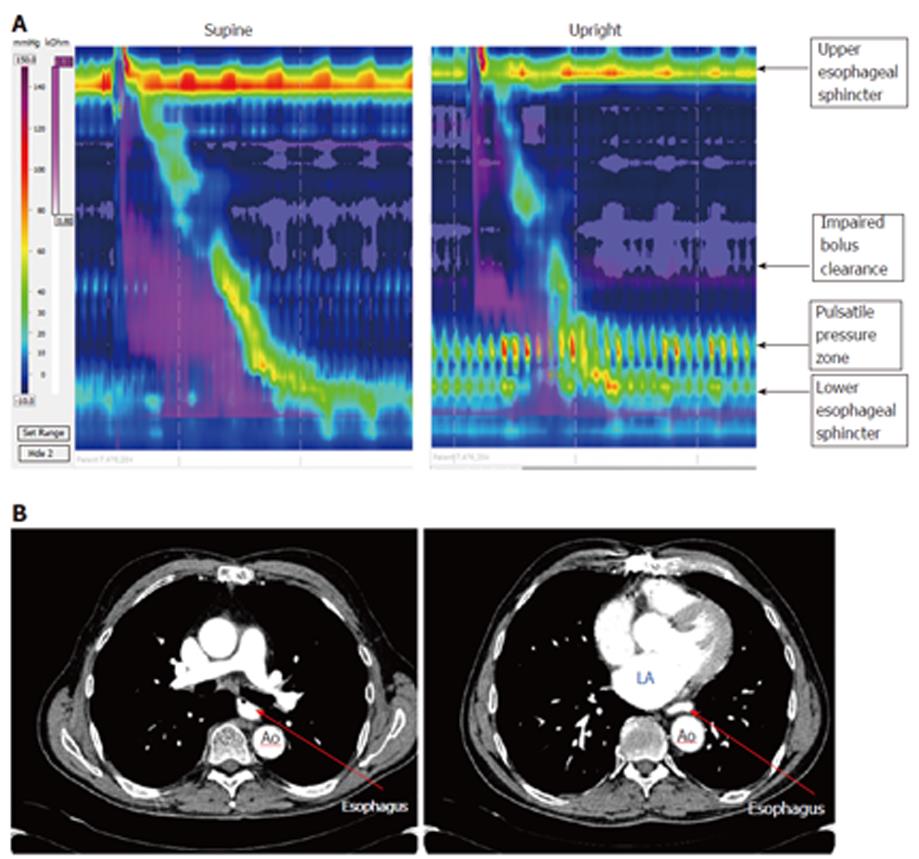
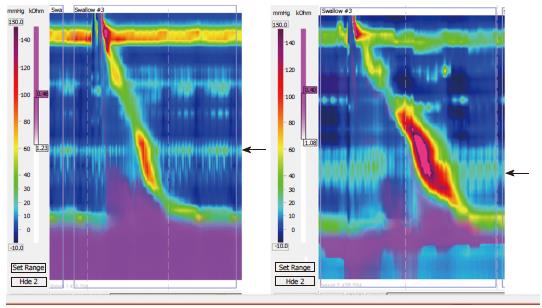
Some patients had impairment in bolus clearance with regards to dominant posture, but there was no clear association between PPZ pressure and Impedance. (Impairment was considered to be > 20% change in bolus clearance on Impedance.) Impaired bolus clearance was observed in 12/50 (24%) dysphagia patients and was associated with the PPZ becoming more prominent. Bolus clearance in these patients decreased by > 20% if PPZ was dominant regardless of upright or supine posture, hence showing no relation to posture itself. Only 3 of these 12 patients had clear evidence of partial compression from external TCV structures on radiology (1 from aberrant subclavian artery with dysphagia lusoria, 2 from dilated left atrium). In the patient with dysphagia lusoria, bolus clearance was 10% in supine swallows and 90% upright swallows (Figure 3). In one of the patients with enlarged left atrium, bolus clearance was 80% in supine swallows and 10 % upright (Figure 4). No impedance data was available for the third patient. PPZ pressure (upright) in these 3 patients ranged from 25 to 35 mmHg. There was no radiological evidence of obstruction caused by TCV structures in the remaining 47/50 patients. Figure 5 shows PPZ’s observed in non-dysphagia patients.
The behavior of PPZ’s was observed with regards to change in posture. In cases, majority (39/70, 56%) of the PPZ’s were observed in both upright and supine postures, 10 in upright, and 21 in supine posture only. Similarly in controls, the majority (38/54, 70%) of them were noted in both upright and supine postures. Hence, this presentation was more common in controls. The average axial length of the PPZ in cases was 2.2 ± 1.4 cm, which was comparable to controls 2.4 ± 1.2 cm.
The aortic arch was related to 34 PPZ’s, 11 of which were in upper and 23 in the middle third. One remaining PPZ in the upper third was due to an aberrant subclavian artery (dysphagia lusoria). The left atrium was related to 35 PPZ’s including all 34 PPZ’s in the lower third and one in the middle third. Hence the characteristics described for the lower PPZ’s in Table 1 can be attributed to the left atrium.
Among the 50 patients with dysphagia, 20 had dysphagia to solids, 4 to both solids and liquids whereas 26 were unspecified per chart review. Esophageal orientation on lower thoracic cavity could be ascertained in 27 patients, 24 of them had a left turn of esophagus causing it to be situated between the descending thoracic aorta and left atrium. This finding was present in all patients with PPZ’s causing bolus impairment on impedance in lower third of esophagus.
Per our results, transmitted cardiovascular pulsations were seen with a comparable frequency in patients with and without dysphagia. However the older age of the dysphagia population indicates that advancing age and/or development of atherosclerosis and valvular heart disease could be a risk factor. The most common causes for a pulsatile pressure zone were the left atrium and the aortic arch likely because they are most closely related CV structures to the esophagus during its thoracic course. Prior studies have reported dysphagia aortica seen in elderly patients especially women with a history of hypertension and kyphosis[12,13]. Even though a majority of the PPZ’s were present in both upright and supine posture, it was apparent that most of these had a more prominent appearance in one posture than the other. We called this the ‘dominant posture’ for the PPZ, and noted that this had some relation with impaired bolus clearance in the dysphagia group. Pressure, likely by itself is not a key determinant of bolus impairment unless there are other factors at play. We opted to measure pressure only in the upright posture (rather than the dominant posture) since it is the physiological posture of swallowing. Hence, pressure from a vascular source of dysphagia would be more relevant if the PPZ was observed in the upright posture. Similar PPZ pressures in non-dysphagia patients had no impact on impedance, whereas they did in some dysphagia patients. More research needs to be done regarding dysphagia in the elderly in correlation with the history of atherosclerosis and cardiomegaly.
HREIM has evolved into quite an important field over the past couple of decades, and has established itself in the research and clinical arena. It provides us with a wealth of information in the realm of esophageal functional disorders and their diagnostics[14]. Transmitted cardiovascular pulsations are commonly encountered by the observing gastroenterologist, however there is still a lack of knowledge regarding their impact in patients with dysphagia. Our results demonstrate that even though there were a few cases of dysphagia secondary to TCV structures, these can only be diagnosed with confidence with the combined evidence of impedance and fluoroscopic studies such as barium swallow[15]. HREIM also does not fully account for patients with dysphagia to solids, which was the case in most of our patients when type of dysphagia was specified.
In elderly patients with dysphagia, the differential diagnosis usually focuses on swallowing difficulties secondary to advancing age or dementia, or intra-esophageal pathology, e.g., strictures, rings. External compression by cardiovascular landmarks can be another possible, yet uncommon, cause of dysphagia in this population. It can cause considerable frustration for both the physician and the patient when an extensive work up is unsuccessful in yielding answers as to the cause of the symptoms. As per literature review an esophagus lying to the left of the vertebral column in the lower thorax has greater chances of being compressed between the left atrium and descending thoracic aorta[2] (two dominant vascular structures). We also identified this trend in our data, however it’s difficult to establish a clear correlation given that this is the pre-dominant anatomical location for the esophagus, and no statistical evidence was determined to the effect.
It is interesting though, how a PPZ caused by the same cardiovascular structure, behaves differently in presentation across the subjects. It has previously been proposed that some patients have an underlying pre-disposition for dysphagia due to CVS causes which becomes manifest with super-imposed factors (pill-induced esophagitis[3], gastritis, atherosclerosis[16], aortic aneurysm[17,18]). No clear association was observed between any specific CV structure with properties of its esophageal transmitted pulsations, e.g., appearance in upright vs supine posture, axial length, PPZ pressure, or presence or absence of impaired bolus clearance. The only obvious anatomical association was the presence of Aortic arch in the middle third of the esophagus on HREIM and the left atrium in the lower third.
When there is pre-existing history of mitral valve stenosis/regurgitation, atrial fibrillation or cardiomegaly[4,6] in a dysphagia patient, a dilated left atrium compressing the esophagus can be the culprit. Cardiac dysphagia was the cause in two of our 3 pts in which we could determine clear association between dysphagia and pressure caused by a cardiovascular structure. The third patient was of dysphagia lusoria due to an aberrant subclavian artery. Treatment of cardiovascular associated dysphagia is initially always dietary modification[13], however some cases have described endovascular stenting[16] as well as surgical options[2] in advanced cases.
The limitations of our study include a smaller control group as compared to cases, which could not be matched on basis of age. Since our study was retrospective and most HREIM’s are performed on patients with dysphagia, we were unable to compensate for this size difference and our control group was limited. Thoracic radiology was also not available for the control group. We were also unable to incorporate the pulse rate and blood pressure of the subjects in the analysis due to the retrospective design. The dysphagia status of the patient was also determined from chart review rather than a standardized patient reported measure. Future research can focus on older populations with underlying cardiovascular disease in the presence of noticeable transmitted CV pulsations on the esophagus and their association with dysphagia.
In conclusion transmitted pulsations from TCV structures are frequently observed on HREIM usually having no significant impact on swallowing. However, evidence of impaired bolus clearance on impedance can be correlated with clinical data and functional esophageal swallow studies in older adults with dysphagia to assess for external compression from cardiovascular structures.
Transmitted pulsations of thoracic cardiovascular (TCV) structures are often visible on high resolution esophageal impedance manometry (HREIM) as pulsatile pressure zones (PPZ’s). However, their significance has never been established especially in light of dysphagia. This study investigated the characteristics of these common observations, to determine their impact on swallowing and dysphagia.
The field of HREIM has shown rapid development in the past decade and its diagnostic importance, especially for swallowing disorders, is without question. There is a close anatomical relationship between thoracic cardiovascular structures and the esophagus, and the advent of HREIM enables us to study their physiological relationship in better detail.
This study investigates a commonly observed, but poorly understood, phenomenon on HREIM and seeks better comprehension of its relevance. This study is one such step in this direction, and seeks to answer questions about transmitted cardiovascular pulsations and dysphagia. This findings point towards an age-related association with dysphagia which is novel.
This study was retrospective in nature, and was carried out with liquid swallows. Future studies, with more control data and a prospective design, can be designed to study the impact of transmitted pulsations on a solid bolus, barium pill, or the impact of posture.
TCV structures: describes the heart and the major vessels. In our study, reference is mostly to arterial structures due to the transmission of pulses across to the esophagus. HREIM and PPZ’s are the representative areas of transmitted arterial pulsations to the esophagus on HREIM.
This is a well written article. It is an interesting topic. High resolution manometry is still evolving and there are various features in it that are not very clear.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Jain M, Saligram S S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Levitt B, Richter JE. Dysphagia Lusoria: a comprehensive review. Dis Esophagus. 2007;20:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Mittal RK, Siskind BN, Hongo M, Flye MW, McCallum RW. Dysphagia aortica. Clinical, radiological and manometric findings. Dig Dis Sci. 1986;31:379-384. [PubMed] |
| 3. | Malhotra A, Kottam RD, Spira RS. Dysphagia Lusoria presenting with pill induced oesophagitis- a case report with review of literature. BJMP. 2010;3:312. |
| 4. | Whitney B, Croxon R. Dysphagia caused by cardiac enlargement. Clin Radiol. 1972;23:147-152. [PubMed] |
| 5. | Raffa GM, Cappai A, Tarelli G. Giant left atrium Syndrome. J Cardiovasc Med (Hagerstown). 2011;12:745-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Monkemuller K, Feldman K, Ulbright L. Cardiac Dysphagia. Clinical Gastroenterology and Hepatology. 2010;8:e41-e42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Arifputera A, Loo G, Chang P, Kojodjojo P. An unusual case of dysphonia and dysphagia. Singapore Med J. 2014;55:e31-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Babaei R, Mittal K. Cardiovascular compression of the esophagus and spread of gastro-esophageal reflux. Neurogastroenterol Motil. 2011;23:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Wilkinson JM, Euinton HA, Smith LF, Bull MJ, Thorpe JA. Diagnostic dilemmas in dysphagia aortica. Eur J Cardiothorac Surg. 1997;11:222-227. [PubMed] |
| 10. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1447] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 11. | Xiao Y, Read A, Nicodème F, Roman S, Kahrilas PJ, Pandolfino JE. The effect of a sitting vs supine posture on normative esophageal pressure topography metrics and Chicago Classification diagnosis of esophageal motility disorders. Neurogastroenterol Motil. 2012;24:e509-e516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Hilliard AA, Murali N, Keller AS. Dysphagia aortica. Ann Intern. Med. 2005;142:230-231. [PubMed] |
| 13. | Karavelioğlu Y, Kalçik M, Yetim M, Sarak T, Bekar L, Doğan T. A Rare Cause of Dysphagia and Weight Loss in a Nonagenarian with Hypertension: Dysphagia Aortica. J Am Geriatr Soc. 2015;63:1488-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Carlson DA, Ravi K, Kahrilas PJ, Gyawali CP, Bredenoord AJ, Castell DO, Spechler SJ, Halland M, Kanuri N, Katzka DA. Diagnosis of Esophageal Motility Disorders: Esophageal Pressure Topography vs. Conventional Line Tracing. Am J Gastroenterol. 2015;110:967-977; quiz 978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Scharitzer M, Pokieser P, Schober E, Schima W, Eisenhuber E, Stadler A, Memarsadeghi M, Partik B, Lechner G, Ekberg O. Morphological findings in dynamic swallowing studies of symptomatic patients. Eur Radiol. 2002;12:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kische S, Werner D, Ince H. A neglected symptom of contained Aortic Laceration- Dysphagia Aortica successfully treated by endovascular stent grafting. Catheter Cardiovasc Interv. 2012;80:1052-1055. |
| 17. | Wang WP, Yan XL, Ni YF, Zhang T, Han Y, Li XF, Lu Q. An unusual cause of dysphagia: thoracic aorta aneurysm. J Thorac Dis. 2013;5:E224-E226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Antón E. Dysphagia aortica: a diagnostic challenge in the elderly. Rev Esp Enferm Dig. 2007;99:362-364. [PubMed] |