Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7765
Peer-review started: August 15, 2017
First decision: August 30, 2017
Revised: September 14, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: November 21, 2017
Processing time: 97 Days and 19.7 Hours
To explore the ability of superb microvascular imaging (SMI) in differential diagnosis of focal liver lesions (FLLs) and to compare SMI morphology findings to those of color Doppler ultrasound and enhanced imaging.
Twenty-four patients with 31 FLLs were included in our study, with diagnoses of hemangioma (HE) (n = 17), hepatocellular carcinoma (HCC) (n = 5), metastatic lesions (n = 5), primary hepatic lymphoma (n = 1), focal nodular hyperplasia (FNH) (n = 2), and adenoma (n = 1). Nine lesions were pathologically diagnosed, and 22 lesions were radiologically confirmed, all of which were evaluated by at least two types of enhanced imaging techniques. All patients had undergone SMI. Patients were divided into subgroups based on pathological and radiological diagnoses to analyze SMI manifestations. We also compared the SMI manifestations of the most common malignant FLLs (HCCs and metastatic lesions) with those of the most common benign FLLs (HEs).
HEs were classified into three SMI subgroups: diffuse dot-like type (n = 6), strip rim type (n = 8), and nodular rim type (n = 3). The sizes of the three types of HEs were significantly different (P = 0.00, < 0.05). HCCs were classified into two subgroups: diffuse honeycomb type (n = 2) and non-specific type (n = 3). Four of the metastatic lesions were the strip rim type, and the other metastatic lesion was the thick rim type, which is the same as that of lymphoma. FNH was described as a spoke-wheel type, and adenoma as a diffuse honeycomb type. The SMI types of HCCs and metastatic lesions were significantly different from those of HEs (P = 0.048, < 0.05).
SMI technology enables microvascular evaluation of FLLs without using any contrast agent. For HEs, lesion size may affect SMI performance. SMI is able to provide useful information for differential diagnosis of HCCs and metastatic lesions from HEs.
Core tip: We utilized a novel ultrasound technique, superb microvascular imaging (SMI), to assess the microvascular morphology of focal liver lesions to provide additional diagnostic information. The focal liver lesions consisted of hemangiomas, hepatocellular carcinomas, metastatic lesions, primary hepatic lymphoma, focal nodular hyperplasia, and adenoma. We also compared SMI manifestations to color Doppler ultrasound and enhanced imaging features.
- Citation: He MN, Lv K, Jiang YX, Jiang TA. Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol 2017; 23(43): 7765-7775
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7765
The detection rate of focal liver lesions (FLLs) is clearly increasing because of the widespread application of imaging techniques, especially ultrasound examinations. Kaltenbach et al[1] investigated 45319 hospitalized patients and found that the prevalence of benign FLLs was 15.1%. Hepatocellular carcinoma (HCC) is the second most common cause of mortality from cancer[2,3]. The current diagnostic challenge not only involves effectively distinguishing between malignant and benign FLLs[4,5] but also precisely identifying the characteristics of all types of FLLs since they require different clinical treatments and have different outcomes. For example, treatments for hemangioma (HE) and adenoma and those for HCC and lymphoma differ significantly.
To address this challenge, it is essential to establish a diagnostic method that is inexpensive, easy to operate, and has high diagnostic accuracy. Contrast-enhanced ultrasound (CEUS) has been gradually recognized as a comparable imaging technique to contrast-enhanced computed tomography (CECT) and contrast-enhanced magnetic resonance imaging (CEMRI) in the diagnosis of FLLs[6,7]. The common advantage of the above three techniques is visualization of the microvascular structure, which is one of the most important elements of the tumor microenvironment[8], plays an important role in the development and progression of lesions, and is essential for their differential diagnosis. However, these techniques also have drawbacks. Some patients may be ineligible for these examinations because of contraindications to contrast agents, such as the risk of triggering or worsening renal failure with the iodinated contrast agents used for computed tomography (CT) and gadolinium diethylene-trianmine pentaacetic acid (Gd-DTPA) used for magnetic resonance imaging (MRI)[9], as well as the risk of hypersensitivity reactions caused by the agents used in CEUS, CECT, and CEMRI[10]. Additionally, these techniques are expensive and time-consuming, limiting their widespread use.
Superb microvascular imaging (SMI) is a novel Doppler technique developed by Toshiba Medical System (Tokyo, Japan)[11], which was designed to simulate CEUS by using advanced clutter elimination to obtain only vascular flow signals without using any contrast agents[12]. Similar to color Doppler and power Doppler imaging, SMI can provide a real-time examination of vascularity in FLLs, but it has the additional advantages of detecting slower blood flow and revealing micro-vessels. Studies on SMI technology in superficial tissues, such as thyroid and breast tumors, have been reported, with some useful information obtained for differential diagnoses[11,13]. Machado et al[13] evaluated the capability of SMI to show microvascular flow in normal thyroid tissue and in thyroid nodules compared with that of CDFI and found that SMI was able to better depict vessel branching. Zhan et al[14] compared the abilities of CDFI and SMI to reveal penetrating vessels (PVs) in breast cancer and discovered that more PVs were evident by SMI than by CDFI. By contrast, very few studies have been conducted on SMI for FLLs[15]. The purpose of this study was to investigate the SMI features of FLLs and to analyze the ability of SMI to provide additional information for the differential diagnosis of FLLs.
This study was performed from November 2016 to March 2017 at Peking Union Medical College Hospital. The study was approved by the ethical committee of the hospital, and informed consent was provided by each patient before examination. Twenty-four patients (mean age, 53.5 ± 12.9 years, range 24-79 years; 13 men and 11 women) with 31 FLLs were included in our study. All FLLs had been detected by at least two modalities of CEUS/CT/MRI. Of all FLLs, nine were pathologically diagnosed, including two HEs in two patients, five HCCs in four patients, one hepatic adenoma, and one primary hepatic lymphoma, and 22 were radiologically confirmed, including 15 HEs in 12 patients (a single lesion in nine patients and two lesions in three patients), which were diagnosed by CECT and/or CEMRI and showed typical manifestations of nodular or strip type, with peripheral to centripetal enhancement and persistent enhancement in the portal venous phase and the delayed phase. Five metastatic masses (one patient had a single lesion and another patient had four lesions) were diagnosed by the primary tumor history and CECT/CEUS, which showed malignant characteristics with arterial enhancement that disappeared quickly in the portal venous phase. Two focal nodular hyperplasia (FNH) lesions were diagnosed by CECT based on persistent enhancement from the arterial phase to the portal venous/delayed phase, clearly defined outlines, and a central scar. Table 1 summarizes the clinical, SMI, and pathologic features of the 31 FLLs.
| No. | Clinical | Pathological | SMI type | Size | Age | Sex | Other |
| diagnosis | diagnosis | (I-VII) | (cm) | (yr) | |||
| 1 | HE | - | I | 1.7 | 79 | M | |
| 2 | HE | - | I | 1.1 | 58 | F | |
| 3 | HE | - | I | 2.6 | 54 | M | |
| 4 | HE | - | I | 2.5 | 52 | F | |
| 5 | HE | - | I | 1.8 | 24 | F | Same person |
| 6 | HE | - | I | 2.7 | 24 | F | |
| 7 | HE | - | II | 2.3 | 63 | M | |
| 8 | HE | - | II | 3.9 | 61 | M | Same person |
| 9 | HE | - | II | 4.8 | 61 | M | |
| 10 | HE | - | II | 3.7 | 61 | F | |
| 11 | HE | - | II | 2.8 | 48 | M | Same person |
| 12 | HE | - | II | 3.1 | 48 | M | |
| 13 | HE | - | II | 3.1 | 41 | M | |
| 14 | HE | - | II | 5.7 | 33 | F | |
| 15 | HE | - | III | 6.3 | 63 | F | |
| 16 | - | HE | III | 8.4 | 51 | F | |
| 17 | - | HE | III | 8.5 | 47 | F | |
| 18 | B-M | - | II | 1.4 | 39 | F | Same person |
| 19 | B-M | - | II | 2.3 | 39 | F | |
| 20 | B-M | - | II | 2.6 | 39 | F | |
| 21 | B-M | - | II | 3.3 | 39 | F | |
| 22 | P-M | - | VI | 3.9 | 64 | M | |
| 23 | - | HCC | IV | 7.4 | 68 | M | |
| 24 | - | HCC | IV | 6.1 | 57 | M | |
| 25 | - | HCC | V | 2.5 | 60 | M | |
| 26 | - | HCC | V | 2.9 | 48 | M | Same person |
| 27 | - | HCC | V | 4.7 | 48 | M | |
| 28 | - | HA | IV | 5.3 | 41 | M | |
| 29 | - | LYM | VI | 5.8 | 71 | M | |
| 30 | FNH | - | VII | 4.1 | 62 | F | |
| 31 | FNH | - | VII | 4.3 | 39 | F |
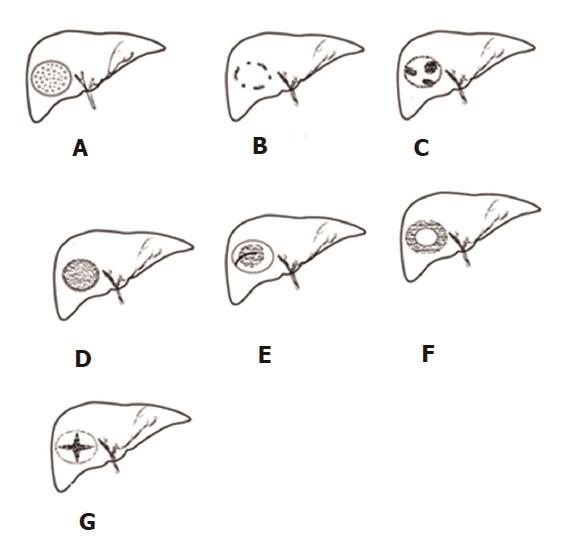
Patients were placed in the supine or left lateral position after 6 h of fasting. All US examinations, including B-mode US, CDFI, and SMI, were performed with a curved transducer (6C1Aplio 500; Toshiba Medical Systems Corporation, Tochigi, Japan). B-mode US was performed first to thoroughly scan the liver for FLLs. Once detected, the general features of FLLs were observed and their sizes (maximal diameter) were measured. Subsequently, conventional CDFI and SMI were performed to observe the vascular structures of the FLLs. For the CDFI examination, the scale was set as low as possible until the appropriate level was reached without any pseudo color flow, such as color flow spillover (the lowest scale was 4 cm/s), and the flow gain was adjusted until noise emerged. For the SMI examination, the parameter settings were as follows: color velocity scale of no more than 2.0 cm/s, frame rate > 30 fps, color frequency 5-7 MHz, and the gain setting adjusted to show optimal imaging. All US examinations were performed by a single operator, and the imaging data were analyzed by two experienced radiologists. Both the operator and readers had > 10 years of experience in liver ultrasound. The readers classified the SMI characteristics of the FLLs into seven types (Figure 1): I, diffuse dot-like type; II, strip rim type; III, nodular rim type; IV, diffuse honeycomb type; V, non-specific type; VI, thick rim type with lymphoma; and VII, spoke-wheel type[15]. If a disagreement occurred, the decision was determined by consensus after consultation with a third experienced doctor. We divided the 31 FLLs into a ≤ 3.0 cm group and a > 3.0 cm group[16], and then compared vascular visibility between CDFI and SMI. We also compared the SMI types of the most common malignant FLLs (HCCs and metastatic lesions) and those of the most common benign FLLs (HEs).
Differences in SMI types between the most common malignant FLLs (HCCs and metastatic lesions) and the most common benign FLLs (HEs) were evaluated by Fisher’s exact test. The χ2 test was used to compare the sex distribution of HEs between different SMI types. Differences in size and age were evaluated by a one-way ANOVA test. P < 0.05 was considered statistically significant. SPSS 16.0 was used for all data analyses.
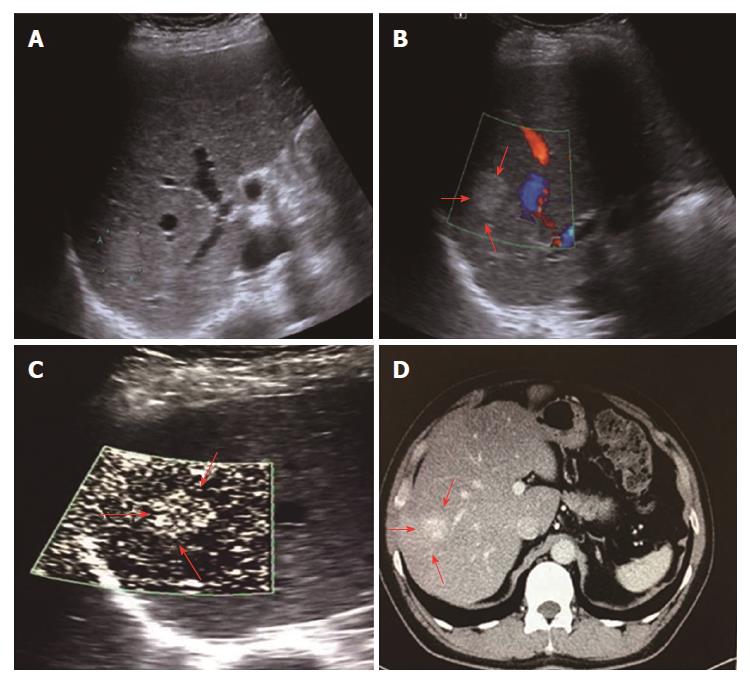
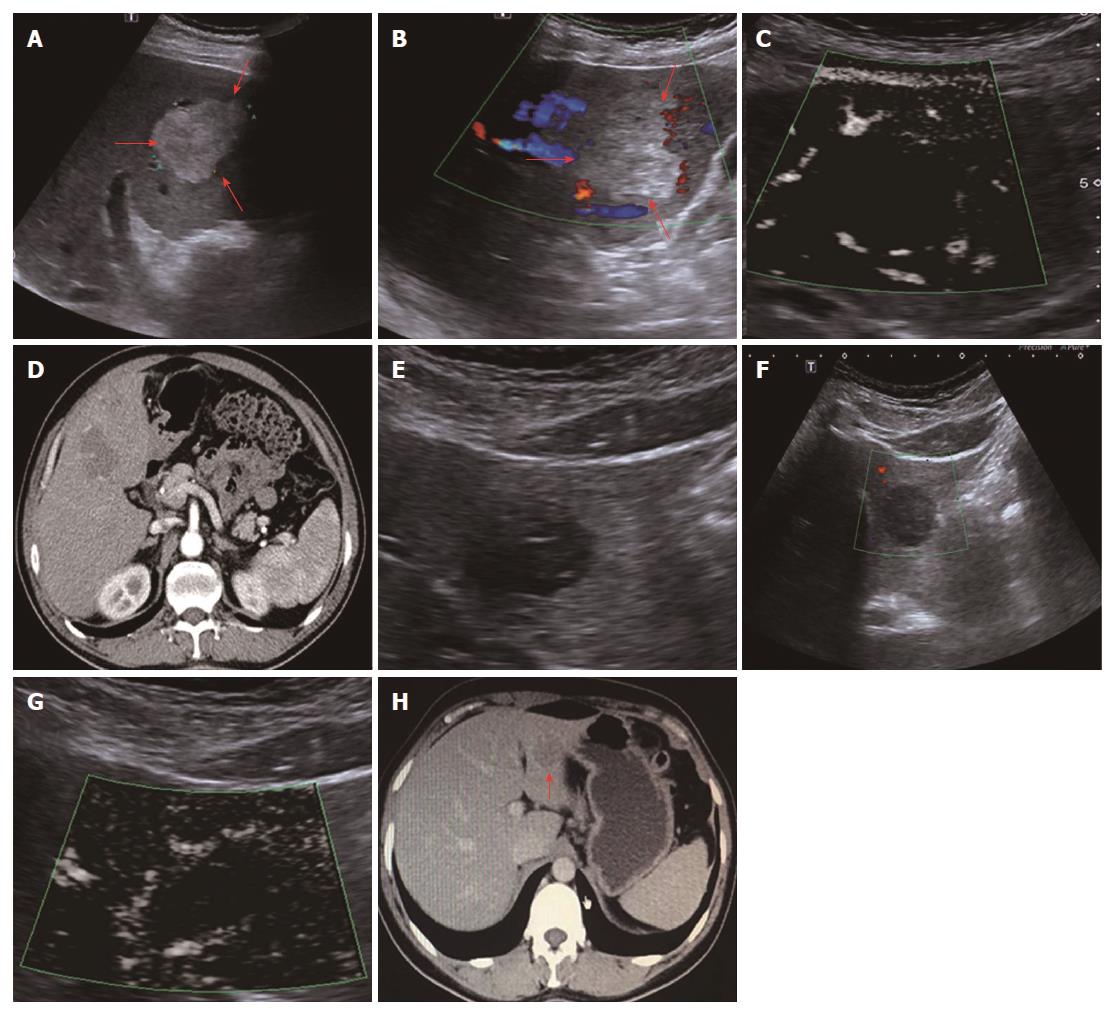
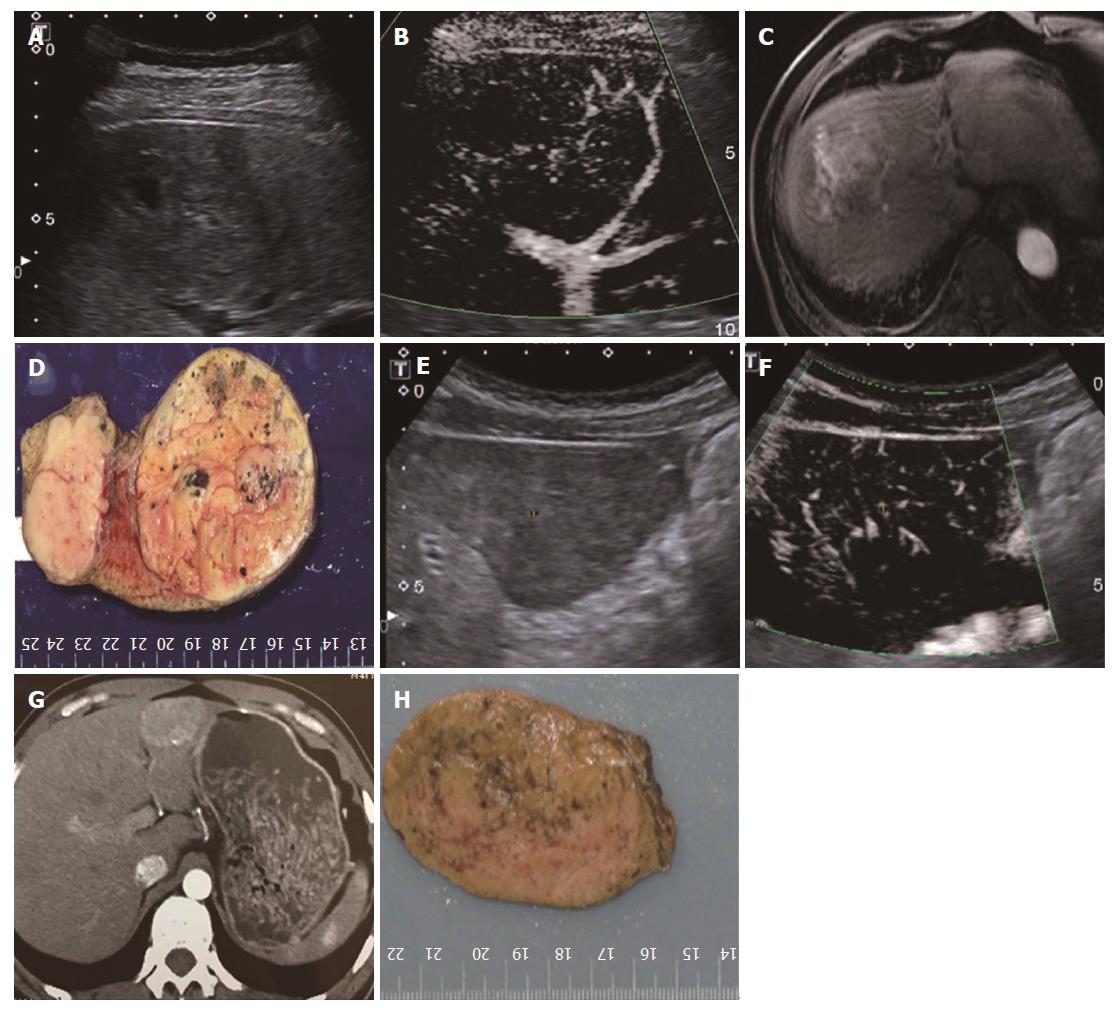
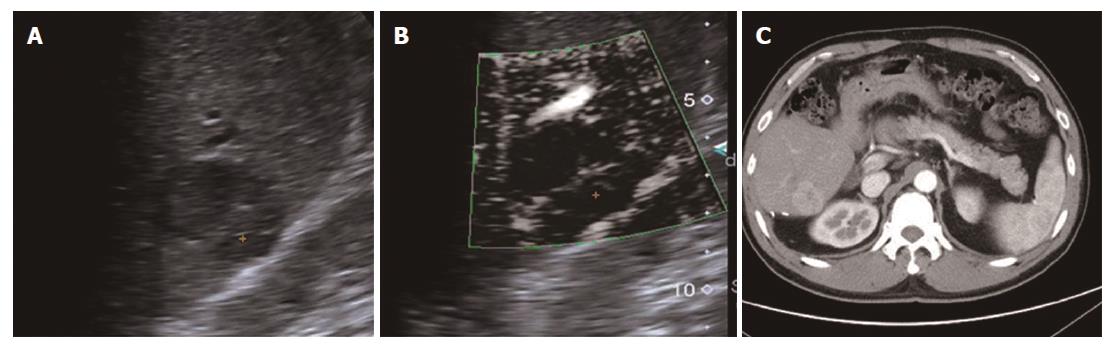
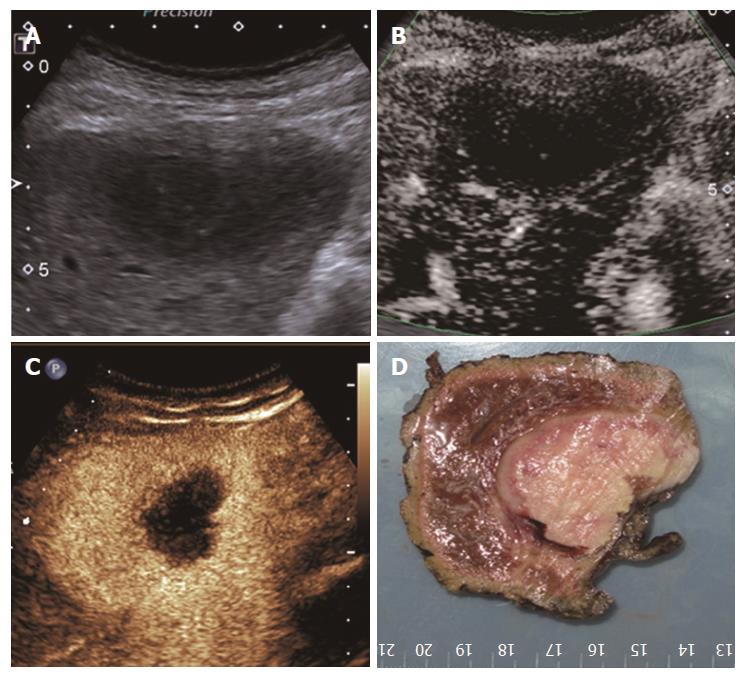
All 31 FLLs underwent successful US examinations, including B-mode US, CDFI, and SMI, and satisfactory images were obtained. Of the 17 HEs, the SMI features could be divided into three types: diffuse dot-like type (Type I; n = 6; Figure 2), strip rim type (Type II; n = 8; Figure 3), and nodular rim type (Type III; n = 3; Figure 4). The sizes of the three types of HEs were significantly different (P = 0.00, < 0.05), but the average age and sex distributions of the patients showed no significant differences (Table 2).
| Type | Size | Yr | Sex |
| I | 2.07 ± 0.63 | 48.5 ± 21.3 | 2\4 |
| II | 3.68 ± 1.12 | 52.0 ± 11.2 | 6\2 |
| III | 7.73 ± 1.24 | 53.7 ± 8.3 | 0\3 |
| P value | 0 | 0.89 | 0.06 |
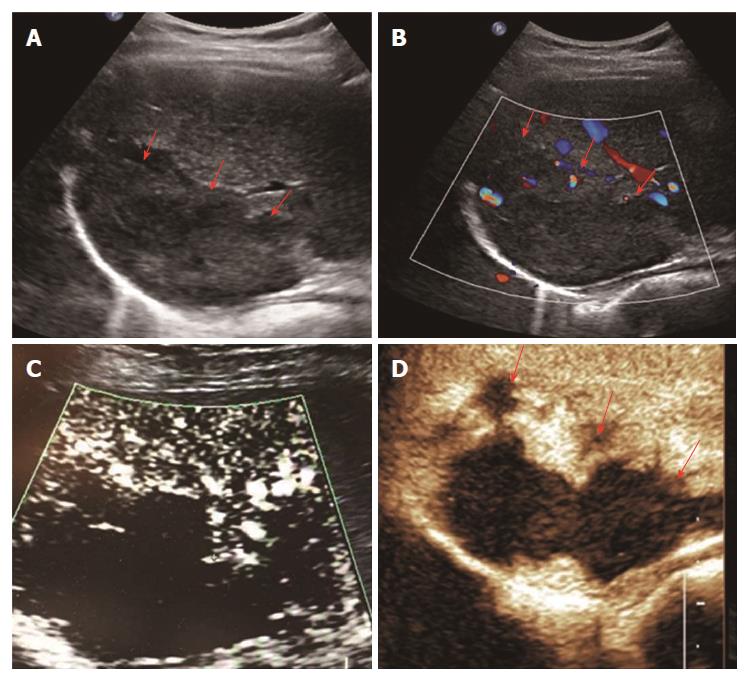
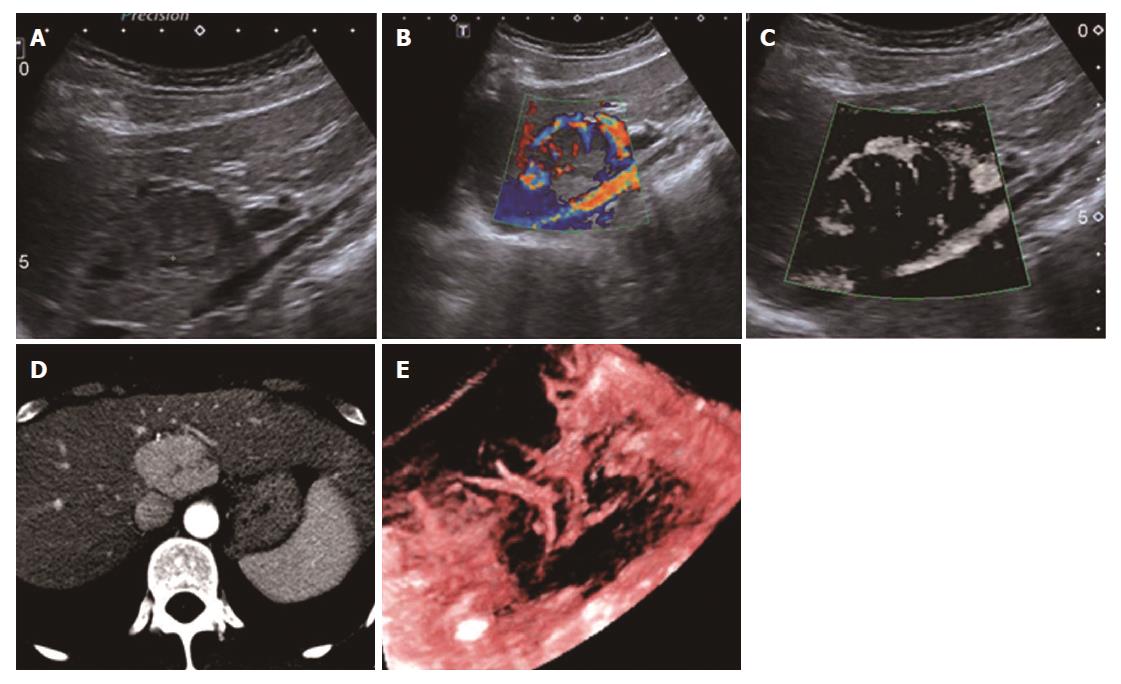
The SMI features of the 14 remaining FLLs were as follows: two of the HCCs were described as the diffuse honeycomb type (Type IV, Figure 5) and three were defined as the non-specific type (Type V, Figure 6). Four of the metastatic lesions were from breast cancer in one patient and were classified as the strip rim type (Type II), and one was from pancreatic cancer and was classified as the thick rim type (Type VI, Figure 7), with the lesion reflecting primary hepatic lymphoma. Two FNH lesions were described as the spoke-wheel type (Type VII, Figure 8), and the adenoma was described as the diffuse honeycomb type (Type V). The distributions of SMI types between the most common malignant FLLs (HCCs and metastatic lesions) and the most common benign FLLs (HEs) were significantly different (P = 0.048, < 0.05) (Table 3), and these morphological findings of SMI types in different FLLs were consistent with the findings from contrast-enhanced ultrasound/CT/MRI. The characteristics of all FLLs and the corresponding patients are summarized in Table 1.
| Group | I | II | III | IV | V | VI | VII |
| HE (17) | 6 (35.2) | 8 (47.1) | 3 (17.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| M (5) | 0 (0) | 4 (80) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) |
| HCC (5) | 0 (0) | 0 (0) | 0 (0) | 2 (40) | 3 (60) | 0 (0) | 0(0) |
| FNH (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| HA (1) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) |
| PHL (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
Among the 31 FLLs, 13 were small FLLs with a maximum diameter of less than 3.0 cm and 17 were larger than 3.0 cm. SMI could detect the vascular structures of all 31 lesions, while CDFI failed to detect the vascular structures of nine (69.2%) lesions in the < 3.0 cm group and two (11.8%) lesions in the > 3.0 cm group.
Angiogenesis, an important part of the tumor microenvironment[17], plays a key role in the development of FLLs. The morphology of blood vessels in lesions is also important for differential diagnoses. CDFI had been widely used to depict tumor vessels, but it is limited in identifying low-speed flow signals because of the wall filter, which suppresses clutter and motion artifacts and results in the loss of low-speed flow signals. SMI, a novel technique, can overcome this limitation and can effectively distinguish low-speed flow signals from artifacts without the use of any contrast agent. In the current study, we compared the abilities of CDFI and SMI to detect the vascular structures of all 31 FLLs, and the results showed that SMI could detect flow information in all lesions, but CDFI failed to detect the vascular structures of nine (69.2%) lesions in the < 3.0 cm group and two (11.8%) lesions in the > 3.0 cm group, suggesting that SMI has obvious advantages in detecting the blood vessels of FLLs. Therefore, SMI overcomes the limitation of CDFI, especially for the description of micro-vessels in small lesions.
Studies on the application of SMI for FLLs are limited, and only two investigations have been reported: one was reported by Wu et al[18], in which SMI clearly demonstrated the typical spoke-wheel vascular type of FNH in the liver without the use of any contrast agent, and the other was reported by Lee et al[15], who used SMI for 29 FLLs, including HE, HCC, and FNH, and concluded that the SMI types were significantly different between FLLs. In the present study, we analyzed the SMI features of 31 FLLs, including HEs, HCC, metastatic lesions, FNH, hepatic adenoma, and primary hepatic lymphoma. We used seven SMI types to depict the vessel distributions and morphologies of the 31 FLLs. We found that the SMI type distribution between the most common malignant FLLs (HCCs and metastatic lesions) and the most common benign FLLs (HEs) differed significantly, which could provide meaningful differential diagnostic information. Meanwhile, we also found that various FLLs shared the same SMI type. For example, the metastatic lesions from breast cancer and HEs shared the strip rim type (Type II), and the other metastatic lesion from pancreatic cancer and primary hepatic lymphoma both showed the thick rim type (Type VI). Hepatic adenoma and some HCCs had the same diffuse honeycomb type (Type IV). Therefore, when an FLL is suspected to be a certain SMI type, other clinical information, such as a medical history, is still needed to determine an accurate diagnosis.
For HEs, the average maximum diameters of type I, type II, and type III lesions were 2.07 ± 0.63 cm, 3.68 ± 1.12 cm, and 7.73 ± 1.24 cm, respectively, and the lesion sizes of the three different SMI types were significantly different. The histology of the HEs can explain the SMI manifestations. First, the original etiology of HEs was not clear, but congenital vessel malformation caused by hyperplastic endothelial cells may be the cause[19]. Consequently, the microscopic features of HEs appeared like a blood pool constituted by cavernous vascular spaces, which were lined by a single layer of flat endothelial cells of different sizes. The vascular cavities varied in size and corresponded to the size of HE lesions. Some HEs may contain a thrombus that will gradually turn into a fibrous scar or nodules, especially in large lesions[20]. Therefore, for larger HEs, the internal blood flow may be slower or even completely replaced by the thrombus, so an enhanced examination can reveal peripheral nodules or ring-enhanced patterns. SMI also reveals these features, especially in type II and type III. For small HEs, both enhanced techniques and SMI could show slow internal vascular signals, as in type I. According to the previous statements, type I and type III had distinctive features enabling the discrimination of HEs from other FLLs.
Regarding the other types of FLLs, the previous SMI study on FLLs by Lee et al[15] showed that the SMI manifestations of HCCs revealed non-specific vascular types, while in the present study, we used two SMI types to summarize HCCs, including type IV, the diffuse honeycomb type, and type V, the non-specific type. The general pathology of the former type showed that the inter-tumor blood vessels were distributed in a grid pattern resembling honeycombs. The SMI feature of the latter type was a strip-like trunk with tiny branches, but we do not have enough evidence from other enhanced techniques or pathology to support it. Therefore, we classified it as the non-specific type. In this research, including two cases of FNH with both CDFI and SMI showing the typical spoke-wheel type without a basic echo of the liver parenchyma, SMI seemed to show the vascular structure more clearly, which is consistent with the previous studies[15,18].
For metastatic lesions, it is always assumed that their imaging results are particularly confusing because metastatic lesions can simulate various other types of FLLs, as in the present study. The SMI types of metastatic lesions were similar to those of HEs and lymphoma. Therefore, in the diagnosis of metastatic lesions, a clinical history including the primary tumor is critical in addition to imaging studies.
Research on the ultrasound features of hepatic adenomas is relatively rare because of the low morbidity compared to other FLLs. Dong et al[21] conducted a retrospective study to analyze differences in ultrasound and CEUS features between hepatic adenoma and HCC, and the results showed that most cases of hepatic adenomas manifested as homogenous, rapid, and complete enhancement in the arterial phase, which is similar to HCC. In this study, our case of hepatic adenoma showed the same SMI type (type IV, diffuse honeycomb type) as some HCCs. This patient was diagnosed with a benign lesion or a relatively mild malignant mass before surgery because of the very clear margin and the slow wash-out pattern on CECT. SMI for this case did not show unique characteristic performance and was limited to the diagnosis of hepatic adenoma, requiring supplementation with an enhanced imaging examination.
Primary hepatic lymphoma (PHL) is also a rare disease, accounting for only 0.016% of all cases of non-Hodgkin’s lymphoma (NHL)[22]. The treatment for PHL includes surgery, chemotherapy, and radiotherapy, which is significantly different from that for HCC or other malignant FLLs. Therefore, an accurate diagnosis before surgery is essential. Research by Lu et al[23] showed that one CECT manifestation of some PHLs was rim-like enhancement, which was similar to the features revealed by CEUS and SMI. Therefore, SMI may provide some helpful information for the diagnosis of some PHLs.
Our study has some limitations. First, for hepatic adenoma and primary hepatic lymphoma, the incidence rates are very low and sample errors are inevitable. Second, only nine lesions were pathologically diagnosed in this study. Therefore, a very low possibility of missed diagnosis remains for the other 22 lesions that were radiologically confirmed because as the results showed, different types of FLLs may have similar imaging manifestations despite the use of at least two kinds of enhanced imaging techniques.
In conclusion, SMI technology allows evaluation of the microvascular structures of FLLs without using any contrast agent. For HEs, the lesion size may affect SMI performance. SMI can overcome the limitation of CDFI, especially for micro-vessel descriptions in small lesions. The SMI characteristics between the most common malignant FLLs (HCCs and metastatic lesions) and the most common benign FLLs (HEs) are significantly different.
The frequency of focal liver lesion (FLL) detection is increasing because of the development and prevalence of imaging technology, especially ultrasound examinations. The subsequent challenge not only involves efficiently distinguishing between malignant and benign FLLs but also precisely identifying the characteristics of all types of FLLs as different clinical treatments and outcomes may be inherent to each type.
Addressing this challenge involves choosing a diagnostic method that requires minimal time and effort, but can achieve high diagnostic accuracy. Some patients may be ineligible for the currently used imaging techniques, such as CEUS/CT/MRI, because of the risk of triggering or worsening renal failure due to contrast agents, such as iodine used for CT and Gd-DTPA (gadolinium diethylene-trianmine pentaacetic acid) used for MRI. In addition, the agents used in CEUS, CECT, and CEMRI are foreign bodies, and each one could cause hypersensitivity reactions. Additionally, the three techniques are expensive and time-consuming, limiting their widespread application.
Superb microvascular imaging (SMI) is a novel Doppler technique that simulates enhanced ultrasound by using advanced clutter elimination to obtain only vascular flow signals without using any contrast agent. The purpose of our study was to investigate the SMI features of focal liver lesions and to analyze their ability to provide additional information for differential diagnoses.
To explore the ability of SMI to differentially diagnose focal liver lesions and compare SMI morphologies to those of color Doppler ultrasound and enhanced imaging.
Twenty-four patients with 31 focal liver lesions (FLLs) were included in our study, with diagnoses of hemangioma (HE) (n = 17), hepatocellular carcinoma (HCC) (n = 5), metastatic lesions (n = 5), primary hepatic lymphoma (n = 1), focal nodular hyperplasia (FNH) (n = 2), and adenoma (n = 1). Nine lesions were pathologically diagnosed, and 22 lesions were radiologically confirmed, all of which were evaluated by at least two types of enhanced imaging techniques. All patients had undergone SMI. Patients were divided into subgroups based on pathological and radiological diagnoses to analyze SMI manifestations. We also compared the SMI manifestations of the most common malignant FLLs of HCCs and metastatic lesions with those of the most common benign FLLs of HEs.
HEs were classified into three SMI subgroups: diffuse dot-like type (n = 6); strip rim type (n = 8); and nodular rim type (n = 3). The sizes of the three types of HEs were significantly different (P = 0.00, < 0.05). HCCs were classified into two subgroups: diffuse honeycomb type (n = 2) and non-specific type (n = 3). Four of the metastatic lesions were the strip rim type of HE, and the other metastatic lesion was the thick rim type, which is the same as that of lymphoma. FNH was described as a spoke-wheel type, and adenoma as a diffuse honeycomb type. The SMI types of HCCs and metastatic lesions were significantly different from that of HEs (P = 0.048, < 0.05).
SMI technology enables microvascular evaluation of focal liver lesions without using any contrast agent. For HEs, lesion size may affect SMI performance. SMI is able to provide useful information for differentially diagnose HCCs and metastatic lesions from HEs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dietrich CF, Eleftheriadis NP, Tarantino G S- Editor: Wei LJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, Graeter T. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY). 2016;41:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World J Exp Med. 2016;6:21-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Chow PK, Choo SP, Ng DC, Lo RH, Wang ML, Toh HC, Tai DW, Goh BK, Wong JS, Tay KH. National Cancer Centre Singapore Consensus Guidelines for Hepatocellular Carcinoma. Liver Cancer. 2016;5:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Dietrich CF, Sharma M, Gibson RN, Schreiber-Dietrich D, Jenssen C. Fortuitously discovered liver lesions. World J Gastroenterol. 2013;19:3173-3188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Collin P, Rinta-Kiikka I, Räty S, Laukkarinen J, Sand J. Diagnostic workup of liver lesions: too long time with too many examinations. Scand J Gastroenterol. 2015;50:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Dietrich CF, Maddalena ME, Cui XW, Schreiber-Dietrich D, Ignee A. Liver tumor characterization--review of the literature. Ultraschall Med. 2012;33 Suppl 1:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Dietrich CF. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012;33 Suppl 1:S22-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 9. | Bedoya M A, White A M, Edgar J C, Pradhan M, Raab EL, MPH1 , Meyer J S. Effect of Intravenous Administration of Contrast Media on Serum Creatinine Levels in Neonates. Radiology. 2017;284:160895. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kolenda C, Dubost R, Hacard F, Mullet C, Le Quang D, Garnier L, Bienvenu J, Piriou V, Bérard F, Bienvenu F. Evaluation of basophil activation test in the management of immediate hypersensitivity reactions to gadolinium-based contrast agents: a five-year experience. J Allergy Clin Immunol Pract. 2017;5:846-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Park AY, Seo BK, Cha SH, Yeom SK, Lee SW, Chung HH. An Innovative Ultrasound Technique for Evaluation of Tumor Vascularity in Breast Cancers: Superb Micro-Vascular Imaging. J Breast Cancer. 2016;19:210-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Ma Y, Li G, Li J, Ren WD. The Diagnostic Value of Superb Microvascular Imaging (SMI) in Detecting Blood Flow Signals of Breast Lesions: A Preliminary Study Comparing SMI to Color Doppler Flow Imaging. Medicine (Baltimore). 2015;94:e1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Machado P, Segal S, Lyshchik A, Forsberg F. A Novel Microvascular Flow Technique: Initial Results in Thyroids. Ultrasound Q. 2016;32:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Zhan J, Diao XH, Jin JM, Chen L, Chen Y. Superb Microvascular Imaging-A new vascular detecting ultrasonographic technique for avascular breast masses: A preliminary study. Eur J Radiol. 2016;85:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Lee DH, Lee JY, Han JK. Superb microvascular imaging technology for ultrasound examinations: Initial experiences for hepatic tumors. Eur J Radiol. 2016;85:2090-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J, Committee G. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 185] [Cited by in RCA: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | Drudi FM, Cantisani V, Gnecchi M, Malpassini F, Di Leo N, de Felice C. Contrast-enhanced ultrasound examination of the breast: a literature review. Ultraschall Med. 2012;33:E1-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Wu L, Yen HH, Soon MS. Spoke-wheel sign of focal nodular hyperplasia revealed by superb micro-vascular ultrasound imaging. QJM. 2015;108:669-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Massironi S, Branchi F, Rossi R E, Fraquelli M, Elli L, Bardella MT, Cavalcoli F, Conte D. Hepatic hemangioma in celiac patients: data from a large consecutive series. Gastroenterol Res Pract. 2015;2015:749235. |
| 20. | Ishak K G, Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995-1013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 298] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Dong Y, Zhu Z, Wang W P, Mao F, Ji ZB. Ultrasound features of hepatocellular adenoma and the additional value of contrast-enhanced ultrasound. Hepatobiliary Pancreat Dis Int. 2016;15:48-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Padhan RK, Das P, Shalimar . Primary hepatic lymphoma. Trop Gastroenterol. 2015;36:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Lu Q, Zhang H, Wang W P, Jin YJ, Ji ZB. Primary non-Hodgkin’s lymphoma of the liver: sonographic and CT findings. Hepatobiliary Pancreat Dis Int. 2015;14:75-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |