Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7756
Peer-review started: July 3, 2017
First decision: August 10, 2017
Revised: August 25, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: November 21, 2017
Processing time: 141 Days and 18.3 Hours
To gain knowledge of xanthelasma, a large population-based study was conducted.
Patients who underwent upper gastrointestinal endoscopy at the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China during Jan 2009 to Nov 2016 were included. General characteristics as well as clinical data were collected, including blood routine, serum biochemical analysis, endoscopic findinds, histological evaluation and comorbiditie. Statistical analyses was performed using SPSS 20.0 software for Windows (IBM Inc., Chicago, IL, United States) using Student’s t-test, Mann-Whitney U test, χ2 test, univariable and multivariable logistic analysis. 2-tailed P value less than 0.05 was considered to be statistically significant.
A total of 176006 endoscopies were retrieved and we included 1370 xanthelasma participants (703 men, 667 women) in this study. Prevalence of xanthelasma was 0.78% with average age of 56.6 ± 11.2 years. Chief complaint of xanthelasma consisted abdominal pain (24.2%), up-abdominal discomfort (14.1%), abdominal distention (10.1%), dyspepsia (9.1%), et al. Most xanthelasma occurred as single lesion in gastric antrum. Xanthelasma patients witnessed higher Helicobacter pylori (H. pylori) infection rate, more of other gastric lesions including atrophy, intestinal metaplasia and dysplasia (P < 0.01). In xanthelasma patients, serum carcinoembryonic antigen, triglyceride, fasting glucose, neutrophil, neutrophil-to-lymphocyte ratio were significantly higher, and high density lipoprotein-cholesterol, lymphocyte was lower (P < 0.05). Xanthelasma accompanied with more fatty liver disease and hepatic cyst, but fewer gallbladder polyp (P < 0.05). In logistic regression, it revealed that fasting plasma glucose (OR = 3.347, 1.170-9.575, P < 0.05), neutrophil (OR = 1.617, 1.003-2.605, P < 0.05), and carcinoembryonic antigen (OR = 2.011, 1.236-3.271, P < 0.01) were all independent risk factors in xanthelasma.
Current study described a large xanthelasma cohort in Chinese population, revealed its relationship with H. pylori infection, carcinogenesis, metabolic dysfunction and inflammation as well.
Core tip: Xanthelasma was a relatively rare endoscopic finding, characterized by accumulation of lipid in histiocytic foam cells in mucosa. Current study described a large xanthelasma cohort in Chinese population and revealed its relationship with Helicobacter pylori infection, atrophy, intestinal metaplasia, dysplasia, and metabolic disorder, indicating role of xanthelasma in both carcinogenesis and metabolic dysfunction.
- Citation: Chen Y, He XJ, Zhou MJ, Li YM. Gastric xanthelasma and metabolic disorders: A large retrospective study among Chinese population. World J Gastroenterol 2017; 23(43): 7756-7764
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7756
Gastric xanthelasma, characterized by lipid accumulation in histiocytic foam cells, was a relatively scarce endoscopic finding, with prevalence varied from 0.8% to 7% in different study population[1-3]. As is frequently observed in the gastric mucosa of patients with early gastric cancer, xanthelasma was assumed to be a predictive biomarker or pre-tumorigenesis change of gastric adenocarcinoma[1,4]. But there is no large descriptional study among Chinese population.
Mechanism of xanthelasma was unknown. Early small sample studies have showed relationship between xanthelasma and Helicobacter pylori (H. pylori) infection[5,6]. Later on, a Korean cohort further supported this finding, indicating a role of H. pylori as well as inflammation in xanthelasma[2]. As these countries were high H. pylori prevalent places, till now, whether H. pylori infection was result of or just casual finding in xanthelasma was not clear, neither was the association between chronic inflammation and xanthelasma.
As xanthelasma was defined as lipid deposit in stomach, its relevance to metobolic disorders was of great interest. Etiologically, these conditions may be associated with a primary dyslipoproteinemic state, such as diabetes, nephrosis, obesity or cholestasis. In two Korean studies, dyslipidemia, representative of lower mean high density lipoprotein (HDL)-cholesterol and higher mean triglyceride levels was found in gastric xanthelasma subjects in comparison with the controls, accompanied with higher body mass index (BMI)[2,7]. But, there were no reports regarding xanthelasma and metabolic factors in Chinese population yet.
So, herein, we conducted a large retrospective study in China. General aspects of xanthelasma were described, including clinical aspect, endoscopic and histological findings. Furthermore, we focused on relationship between xanthelasma and metabolic disorders, as well as inflammation property.
This study was performed among adults who underwent upper gastrointestinal endoscopy at the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China during Jan., 2009 to Nov., 2016. Endoscopic xanthelasma group was defined as patients with xanthelasma as one of their endoscopic findings. Biopsy-proven xanthelasma were those who did biopsy at suspicious xanthelasma lesion and histological staining showed typical foam cells which supported diagnosis of xanthelasma.
The study protocol was approved by the Hospital Ethics Committee and performed in accordance with Declaration of Helsinki. All persons gave their informed consent prior to their inclusion. Patients with history of gastric cancer, gastrectomy, proton pump inhibitor use within the last 2 wk, antibiotics use within the last 4 wk, poor general condition not suitable for prolonged procedure time, and surveillance or inability to give informed consent were excluded.
Clinical evaluations were performed according to procedures as previously described[8]. In brief, demographic data, health habits and outpatient records (chief complaint, medical history, home medications, et al) were collected by trained physicians. Standing height and body weight without shoes and with light clothes were measured using standard procedures. BMI was calculated as body weight (kg) divided by square of height (m). Waist circumference was measured at the level of the narrowest point between iliac crest and rib cage using a non-stretchable tape. Systolic and diastolic blood pressures were measured using an automated sphygmomanometer, with participants in sitting position. Overnight fasting blood samples were obtained. Blood routine, as well as biochemical factors, including liver enzymes, serum lipids, glucose, and uric acid, was measured as previously described[9,10]. Carbohydrate antigen 199 (CA199) level and carcinoembryonic antigen (CEA) was measured by ECLIA on a Modular Analytics E module (Roche Diagnostics Co., Tokyo, Japan).
Procedure of upper gastrointestinal endoscopy was described in previous study[11]. Two endoscopists worked together on each patient during the whole procedure including biopsy. For controversial or non-classifiable cases, consensus was reached by discussion with one or more senior endoscopists. Gastric antrum was routine biopsies site. Additional biopsy were collected where lesion was found. The biopsy samples were fixed in formalin and sent to pathology laboratory in two separate vials, labeled according to their demographic data and anatomic sites.
Endoscopic xanthelasma diagnosis was based on observation of typical lesions appearing as a yellow-white nodule or plaque. Anatomic location and size of all xanthelasma lesion were recorded during procedure. Size was estimated by comparing it with open biopsy forceps[12]. Non-gastric gastrointestinal xanthelasma refers to xanthelasma within GI tract, but not located in stomach; extra-gastrointestinal xanthelasma refers to xanthelasma other than GI tract, such as skin xanthelasma or eye xanthelasma.
Histology of each specimen was determined by two experienced pathologists independently, who were blinded to endoscopic findings. Procedures and definitions of different lesion were described in previous reports[11,12].
H. pylori status was evaluated by modified Giemsa staining. Dysplasia refers to phenotypically neoplastic epithelium confined to glandular structures inside the basement membrane[13]. Atrophy of gastric mucosa indicates that the gastric glands proper in the gastric mucosa become sparse.
Foamy cells were typical histological findings and diagnostic prior criteria of xanthelasma[2]. The histologic appearance of lipid islands was described as lamina propria occupied by large ovoid to polygonal histiocytes with an abundant, finely vacuolated (foamy) cytoplasm staining lightly with eosin. Nuclei of foam cell were regular, round to ovoid, and occupied a small portion of cell area. Intestinal metaplasia was characterized by presence of histologically typical goblet cells. Grade of intestinal metaplasia was classified according to goblet cell density (low for 1-10, medium for 10-50 and high as > 50 goblet cells per low power field). If any inconsistence of two pathologists, the biopsy specimens were assessed by a third experienced pathologist, and the final diagnosis was based on diagnosis of the majority.
Statistical analyses was performed using SPSS 20.0 software for Windows (IBM Inc., Chicago, IL, United States). Continuous variables were shown as mean and standard deviation and compared using Student’s t-test or Mann-Whitney U test. Categorical variables were analyzed using χ2 test. Univariable and multivariable logistic analysis were adopted in constructing a predicting model of xanthelasma. 2-tailed P value less than 0.05 was considered as statistically significant.
From 2009 to 2016, a total of 176006 endoscopies was retrieved in the First Affiliated Hospital. Among them, 1370 xanthelasma were identified, with prevalence being 0.78%. Proportion of female and male was almost equivalent (667 vs 703). Xanthelasma was seen in a relative elderly group, with average age of 56.6 ± 11.2 years (57.2 ± 11.5 for male and 55.9 ± 10.9 for female).
Among all patients with xanthelasma, 24.2% complained of abdominal pain, 9.1% visited hospital for dyspepsia, 10.1% came with abdominal distention, and 14.1% for up-abdominal discomfort. Other symptoms like constipation, regurgitation, and diarrhea were seen in minority.
Xanthelasma appeared in most cases as single lesion, 6.1% were found as double or multiple. Average size of xanthelasma was 0.40 ± 0.24 cm. Most common site of xanthelasma was antrum (as high as 70.7%), corpus being the second (15.2%), and angulus xanthelasma accounted for 10.1% patients. None was seen in the fundus. Among all antrum xanthelasma, 35.7% were found in greater curvature, 26.3% in less curvature, 11.1% in the frontier wall and the rest in the posterior. No non-gastric gastrointestinal xanthelasma or extra-gastrointestinal xanthelasma was identified in current study.
In order to investigate relationship between xanthelasma and other gastric lesion (such as atrophy, H. pylori infection, intestinal metaplasia and dysplasia), 207 patients without biopsy were excluded in current analysis (Table 1). Among all 1163 xanthelasma, H. pylori infection rate was 30.8%. In 13.3% xanthelasma patients, atrophy was identified. Prevalence of dysplasia was 2.1%. To look deep into transition from gastritis to dysplasia, intestinal metaplasia was investigated. Pathological evaluation of intestinal metaplasia was conducted and sorted into three degrees, and it showed that high, medium and low grade intestinal metaplasia was found in 28.5%, 17.1% and 4.0% xanthelasma population, respectively.
| Variables | Xanthelasma | Non-xanthelasma | χ2 | P |
| n (male/female) | 1163 (602/561) | 1163 (602/561) | - | 1 |
| Age (yr) | 57 ± 11 | 56 ± 13 | - | 0.619 |
| Helicobacter pylori infection | 358 (30.8) | 341 (29.4) | 0.269 | 0.603 |
| Atrophy | 155 (13.3) | 58 (4.9) | 13.1 | < 0.01 |
| Intestinal metaplasia | 576 (49.5) | 166 (14.3) | 44.6 | < 0.001 |
| Low grade | 331 (28.5) | 119 (10.3) | 15.6 | < 0.001 |
| Medium grade | 199 (17.1) | 43 (3.7) | 27.1 | < 0.001 |
| High grade | 46 (4.0) | 4 (0.3) | 13.7 | < 0.001 |
| dysplasia | 24 (2.1) | 36 (3.1) | 14.3 | < 0.01 |
To dig deep into relationship of these findings with xanthelasma, 1163 patients without endoscopic xanthelasma were also included among people who underwent endoscopy in our hospital (Table 1). Comparing with control group, patients with xanthelasma showed a slightly higher rate of H. pylori infection but not significant. Atrophy was more prevalent in xanthelasma (13.3% vs 4.9% in control group, P < 0.01). Similarly, all three grades of intestinal metaplasia was seen more common in xanthelasma. Prevalence of dysplasia was not seven-fold higher in xanthelasma than non-xanthelasma group (2.1% vs 0.3% in control group, P < 0.001).
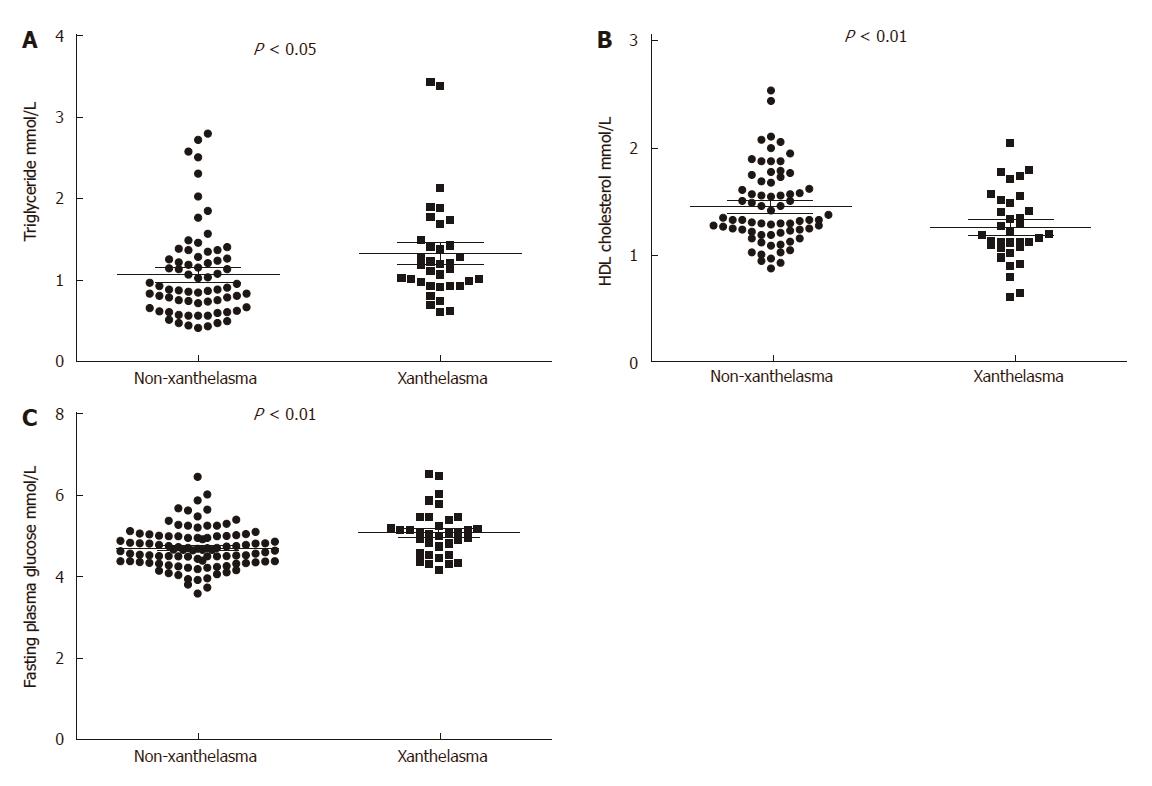
Among 1370 endoscopic diagnosed xanthelasma, 99 were biopsy-proven, which were included in further analysis, with 41 being male and 58 female (Table 2, Figure 1). 99 healthy controls were collected from annual health examination who underwent endoscopy with negative finding of xanthelasma. Mean age in xanthelasma was 56 ± 11 years, and 55 ± 13 in non-xanthelasma. Body mass index was similar in these two group (23.2 ± 2.3 in xanthelasma vs 22.1 ± 2.3 in non-xanthelasma), as well as blood pressure (systolic 117 ± 13 and diastolic 73 ± 13 in xanthelasma vs systolic 119 ± 16 and 73 ± 10 in non-xanthelasma).
| Variables | Non-xanthelasma | Xanthelasma | P |
| n (male/female) | 99 (41/58) | 99 (41/58) | 1.000 |
| Age (yr) | 55 ± 13 | 56 ± 11 | 0.537 |
| Body mass index (kg/m2) | 22.1 ± 2.3 | 23.2 ± 2.3 | 0.288 |
| Systolic blood pressure | 119 ± 16 | 117 ± 13 | 0.818 |
| Diastolic blood pressure | 73 ± 10 | 73 ± 13 | 0.785 |
| Alanine aminotransferase | 20 ± 13 | 20 ± 9 | 0.953 |
| Aspartate aminotransferase (U/L) | 21 ± 6 | 21 ± 4 | 0.669 |
| Alkaline phosphatase (U/L) | 67 ± 18 | 72 ± 21 | 0.202 |
| γ-Glutamyltransferase (U/L) | 23 ± 18 | 27 ± 32 | 0.327 |
| Total bilirubin (μmol/L) | 12.2 ± 3.8 | 13.4 ± 8.5 | 0.246 |
| Triglyceride (mmol/L) | 1.07 ± 0.55 | 1.33 ± 0.63 | < 0.05 |
| Albumin (g/L) | 47.5 ± 3.2 | 46.2 ± 3.8 | 0.063 |
| Total cholesterol (mmol/L) | 4.51 ± 0.77 | 4.59 ± 0.78 | 0.614 |
| HDL-cholesterol (mmol/L) | 1.45 ± 0.35 | 1.26 ± 0.33 | < 0.01 |
| LDL cholesterol (mmol/L) | 2.42 ± 0.61 | 2.56 ± 0.52 | 0.259 |
| Fasting plasma glucose (mmol/L) | 4.71 ± 0.50 | 5.08 ± 0.58 | < 0.001 |
| Hb1Ac (%) | 5.49 ± 1.19 | 5.5 ± 0.17 | 0.984 |
| Serum uric acid (μmol/L) | 303 ± 79 | 328 ± 91 | 0.119 |
| CEA (ng/mL) | 1.8 ± 1.2 | 2.8 ± 1.6 | < 0.001 |
| CA199 (U/mL) | 8.3 ± 9.5 | 9.4 ± 12 | 0.780 |
Serum lipid profile was found distinct between two groups (Figure 1). Xanthelasma patients showed significant higher TG (1.33 ± 0.63 vs 1.07 ± 0.55 in non-xanthelasma, P < 0.05) and lower HDL-cholesterol (1.45 ± 0.35 vs 1.26 ± 0.33 in non-xanthelasma, P < 0.01), but no difference in LDL-cholesterol or total cholesterol. As for glucose metabolism, serum fasting glucose was increased in xanthelasma (5.08 ± 0.58 vs 4.71 ± 0.49 in non-xanthelasma, P < 0.001), but Hb1Ac was similar.
Tumor marker CEA was higher in xanthelasma group (2.8 ± 1.6 vs 1.8 ± 1.2 in non-xanthelasma, P < 0.001), but CA199 was similar in two groups (9.4 ± 12 vs 8.7 ± 9.5 in non-xanthelasma).
No significant disparity was seen in liver enzyme, including ALT, AST, ALP and GGT. Other factors, such as total bilirubin, albumin and serum uric acid were not different in two groups.
Data of metabolic comorbidities were collected in xanthelasma and non-xanthelasma group (Table 3). In xanthelasma, fatty liver was more prevalent than non-xanthelasma (28.9% vs 15.2% in non-xanthelasma). Liver cyst was found more frequent in xanthelasma (10.1% vs 17% in non-xanthelasma) and fewer gallbladder polyp was discovered in xanthelasma (9.1% vs 6.8% in xanthelasma). Prevalence of other diseases, including chronic liver disease, hemangioma, gallstone coronary artery disease, atrial fibrillation, carotid atherosclerosis, stroke, thyroid disease and diabetes mellitus were similar in two groups.
| Metabolic comorbidity | Non-xanthelasma | Xanthelasma | P |
| Biliary and liver diseases | |||
| Chronic liver disease | 2 (2.0) | 4 (8.9) | 0.911 |
| Fatty liver | 15 (15.2) | 13 (28.9) | < 0.05 |
| Hemangioma | 1 (1.9) | 1 (2.1) | 0.568 |
| Cyst | 10 (10.1) | 8 (17.0) | < 0.05 |
| Post-cholecystectomy | 2 (2.0) | 4 (8.5) | 0.911 |
| Gallstone | 4 (4.0) | 3 (6.8) | 0.132 |
| Gallbladder polyp | 9 (9.1) | 3 (6.8) | < 0.01 |
| Cardio-cerebral-vascular disease | |||
| Coronary artery disease | 1 (1.0) | 2 (4.9) | 0.877 |
| Atrial fibrillation | 0 (0) | 1 (2.4) | 0.522 |
| Carotid atherosclerosis | 5 (5.1) | 6 (14.6) | 0.223 |
| Stroke | 0 (0) | 1 (2.4) | 0.522 |
| Thyroid disease | 4 (4.0) | 4 (9.8) | 0.187 |
| Diabetes mellitus | 1 (1.0) | 2 (4.9) | 0.877 |
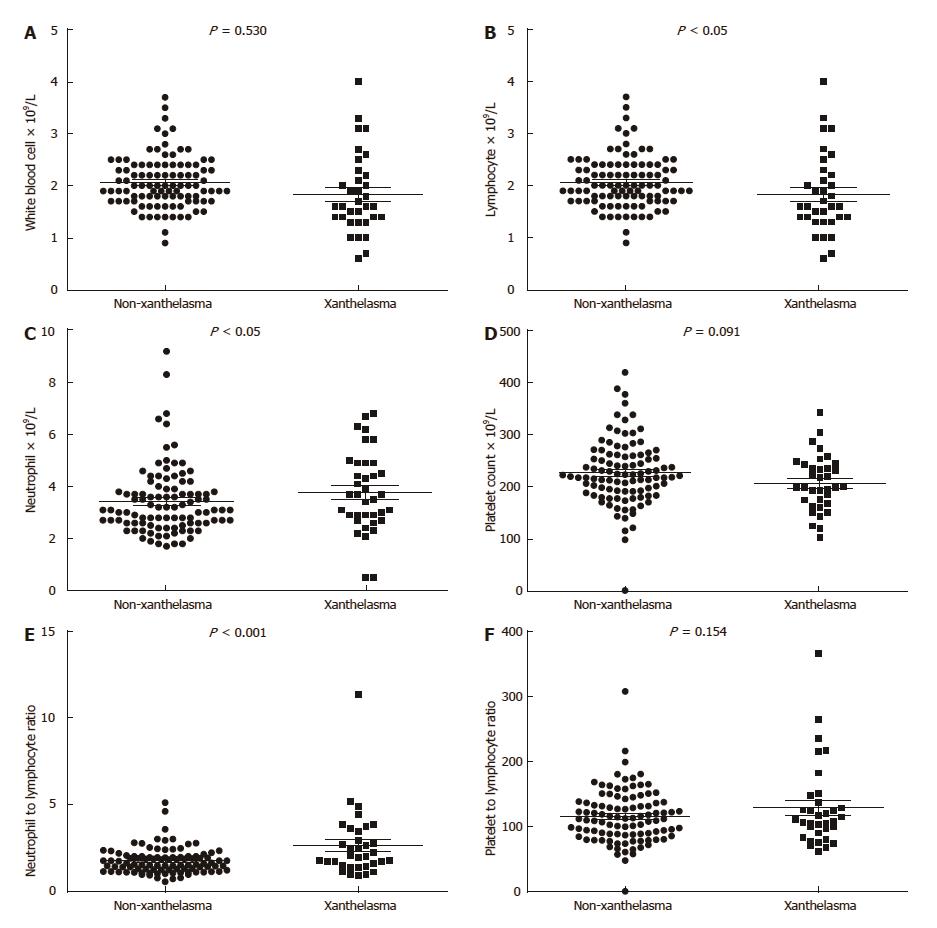
Inflammation markers were analyzed in our study as well (Table 4, Figure 2). In xanthelasma group, neutrophil was higher (3.4 ± 1.3 vs 4.0 ± 1.3 in non-xanthelasma, P < 0.05), lymphocyte was lower (2.1 ± 0.5 vs 1.8 ± 0.8 in non-xanthelasma, P < 0.05), newly developed inflammation related index NLR (Neutrophil-to-lymphocyte ratio) was significantly increased (1.74 ± 0.74 vs 2.62 ± 1.92 in non-xanthelasma, P < 0.001), but C-reactive protein (CRP) was similar (2.4 ± 4.6 vs 2.4 ± 2.8 in non-xanthelasma, P = 0.124).
| Variables | Non-xanthelasma | Xanthelasma | P |
| White blood cell (× 109/L) | 6.0 ± 1.5 | 5.8 ± 1.6 | 0.530 |
| Neutrophil (× 109/L) | 3.4 ± 1.3 | 4.0 ± 1.3 | < 0.05 |
| Lymphocyte (× 109/L) | 2.1 ± 0.5 | 1.8 ± 0.8 | < 0.05 |
| Hemoglobin (g/L) | 141 ± 15 | 141 ± 17 | 0.950 |
| Platelet count (× 109/L) | 228 ± 63 | 208 ± 53 | 0.091 |
| NLR | 1.74 ± 0.74 | 2.62 ± 1.92 | < 0.001 |
| PLR | 116 ± 41 | 129 ± 65 | 0.154 |
| CRP (mg/L) | 2.4 ± 4.6 | 2.4 ± 2.8 | 0.124 |
In order to investigate diagnostic and predictive role of metabolic and inflammatory factors in xanthelasma, univariable logistic analysis was adopted. It turned out that fasting plasma glucose (OR = 3.740, 95%CI: 1.721-8.129, P < 0.01), neutrophil (OR = 1.329, 95%CI: 1.004-1.758, P < 0.05), NLR (OR = 2.097, 95%CI: 1.234-3.564, P < 0.01), and CEA (OR = 1.882, 95%CI: 1.195-2.964, P < 0.01) were significantly associated with gastric xanthelasma (Table 5).
| Variables | OR | 95%CI | P |
| n (male/female) | 1.006 | 0.571-1.773 | 0.983 |
| Age (yr) | 1.007 | 0.984-1.131 | 0.535 |
| Body mass index (kg/m2) | 1.188 | 0.866-1.629 | 0.286 |
| Systolic blood pressure | 0.972 | 0.904-1.045 | 0.437 |
| Diastolic blood pressure | 1.045 | 0.938-1.163 | 0.425 |
| Alanine aminotransferase | 1.051 | 0.975-1.131 | 0.192 |
| Aspartate aminotransferase (U/L) | 0.911 | 0.801-1.037 | 0.159 |
| Alkaline phosphatase (U/L) | 1.011 | 0.986-1.036 | 0.393 |
| γ-Glutamyltransferase (U/L) | 0.992 | 0.961-1.024 | 0.623 |
| Total bilirubin (μmol/L) | 1.071 | 0.971-1.180 | 0.171 |
| Triglyceride (mmol/L) | 1.145 | 0.361-3.632 | 0.818 |
| Albumin (g/L) | 0.902 | 0.805-1.009 | 0.072 |
| Total cholesterol (mmol/L) | 0.178 | 0.010-3.133 | 0.238 |
| HDL-cholesterol (mmol/L) | 9.130 | 0.448-185.8 | 0.150 |
| LDL-cholesterol (mmol/L) | 4.764 | 0.183-123.9 | 0.348 |
| Fasting plasma glucose (mmol/L) | 3.740 | 1.721-8.129 | < 0.01 |
| Hb1Ac (%) | 1.011 | 0.350-2.924 | 0.983 |
| Serum uric acid (μmol/L) | 1.006 | 0.999-1.012 | 0.088 |
| White blood cell (× 109/L) | 0.919 | 0.708-1.194 | 0.527 |
| Neutrophil (× 109/L) | 1.329 | 1.004-1.758 | < 0.05 |
| Lymphocyte (× 109/L) | 0.471 | 0.222-1.000 | 0.050 |
| Hemoglobin (g/L) | 1.012 | 0.955-1.072 | 0.695 |
| Platelet count (× 109/L) | 0.971 | 0.927-1.016 | 0.200 |
| NLR | 2.097 | 1.234-3.564 | < 0.01 |
| PLR | 1.071 | 0.995-1.152 | 0.069 |
| CRP (mg/L) | 0.998 | 0.838-1.188 | 0.980 |
| CEA (ng/mL) | 1.882 | 1.195-2.964 | < 0.01 |
| CA199 (U/mL) | 1.007 | 0.961-1.054 | 0.777 |
To gain more specific knowledge of whether these four factors are independent, multivariable logistic regression was used. As NLR was a calculated index from neutrophil, we herein included fasting plasma glucose, neutrophil, and CEA in further analysis to avoid potential confounding. It manifested that fasting plasma glucose (OR = 3.347, 95%CI: 1.170-9.575, P < 0.05), neutrophil (OR = 1.617, 95%CI: 1.003-2.605, P < 0.05), and CEA (OR = 2.011, 95%CI: 1.236-3.271, P < 0.01) were all independent risk factors in xanthelasma (Table 6).
| Variables | OR | 95%CI | P |
| Fasting plasma glucose (mmol/L) | 3.347 | 1.170-9.575 | < 0.05 |
| Neutrophil (× 109/L) | 1.617 | 1.003-2.605 | < 0.05 |
| CEA (ng/mL) | 2.011 | 1.236-3.271 | < 0.01 |
Current study was the first populational based cohort of xanthelasma in China. This retrospective study collected a large xanthelasma group in Chinese population. Prevalence of endoscopic xanthelasma was 0.78% in our cohort with average age of 56.6 ± 11.2. Reasons of hospital visiting included abdominal pain (24.2%), up-abdominal discomfort (14.1%), abdominal distention (10.1%), and dyspepsia (9.1%), et al. Xanthelasma was most frequently seen in antrum as a single lesion. Chi-square analysis revealed positive relationship between prevalence of xanthelasma and H. pylori infection rate, more atrophy, intestinal metaplasia, dysplasia respectively. Metabolic and inflammatory factors were found associated with xanthelasma, as with higher CEA, TG, fasting glucose, neutrophil, NLR index, fatty liver comorbidity and lower HDL-cholesterol, lymphocyte. Among all these factors, fasting plasma glucose, neutrophil, and CEA were proved to be independent factors in xanthelasma.
Current analysis revealed co-existence of atrophy, intestinal metaplasia and dysplasia in xanthelasma, indicating a potential pro-tumorigenesis role in gastric pathology. In previous prospective study, age/sex/atrophy-matched control analysis demonstrated that the presence of gastric xanthelasma was significantly associated with the presence of gastric cancer, indicating that xanthelasma could serve as a warning sign of gastric cancer[4]. Furthermore, in a Japanese cohort, with follow-up being more than three years, gastric xanthelasma was shown to be a useful marker for gastric cancer development[1]. Other than endoscopic diagnosis of gastric cancer, serum CEA level in xanthelasma group was significantly elevated. The exact mechanism behind this remained unknown. But it was speculated that reactive oxygen species (ROS) might be the mediator. First, increased release of ROS may be involved in accumulation of oxidized LDL cholesterol and development of gastric xanthelasma[14]. On this basis, ROS can cause DNA damage and associated oncogenic changes, thus lead to tumorigenesis[15,16]. However, molecular and cell biology experiments were needed to support the idea.
This study identified a slightly higher H. pylori infection rate in gastric xanthelasma. Recent years witnessed rapid increase rate in infection, especially in China[17]. As high as 57.6% people in population carried H. pylori[18]. H. pylori is a well-known cause of various gastrointestinal issues, such as peptic ulcer disease, gastroesophageal reflux disease and chronic active gastritis as well[19]. Chronic gastritis is thought to be involved in gastric glandular atrophy and intestinal metaplasia, which is considered as a precursor of gastric tumorigenesis[20]. In consistent with our findings, Hori and Tsutsumi first reported that H. pylori infection was seen on the surface of foveolar cells in 48% of biopsy samples of xanthelasma in 1996[5]. To further support that, Isomoto, in 1999, also reported a close relationship among H. pylori infection, xanthelasma, and atrophic gastritis[6]. Similarly, a large cohort in Korea also identified this association and proposed that xanthelasma may be provoked by H. Pylori infection[2].
In order to better understand role of chronic inflammation and xanthelasma, this study also investigated relationship between serum inflammatory biomarkers and xanthelasma, which revealed an increasing trend of neutrophil and a decrease in lymphocyte with negative relationship of CRP, an acute phase protein. Moreover, NLR index, defined as neutrophil counts divided by lymphocyte counts, was introduced. In most cases, lymphopenia well reflects impaired cell-mediated immunity, while neutrophilia represents a response to systematic inflammation, so high NLR represents systemic and local inflammatory state[21]. Emerging evidences have shown an important role of NLR in different cancer types and metabolic disease such as cardiovascular disease as well[22,23]. These results further supported an inflamed environment in xanthelasma.
Xanthelasma was characterized by deposit of lipid in gastrointestinal tract. In this study, we found a positive association between xanthelasma and serum TG level accompanied by decreased HDL cholesterol. As a major component in metabolic syndrome, fatty liver was seen more frequent in xanthelasma patients. Glucose metabolism was also impaired in xanthelasma, supported by significantly higher fasting plasma glucose. However, Hb1Ac did not differ between groups. This indicated a scenario of slight fasting glucose intolerance, rather than well-established diabetes. Moreover, the amount of epicardial adipose tissue found in subjects with xanthelasma was higher and, the presence of xanthelasma was independently associated with supramedian epicardial fat thickness[24]. Whether these were random findings or potential causal relationship in pathogenesis still need further studies.
However, this study has several limitations. Firstly, with the nature of retrospective case-control study, no causal relationship can be identified between either xanthelasma and other gastric pathology or xanthelasma with metabolic disorders. Further prospective studies are needed in this concern and in other groups to improve generalizability. Secondly, as was a retrospective study, no standard procedure of xanthelasma treatment was set. Difference might existed among endoscopists. Rate of xanthelasma biopsy would be underestimated.
Current study described a large xanthelasma cohort in Chinese population, and revealed its positive relationship with H. pylori infection, atrophy, intestinal metaplasia, dysplasia, and metabolic disorder as well, indicating an important role of xanthelasma in both carcinogenesis and metabolic dysfunction. Logistic regression revealed role of fasting plasma glucose, neutrophil, and CEA as independent risk factors in xanthelasma.
Xanthelasma was a relatively rare endoscopic finding, characterized by accumulation of lipid in histiocytic foam cells in mucosa. To gain knowledge of xanthelasma, a large population-based study was conducted.
As xanthelasma was defined as lipid deposit in stomach, its relevance to metobolic disorders was of great interest. Dyslipidemia, representative of lower mean high density lipoprotein-cholesterol and higher mean triglyceride levels was found in two Korean studies, accompanied with higher body mass index in gastric xanthelasma subjects in comparison with the controls. But, there were no reports in this concern in Chinese population yet.
Current study described a large xanthelasma cohort in Chinese population, revealed its positive relationship with H. pylori infection, atrophy, intestinal metaplasia, dysplasia, and metabolic disorder as well, indicating an important role of xanthelasma in both carcinogenesis and metabolic dysfunction.
With better understanding of xanthelasma pathogenesis and its relationship with metabolic, immunogenic and carcinogenic factors, clinical value of xanthelasma as predicting factor will be envisaged.
The authors studied the relationship between gastric xanthelasma and metabolic disorders in Chinese patients. They collected patient information from endoscopy archives and analysis the relationship of xanthelasma with several biochemical, metabolic and tumor markers.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ding SZ, Lankarani KB, Lee CL S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
| 1. | Sekikawa A, Fukui H, Sada R, Fukuhara M, Marui S, Tanke G, Endo M, Ohara Y, Matsuda F, Nakajima J. Gastric atrophy and xanthelasma are markers for predicting the development of early gastric cancer. J Gastroenterol. 2016;51:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Yi SY. Dyslipidemia and H pylori in gastric xanthomatosis. World J Gastroenterol. 2007;13:4598-4601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Dhakal M, Dhakal OP, Bhandari D, Gupta A. Gastric xanthelasma: an unusual endoscopic finding. BMJ Case Rep. 2013;2013:bcr2013201017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Sekikawa A, Fukui H, Maruo T, Tsumura T, Kanesaka T, Okabe Y, Osaki Y. Gastric xanthelasma may be a warning sign for the presence of early gastric cancer. J Gastroenterol Hepatol. 2014;29:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Hori S, Tsutsumi Y. Helicobacter pylori infection in gastric xanthomas: immunohistochemical analysis of 145 lesions. Pathol Int. 1996;46:589-593. [PubMed] |
| 6. | Isomoto H, Mizuta Y, Inoue K, Matsuo T, Hayakawa T, Miyazaki M, Onita K, Takeshima F, Murase K, Shimokawa I. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J Gastroenterol. 1999;34:346-352. [PubMed] |
| 7. | Chang FY, Shih CY, Lee SD. Abnormal serum lipid levels in subjects with gastric xanthoma. Clin Chim Acta. 1993;217:233-235. [PubMed] |
| 8. | Xu C, Chen Y, Xu L, Miao M, Li Y, Yu C. Serum complement C3 levels are associated with nonalcoholic fatty liver disease independently of metabolic features in Chinese population. Sci Rep. 2016;6:23279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, Li Y. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep. 2015;5:16494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Wang J, Zhu W, Huang S, Xu L, Miao M, Wu C, Yu C, Li Y, Xu C. Serum apoB levels independently predict the development of non-alcoholic fatty liver disease: A 7-year prospective study. Liver Int. 2017;37:1202-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, Wang C, Liu Y, Sha W. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Köksal AŞ, Suna N, Kalkan İH, Eminler AT, Sakaoğulları ŞZ, Turhan N, Saygılı F, Kuzu UB, Öztaş E, Parlak E. Is Gastric Xanthelasma an Alarming Endoscopic Marker for Advanced Atrophic Gastritis and Intestinal Metaplasia? Dig Dis Sci. 2016;61:2949-2955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167-176. [PubMed] |
| 14. | Kaiserling E, Heinle H, Itabe H, Takano T, Remmele W. Lipid islands in human gastric mucosa: morphological and immunohistochemical findings. Gastroenterology. 1996;110:369-374. [PubMed] |
| 15. | Ding Y, Wang H, Niu J, Luo M, Gou Y, Miao L, Zou Z, Cheng Y. Induction of ROS Overload by Alantolactone Prompts Oxidative DNA Damage and Apoptosis in Colorectal Cancer Cells. Int J Mol Sci. 2016;17:558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Sridharan DM, Asaithamby A, Bailey SM, Costes SV, Doetsch PW, Dynan WS, Kronenberg A, Rithidech KN, Saha J, Snijders AM. Understanding cancer development processes after HZE-particle exposure: roles of ROS, DNA damage repair and inflammation. Radiat Res. 2015;183:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016;21 Suppl 1:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, Wang JX, Zhang L, Zhang Y, Bajbouj M. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Tsukanov VV, Kasparov EV, Tonkikh JL, Shtygasheva OV, Butorin NN, Amelchugova OS, Vasyutin AV, Bronnikova EP, Fassan M, Rugge M. Peptic Ulcer Disease and Helicobacter pylori Infection in Different Siberian Ethnicities. Helicobacter. 2017;22:Epub 2016 Jun 6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Sokic-Milutinovic A, Alempijevic T, Milosavljevic T. Role of Helicobacter pylori infection in gastric carcinogenesis: Current knowledge and future directions. World J Gastroenterol. 2015;21:11654-11672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 21. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8174] [Article Influence: 544.9] [Reference Citation Analysis (0)] |
| 22. | Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, Shao B. Neutrophil-to-Lymphocyte Ratio Is a Prognostic Marker in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2017;26:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Hu K, Lou L, Ye J, Zhang S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open. 2015;5:e006404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Akyüz AR, Ağaç MT, Turan T, Şahin S, Kul S, Korkmaz L, Erkuş ME, Erkan H, Çelik Ş. Xanthelasma Is Associated with an Increased Amount of Epicardial Adipose Tissue. Med Princ Pract. 2016;25:187-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |