Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7716
Peer-review started: July 30, 2017
First decision: August 29, 2017
Revised: September 25, 2017
Accepted: September 28, 2017
Article in press: September 28, 2017
Published online: November 21, 2017
Processing time: 114 Days and 22.7 Hours
To investigate the epidemiology and natural history of Wilson’s disease in the Chinese.
Data were retrieved via electronic search of hospital medical registry of the Hong Kong Hospital Authority, which covers all the public healthcare services. We identified cases of Wilson’s disease between 2000 and 2016 by the International Classification of Diseases (ICD)-9 code. We analyzed the incidence rate, prevalence and adverse outcomes of Wilson’s disease.
We identified 211 patients (male cases 104; female cases 107; median age 27.2 years, IQR: 17.1-38.6 years; duration of follow-up 8.0 years, IQR: 5.0-14.0 years). The average annual incidence rate was 1.44 per million person-years while the prevalence was 17.93 per million. Between 2000 and 2016, there was a decrease in the annual incidence rate from 1.65 to 1.23 per million person-years (P = 0.010), whereas there was an increase in the annual prevalence from 7.80 to 25.20 per million (P < 0.001). Among the 176 cases with hepatic involvement, 38 (21.6%) had cirrhosis, three (1.7%) developed hepatocellular carcinoma, 24 (13.6%) underwent liver transplantations, and 26 (14.8%) died. Seven patients had concomitant chronic viral hepatitis B or C. The 5-year and 10-years rates of overall survival were 92.6% and 89.5%, and for transplant-free survival rates 91.8% and 87.4%, respectively. Cirrhosis and possibly chronic viral hepatitis were associated with poorer overall survival.
There was a significant increase in the prevalence of Wilson’s disease in Hong Kong. The prognosis was favorable except for those with cirrhosis or concomitant viral hepatitis.
Core tip: There are few studies on the epidemiology and natural history of Wilson’s disease in Asia. The present territory-based study was the first to describe both the epidemiology and natural history of Wilson’s disease over a long period of time (a span of 17 years from 2000 to 2016) in the Chinese. There was a significant increase in the number of cases of Wilson’s disease in Hong Kong. The prognosis was favorable except for those with cirrhosis or concomitant viral hepatitis.
- Citation: Cheung KS, Seto WK, Fung J, Mak LY, Lai CL, Yuen MF. Epidemiology and natural history of Wilson’s disease in the Chinese: A territory-based study in Hong Kong between 2000 and 2016. World J Gastroenterol 2017; 23(43): 7716-7726
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7716
Wilson’s disease is an autosomal recessive disease with impairment of hepatic excretion of copper, leading to excessive copper accumulation in various organs and tissues[1]. There is a wide spectrum of clinical manifestations including hepatic, neurological, psychiatric and ophthalmological involvements. Nevertheless, some patients may be asymptomatic, and mutational analysis may be needed for a definitive diagnosis[2]. Untreated patients with hepatic involvement carry significant morbidities including cirrhosis and hepatocellular carcinoma (HCC)[3-5]. Therapies which either chelate the copper (D-penicillamine and trientine) or inhibit its absorption (zinc) are proven to be effective in controlling the disease[6].
There are a few population-based studies on the epidemiology of Wilson’s disease in the western population, but the majority were performed before the 1990s. The incidence rate ranges from 17 to 29 per million person-years[7-10], while the prevalence is reported to be 15-30 cases per million population[1,11,12]. Recently, a genetic study of Wilson’s disease concluded that prevalence of Wilson’s disease may actually be even higher (1 in 72016)[13]. Studies are even more scarce in Asia, except for one population-based study in Taiwan reporting a low incidence (average annual incidence rate of 2.7 per million person-years) and prevalence (ranging from 8.4 to 16.0 per million) of Wilson’s disease between 2000 and 2005[14]. However, the study observation period was short, and prognostic risk factors were not determined.
The long-term outcomes of patients with Wilson’s disease are reported in the western population but not in the Asians[3,4]. However, these are retrospective studies and not population-based. In addition, the effect of concomitant chronic viral hepatitis B or C infection on the outcomes of patients with Wilson’s disease has not been examined. This is an important factor since around 248 million and 150 million people worldwide have chronic hepatitis B (CHB) and C (CHC) infection, respectively[15,16] While metabolic risk factors (diabetes mellitus, obesity, hypertension as well as metabolic syndrome) predispose CHB and CHC patients to an increased risk of fibrosis/cirrhosis as well as HCC[17,18], whether it will lead to a poorer prognosis in patients with Wilson’s disease remains unknown.
Therefore, there is an unmet need for an update of the epidemiology of Wilson’s disease as well as more comprehensive identification of risk factors for adverse outcomes in the Asians. This will shed light on the better management of affected patients.
The aims of the present study were to determine the epidemiology and the natural history of Wilson’s disease in the Chinese population in Hong Kong. In addition, risk factors for liver transplantation requirement and mortality were determined.
We retrieved data from the Clinical Data Analysis and Reporting System (CDARS), an electronic database managed by the Hong Kong Hospital Authority, which is the sole provider of public healthcare services in Hong Kong with about 90% of the healthcare services being covered[19,20]. Important clinical information are all available in the CDRS, which includes patient demographics, death, diagnoses, admissions, clinic visits, procedures, laboratory results, medication prescription and dispensing history[21]. There has been an increasing number of territory-based studies performed using this electronic database[22-27], with high accuracies of the coding system demonstrated[24,28].
All patients were anonymized in the CDARS, with a unique reference key assigned to each individual. Ethics approval was issued by the Institutional Review Board, The University of Hong Kong and West Cluster of Hospital Authority, Hong Kong.
The study period commenced from 2000 and ended in 2016. Cases of Wilson’s disease from 1999 to 2016 were recognized from the CDARS by using the International Classification of Diseases (ICD)-9 code of 275.1.
Demographics (age, sex, nationality, diagnosis date and death) were retrieved. In addition, information on all diagnoses (including various adverse events and comorbidities) of patients were available for data retrieval.
The incidence rate as well as prevalence of Wilsons’s disease were calculated. Adverse liver events (cirrhosis, hepatic complications, HCC and liver transplantation), non-liver outcomes, and mortality were also studied. Risk factors (including concomitant chronic viral hepatitis and metabolic factors) associated with overall and transplant-free survival were determined.
Cases with cirrhosis were identified by the ICD-9 code of 571.5 and the presence of portal hypertension, splenomegaly or cirrhotic complications. Cirrhotic complications were defined with the existence of at least one of the following: ascites, spontaneous bacterial peritonitis, gastroesophageal varices, hepatic encephalopathy, or hepatorenal syndrome. Chronic viral hepatitis referred to either CHB or CHC infection. Metabolic factors included diabetes mellitus, hypertension, dyslipidemia, obesity and alcoholism. Obesity was diagnosed with a body mass index of 25 kg/m2 or more in the Asian population[29]. Dyslipidemias are defined as disorders of lipoprotein metabolism with high levels of triglycerides, low-density or non-high-density lipoprotein cholesterol, or low level of high-density lipoprotein cholesterol. The cutoff value depends on the individual patient’s cardiovascular risk profile[30]. Smoking status was not usually entered into the electronic database system. Table 1 illustrates the identification of outcomes and covariates.
| Impaired or abnormal liver function | 573.8, 794.8 |
| Hepatitis | 573.3, 571.40, 571.41 |
| Chronic liver disease | 571.9, 573.9 |
| Acute liver failure | 570 |
| Chronic liver failure | 571.8 |
| Hepatocellular carcinoma | 155.0 |
| Cirrhosis | 571.5 |
| Viral hepatitis B carrier | V02.61:0 |
| Viral hepatitis C carrier | V02.62:0 |
| Sequalae of chronic liver disease | 572.8 |
| Esophageal varices | 456.0, 456.1 |
| Gastric varices | 456.8 |
| Ascites | 789.5 |
| Spontaneous bacterial peritonitis | 567.2 |
| Portal hypertension | 572.3 |
| Splenomegaly/Hypersplenism | 789.1, 789.2, 789.4 |
| Hepatic encephalopathy | 572.2 |
| Hepatorenal syndrome | 572.4 |
| Liver transplantation | V42.7 |
| Parkinsonism | 332 |
| Tremor | 781.0 |
| Dystonic disorder | 333.6 |
| Convulsion | 780.32 |
| Tics | 307.20 |
| Dementia | 290 |
| Bipolar affective disorder | 296.7 |
| Depression | 296.2, 296.3, 311 |
| Anxiety | 300.0 |
| Organic affective syndrome | 293.83 |
| Psychosis | 295, 293.83 |
| Personality disorder | 301 |
| Suicide | E950 |
| Hemolytic anemia | 283.10 |
| Alcoholism | 291, 571.0, 571.1, 571.2, 571.3, 303, 305.0, 980.8, 980.9 |
| Obesity | 278.0, 278.1 |
| Diabetes mellitus | 249, 250 |
| Hypertension | 401-405 |
| Dysplipidemia | 272.0-272.4 |
Diagnosis coding accuracy of the CDARS data was validated by reviewing the eletronic medical records of patients from our center, Queen Mary Hospital. Queen Mary Hospital is a hospital which provides tertiary healthcare services including liver transplantation in Hong Kong.
Statistical analyses were done by R version 3.2.3 (R Foundation for Statistical Computing) statistical software. We expressed continuous variables in terms of median with interquartile range (IQR), and used Mann-Whitney U-test to assess the difference in continuous variables between two groups. We used χ2 test or Fisher’s exact test for comparing categorical variables. The incidence rate and prevalence of Wilson’s disease between 2000 and 2016 were calculated. Poisson regression model was used to evaluate the temporal trends of count data. Variables associated with adverse events were identified by Cox proportional hazards model. The adverse events were analysed by Kaplan-Meier method with statistical significance determined by log-rank test. A two-tailed P value < 0.05 was regarded as statistically significant.
Two hundred and eleven patients with Wilson’s disease were identified. Patient demographics are shown in Table 2. Male sex accounted for 49.3% of the cases (male cases 104; female cases 107). There were 170 newly diagnosed cases (male, 84; female, 86). The median follow-up duration was 8.0 years (IQR 5.0 to 14.0 years), with a total follow-up of 1559.5 person-years. Patients were diagnosed with Wilson’s disease at a median age of 27.2 years (IQR 17.1 to 38.6 years). Sixty-five patients (30.8%) were diagnosed before the age of 20. Men were diagnosed with Wilson’s disease at a similar age as women [28.5 years (IQR 19.0 to 38.8 years) and 25.0 years (IQR 15.9 to 38.6 years) for men and women, respectively; P = 0.326]. On presentation, nine patients had end-stage cirrhosis and nine had acute liver failure, requiring liver transplantation (8.5% of the total cases). One hundred and seventy-six patients (83.4%) had liver involvement. Of these, 21.6% developed cirrhosis (n = 38). Forty-nine patients (23.2%) had neurological and/or psychiatric involvement. Thirty patients (14.2%) had neurological involvement, with parkinsonism being the commonest manifestation (n = 15; 50.0%). Twenty-four patients (11.4%) had psychiatric manifestation, with depression being most prevalent (n = 9; 37.5%). Four patients (16.7%) had history of suicide. Twenty-one patients (10.0%) had both hepatic and neurological or psychiatric involvement. Non-immune mediated hemolytic anemia occurred in eight patients (3.8%). Seven patients (3.3%) had concomitant chronic viral hepatitis (CHB, 5; CHC, 2), and 17 (8.1%) had metabolic factors.
| Variables | Clinical characteristics |
| Age | 27.9 (18.2-39.7) |
| Male | 104 (49.3) |
| Drug | 193 (91.5) |
| D-penicillamine1 | 129 (61.6) |
| Trientine2 | 22 (10.4) |
| Zinc | 42 (19.9) |
| Hepatic disease | 176 (83.4) |
| Cirrhosis | 38 (21.6) |
| Cirrhotic complications | 19 (10.8) |
| Ascites | 10 (5.7) |
| Spontaneous bacterial peritonitis | 5 (2.8) |
| Esophageal varices | 10 (5.7) |
| Gastric varices | 5 (2.8) |
| Hepatic encephalopathy | 8 (4.5) |
| Hepatocellular carcinoma | 3 (1.7) |
| Neurological disease | 30 (14.2) |
| Parkinsonism | 15 (50.0) |
| Tremor | 3 (10.0) |
| Dystonia | 4 (13.3) |
| Tics | 1 (3.3) |
| Dementia | 2 (6.7) |
| Seizure | 7 (23.3) |
| Psychiatric disease | 24 (11.4) |
| Depression | 9 (37.5) |
| Anxiety | 2 (8.3) |
| Bipolar disorder | 4 (16.7) |
| Organic affective syndrome | 3 (12.5) |
| Psychosis | 4 (16.7) |
| Personality disorder | 3 (12.5) |
| Suicide | 4 (16.7) |
| Concomitant hepatic and neurological/psychiatric diseases | 21 (10.0) |
| Non-immune hemolytic anemia | 8 (3.8) |
| Viral hepatitis | 7 (3.3) |
| Chronic hepatitis B infection | 5 (71.4) |
| Chronic hepatitis C infection | 2 (28.6) |
| Metabolic factors | 17 (8.1) |
| Diabetes mellitus | 3 (17.6) |
| Hypertension | 7 (41.2) |
| Dyslipidemia | 4 (23.5) |
| Obesity | 4 (23.5) |
| Alcoholism | 4 (23.5) |
| Liver transplantation | 24 (11.3) |
| Death | 26 (12.3) |
Table 3 shows the different medications used in the patients with neurological and/or psychiatric involvement, including anti-parkinson agents, anticonvulsants, benzodiazepines, propranolol, baclofen, tetrabenazine, antidepressants, antipsychotics and lithium.
| Drugs |
| Anticonvulsants (carbamazepine and valproate) |
| Antidepressants (citalopram and fluoxetine) |
| Anti-parkinson agents (levodopa, bromocriptine, amantadine, ropinorole and benzhexol) |
| Antipyschotics (clozapine, olanzapine, quetiapine, perphenazine, paliperidone and trifluoperazine) |
| Baclofen |
| Benzodiazepines (clonazepam, diazepam and lorazepam) |
| Lithium |
| Propranolol |
| Tetrabenazine |
When patients were divided into three age strata (< 20, 20 - 39.9, ≥ 40 years), HCC and mortality were significantly higher in older age groups, while there were no significant differences for other hepatic, neurological or psychiatric manifestations (Table 4).
| Age < 20 (n = 65) | Age 20-39.9 (n = 94) | Age ≥ 40 (n = 52) | P value | |
| Hepatic involvement | 58 (89.2) | 74 (78.7) | 44 (84.6) | 0.201 |
| Cirrhosis | 7 (10.8) | 18 (19.1) | 13 (25.0) | 0.128 |
| Cirrhotic complications | 4 (6.2) | 9 (9.6) | 6 (11.5) | 0.584 |
| Hepatocellular carcinoma | 0 | 0 | 3 (11.5) | 0.014 |
| Neurological involvement | 9 (13.8) | 16 (17.0) | 5 (9.6) | 0.469 |
| Psychiatric involvement | 6 (9.2) | 12 (12.8) | 6 (11.5) | 0.787 |
| Liver transplantation | 11 (16.9) | 11 (11.7) | 2 (3.8) | 0.066 |
| Death | 4 (6.2) | 9 (9.6) | 13 (25.0) | 0.005 |
Between 2000 and 2016, the average annual incidence rate of Wilson’s disease was 1.44 per million person-years. There was a decrease in the annual incidence rate from 1.65 to 1.23 per million person-years (Poisson P = 0.010) (Table 5). The average annual prevalence was 17.93 per million. The annual prevalence increased from 7.80 to 25.20 per million (Poisson P < 0.001).
| Yr | Total population (million) | New cases | Total cases | Incidence rate (per million person-years) | Prevalence (per million) | Prevalence ratio of M/F | ||||||||||
| M | F | T | M | F | T | M | F | T | M | F | T | M | F | T | ||
| 2000 | 3.28 | 3.39 | 6.67 | 4 | 7 | 11 | 24 | 28 | 52 | 1.22 | 2.06 | 1.65 | 7.32 | 8.26 | 7.80 | 0.89 |
| 2001 | 3.28 | 3.43 | 6.71 | 6 | 9 | 15 | 28 | 36 | 64 | 1.83 | 2.62 | 2.24 | 8.54 | 10.5 | 9.54 | 0.81 |
| 2002 | 3.28 | 3.56 | 6.74 | 3 | 8 | 11 | 31 | 44 | 75 | 0.91 | 2.25 | 1.63 | 9.45 | 12.36 | 11.13 | 0.76 |
| 2003 | 3.26 | 3.47 | 6.73 | 11 | 7 | 18 | 40 | 51 | 91 | 3.37 | 2.02 | 2.67 | 12.27 | 14.7 | 13.52 | 0.83 |
| 2004 | 3.27 | 3.52 | 6.78 | 9 | 4 | 13 | 47 | 54 | 101 | 2.75 | 1.14 | 1.92 | 14.37 | 15.34 | 14.90 | 0.94 |
| 2005 | 3.26 | 3.55 | 6.81 | 4 | 5 | 9 | 51 | 57 | 108 | 1.23 | 1.41 | 1.32 | 15.64 | 16.06 | 15.86 | 0.97 |
| 2006 | 3.27 | 3.59 | 6.86 | 2 | 3 | 5 | 52 | 60 | 112 | 0.61 | 0.84 | 0.73 | 15.90 | 16.71 | 16.33 | 0.95 |
| 2007 | 3.28 | 3.63 | 6.92 | 2 | 3 | 5 | 54 | 63 | 117 | 0.61 | 0.83 | 0.72 | 16.46 | 17.36 | 16.91 | 0.95 |
| 2008 | 3.29 | 3.67 | 6.96 | 2 | 2 | 4 | 54 | 63 | 117 | 0.61 | 0.54 | 0.57 | 16.41 | 17.17 | 16.81 | 0.96 |
| 2009 | 3.28 | 3.69 | 6.97 | 6 | 10 | 16 | 60 | 72 | 132 | 1.83 | 2.71 | 2.30 | 18.29 | 19.51 | 18.94 | 0.94 |
| 2010 | 3.29 | 3.73 | 7.02 | 9 | 8 | 17 | 68 | 80 | 148 | 2.74 | 2.14 | 2.42 | 20.67 | 21.45 | 21.08 | 0.96 |
| 2011 | 3.30 | 3.77 | 7.07 | 8 | 3 | 11 | 75 | 83 | 158 | 2.42 | 0.80 | 1.56 | 22.73 | 22.02 | 22.35 | 1.03 |
| 2012 | 3.33 | 3.83 | 7.15 | 3 | 2 | 5 | 77 | 85 | 162 | 0.90 | 0.52 | 0.70 | 23.12 | 22.19 | 22.66 | 1.04 |
| 2013 | 3.33 | 3.86 | 7.19 | 2 | 3 | 5 | 79 | 87 | 166 | 0.60 | 0.78 | 0.70 | 23.72 | 22.54 | 23.09 | 1.05 |
| 2014 | 3.35 | 3.90 | 7.24 | 2 | 8 | 10 | 82 | 94 | 175 | 0.60 | 2.05 | 1.38 | 24.48 | 24.10 | 24.17 | 1.02 |
| 2015 | 3.37 | 3.94 | 7.31 | 6 | 0 | 6 | 86 | 93 | 179 | 1.78 | 0.00 | 0.82 | 25.52 | 23.60 | 24.49 | 1.08 |
| 2016 | 3.38 | 3.96 | 7.34 | 5 | 4 | 9 | 89 | 96 | 185 | 1.48 | 1.01 | 1.23 | 26.33 | 24.24 | 25.20 | 1.09 |
Wilson’s disease was more commonly diagnosed in the younger age groups, with the incidence rate peaking in the 15- to 19-year age group (Table 6). For patients aged less than 25 years, the average annual incidence rate ranged from 2.02 to 3.06 per million person-years. For those aged between 25 and 44 years, the average annual incidence rate ranged from 1.71 to 1.97 per million person-years. Patients were less likely to have disease onset at 45 years or older (age group 45-49: 0.86 per million person-years; age group ≥ 50: 0.29 per million person-years).
| Case number | Incidence rate (per million person-years) | |||||
| Age group | M | F | T | M | F | T |
| 0-4 | 6 | 7 | 13 | 2.81 | 3.38 | 2.61 |
| 5-9 | 3 | 7 | 10 | 1.35 | 2.73 | 2.02 |
| 10-14 | 4 | 9 | 13 | 1.30 | 2.94 | 2.09 |
| 15-19 | 10 | 12 | 22 | 2.70 | 3.43 | 3.06 |
| 20-24 | 10 | 10 | 20 | 2.64 | 2.52 | 2.58 |
| 25-29 | 9 | 6 | 15 | 2.33 | 1.21 | 1.71 |
| 30-34 | 11 | 8 | 19 | 2.72 | 1.45 | 1.97 |
| 35-39 | 9 | 10 | 19 | 1.92 | 1.70 | 1.80 |
| 40-44 | 10 | 9 | 19 | 1.92 | 1.54 | 1.74 |
| 45-49 | 4 | 5 | 9 | 0.81 | 0.92 | 0.86 |
| ≥ 50 | 7 | 4 | 11 | 0.36 | 0.21 | 0.29 |
Among the 176 patients with hepatic involvement, 38 had cirrhosis (21.6%). Nineteen patients (10.8%) had cirrhotic complications, with the two most common being the development of ascites (n = 10; 5.7%) and esophageal varices (n = 10; 5.7%). Around 20% of the cirrhotic cases were men (21 out of 104), while 15.9% were women (17 out of 107) (P = 0.416). The median age for the diagnosis of cirrhosis was 33.4 years [IQR 23.9 to 42.8 years; 32.4 years (IQR 23.7 to 45.5 years) and 34.1 years (IQR 24.4 to 41.4 years) in men and women, respectively; P = 0.772].
Three cases were newly diagnosed with HCC (1.7% of cases with hepatic involvement), all of which developed in men (age at diagnosis ranged from 45.8 to 56.0 years). The 5-year and 10-year cumulative incidences of HCC were 1.3% (95%CI: 0%-3.1%) and 2.3% (95%CI: 0%-4.8%), respectively. The time from diagnosis of Wilson’s disease to HCC development ranged from 1.7 to 8 years. All three patients had underlying cirrhosis, and one of them had concomitant CHB infection. None of them received liver transplantation, and two died at the age of 47.2 and 56.8 years.
Liver transplantations were performed in 24 patients (11.3% of all cases; 13.6% of cases with hepatic involvement) -- 14 for cirrhosis and 10 for acute liver failure. Twelve out of 104 were men (11.1%) and 12 out of 107 were women (11.2%). Five liver transplantations were newly performed between 2000 and 2016. The 5-year and 10-year cumulative incidences of liver transplantation were 1.5% (95%CI: 0%-3.5%) and 4.2% (95%CI: 0%-8.4%), respectively. Patients underwent liver transplantation at a median age of 24.4 years (IQR 18.4 to 37.4 years). There were eight liver transplantation cases in patients aged less than 20 years, 11 cases in those aged between 20 and 39 years, and five cases in those aged between 40 and 59 years.
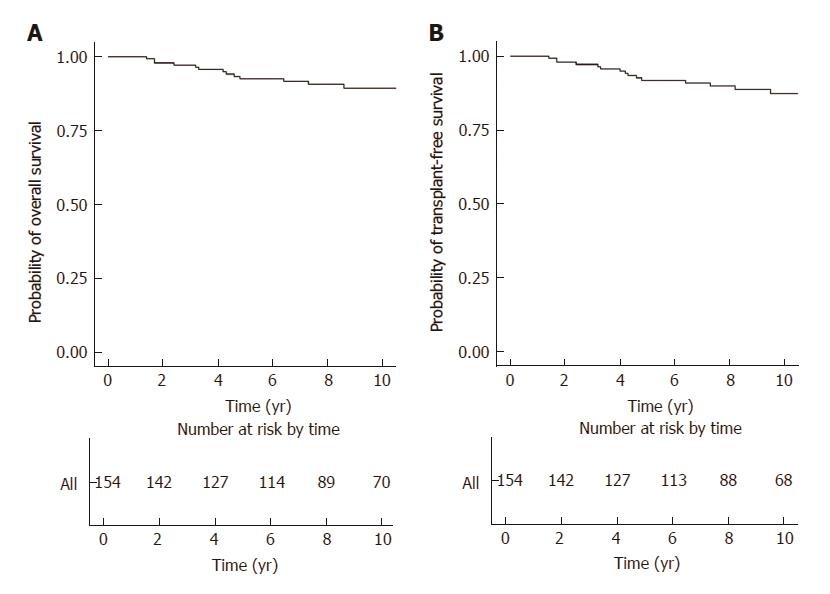
Twenty-six patients died between 2000 and 2016 [male cases 16 (15.0%), female cases 10 (9.6%); P = 0.182]. The median time from diagnosis to death for these 26 patients was 4.2 years (IQR 2.1 to 5.0 years). For those newly diagnosed patients receiving long-term treatment (n = 154), the 5-year and 10-year rates of overall survival were 92.6% (95%CI: 88.3%-97.1%) and 89.5% (95%CI: 84.1%-95.2%), respectively (Figure 1A). By univariate analysis, older age at diagnosis, cirrhosis, HCC , concomitant chronic viral hepatitis and metabolic factors were significant risk factors. By multivariate analysis, cirrhosis (HR 21.75; 95% CI 5.62 to 84.18) was the only independent risk factor, while concomitant chronic viral hepatitis was of borderline significance (HR 7.04; 95% CI 0.94 to 52.81)(Table 7).
| Univariate analysis | Multivariate analysis | |||
| HR | 95%CI | HR | 95%CI | |
| Overall survival | ||||
| Age1 | 1.04 | 1.02-1.07 | 1.03 | 0.99-1.08 |
| Male sex | 1.49 | 0.52-4.29 | ||
| Hepatic disease | 0.92 | 0.21-4.13 | ||
| Cirrhosis | 15.42 | 5.15-46.18 | 21.75 | 5.62-84.18 |
| Hepatocellular carcinoma | 12.42 | 2.71-56.93 | 2.23 | 0.38-13.32 |
| Chronic viral hepatitis2 | 4.95 | 1.09-22.44 | 7.04 | 0.94-52.81 |
| Neurological or psychiatric disease | 1.11 | 0.35-3.55 | ||
| Metabolic factors3 | 4.37 | 1.37-13.96 | 2.68 | 0.59-12.17 |
| Drug | ||||
| Penicillamine | Reference | - | ||
| Trientine | 2.30 | 0.61-8.63 | ||
| Zinc | 0.30 | 0.04-2.39 | ||
| transplant-free survival | ||||
| Age1 | 1.04 | 1.01-1.07 | 1.03 | 0.98-1.07 |
| Male sex | 1.45 | 0.54-3.88 | ||
| Hepatic disease | 1.09 | 0.25-4.80 | ||
| Cirrhosis | 21.34 | 7.34-61.99 | 32.16 | 8.58-120.52 |
| Hepatocellular carcinoma | 11.25 | 2.48-50.92 | 1.93 | 0.34-11.07 |
| Chronic viral hepatitis2 | 4.48 | 0.998-20.09 | 9.97 | 1.36-73.05 |
| Neurological or psychiatric disease | 1.27 | 0.44-3.66 | ||
| Metabolic factors3 | 3.59 | 1.16-11.16 | 2.51 | 0.60-10.54 |
| Drug | ||||
| D-penicillamine | Reference | - | ||
| Trientine | 1.97 | 0.54-7.17 | ||
| Zinc | 0.26 | 0.03-1.97 | ||
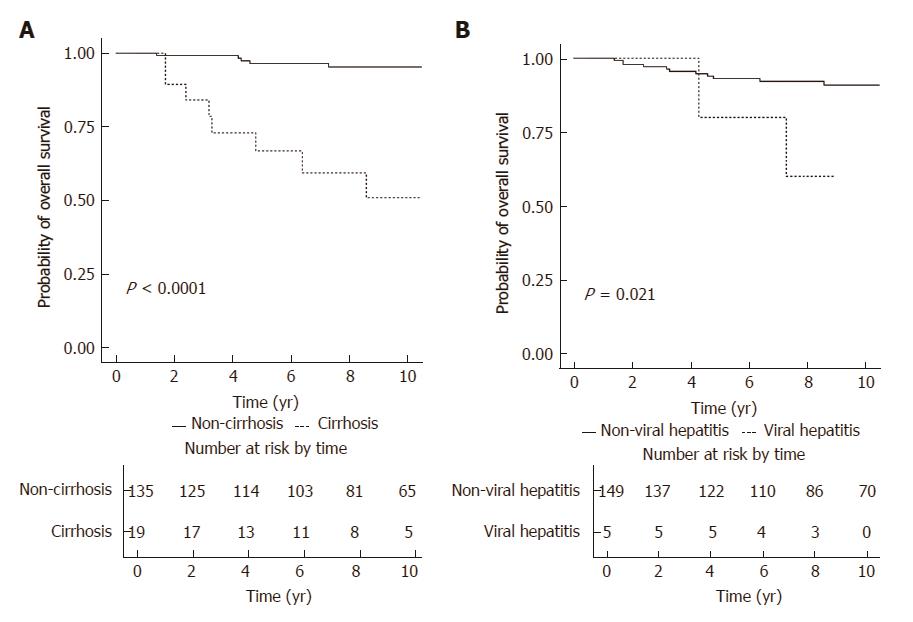
Figure 2A illustrates the comparison of overall survival of patients according to their baseline cirrhosis. Patients without cirrhosis had a significantly higher rate of overall survival (log-rank P < 0.001), with the 5-year and 10-year rates of overall survival being 96.5% (95%CI: 93.2%-99.9%) and 95.3% (95%CI: 91.4%-99.5%), respectively. For patients with cirrhosis, the 5-year and 10-year rates of overall survival were 66.9% (95%CI: 48.3%-92.7%) and 51.0% (95%CI: 30.9%-84.1%), respectively. Figure 2B illustrates the comparison of overall survival of patients according to the presence of chronic viral hepatitis. Patients without chronic viral hepatitis had a significantly higher rate of overall survival (log-rank P = 0.021), with the 5-year and 10-year rates of overall survival being 93.1% (95%CI: 88.9%-97.6%) and 90.9% (95%CI: 85.8%-96.3%), respectively. For patients with chronic viral hepatitis, the 5-year and 10-year rates of overall survival were 80.0% (95%CI: 51.6%-100%) and 60.0% (95%CI: 29.3%-100%), respectively.
The 5-year and 10-year rates of transplant-free survival were 91.8% (95%CI: 87.3%-96.6%) and 87.4% (95%CI: 81.5%-93.8%), respectively (Figure 1B). By univariate analysis, older age at diagnosis, cirrhosis, HCC, concomitant chronic viral hepatitis and metabolic factors were significant risk factors. By multivariate analysis, cirrhosis (HR = 32.16; 95%CI: 8.58-120.52) and concomitant chronic viral hepatitis (HR = 9.97; 95%CI: 1.36-73.05) were independent risk factors (Table 7).
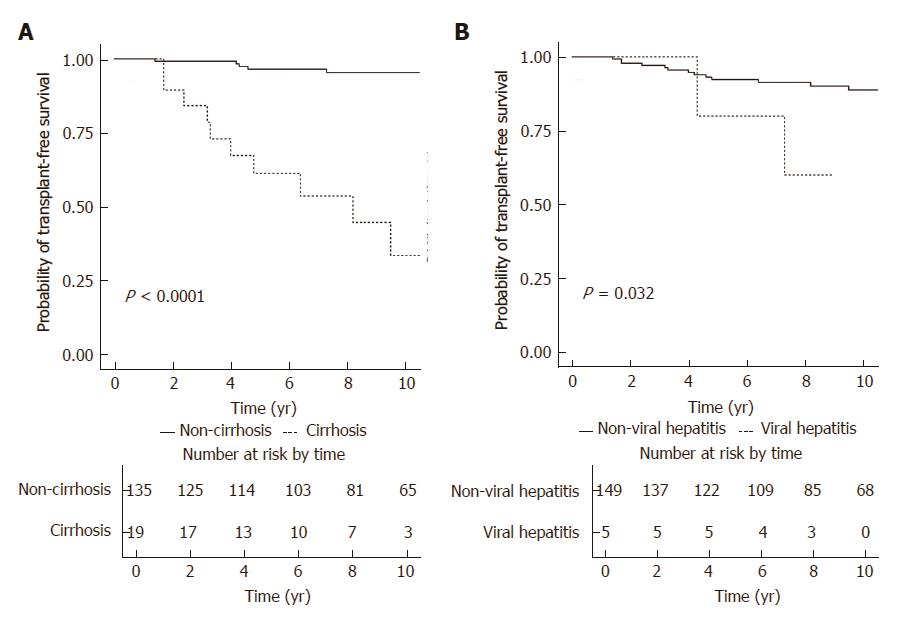
Figure 3A illustrates the transplant-free survival of patients with and without baseline cirrhosis. Patients without cirrhosis had a significantly higher rate of transplant-free survival (log-rank P < 0.001), with the 5-year and 10-year rates of transplant-free survival being 96.5% (95%CI: 93.2%-99.9%) and 95.3% (95%CI: 91.4%-99.5%), respectively. For patients with cirrhosis, the 5-year and 10-year rates of transplant-free survival were 61.2% (95%CI: 42.3%-88.7%) and 33.5% (95%CI: 14.9%-75.2%), respectively. Figure 3B illustrates the transplant-free survival of patients with and without chronic viral hepatitis. Patients without chronic viral hepatitis had a significantly higher rate of transplant-free survival (log-rank P = 0.032), with the 5-year and 10-year rates of transplant-free survival being 92.3% (95%CI: 87.9%-97.0%) and 88.8% (95%CI: 83.1%-94.9%), respectively. For patients with chronic viral hepatitis, the 5- and 10-year rates of transplant-free survival were 80.0% (95%CI: 51.6%-100%) and 60.0% (95%CI: 29.3%-100%), respectively.
Of the 211 patients with Wilson’s disease identified, 80 patients (37.9%) had follow-up in our hospital. After cross validating with the medical records, 77 were confirmed to have Wilson’s disease (positive predictive value (PPV) of 96.3%).
Of the 77 patients, we could not ascertain in 10 patients whether they were index cases or diagnosed by family screening. For the remaining 67 cases, 59 (88.1%) were index cases and 8 (11.9%) were diagnosed by family screening. All cases were diagnosed of Wilson’s disease in accordance to the AALSD or EASL guidelines. Sixty-five patients (84.4%) had liver manifestations, while 25 (32.5%) had neurological and/or psychiatric manifestations [18 (23.4%) and 11 (14.3%) had neurological and psychiatric manifestations, respectively].
Currently, population-based studies on the epidemiology as well as the natural history of Wilson’s disease are lacking in both the western and Asian populations, and the majority of them were done before the 1990s. The present territory-based study was the first to describe both the epidemiology and natural history of Wilson’s disease over a long period of time (a span of 17 years from 2000 to 2016) in the Chinese. The population-based nature of this study, in which the data were retrieved via electronic search of hospital medical registry, enables complete capture of all cases under the care of our public health system. This helps to minimize selection bias inherent in studies conducted in tertiary referral centers or selected hospitals. Cases were identified by ICD-9 coding, with local studies showing high coding accuracies[24,28]. We also confirmed a PPV of 96.3% for the coding accuracy of Wilson’s disease in the CDARS.
The incidence rate and prevalence of Wilson’s disease were estimated to be 17 to 29 per million person-years[8,9] and 30 per million, respectively[1,11] In comparison, the estimates from our study were lower, with an average annual incidence rate of 1.44 per million person-years, and an average annual prevalence of 17.93 per million. These estimates were in line with what were reported in Taiwan between 2000 and 2005 (an average annual incidence rate of 2.7 per million person-years and a prevalence ranging from 8.4 to 16.0 per million). The lower incidence rate may be partly due to under diagnosis. However, whether Wilson’s disease is genuinely less common in Asia requires confirmation from more population-based studies.
From the present study, the annual incidence rate of Wilson’s disease in Hong Kong had been decreasing, from 1.65 to 1.23 per million person-years between 2000 and 2016. This is likely attributed to the ageing population. As shown in Table 4, the incidence rate of Wilson’s disease peaked in the 15- to 19-year age group (3.06 per million person-years), while it was extremely low among individuals aged 45 or more. Between 2000 and 2016, population aged less than 20 has decreased from 1.59 million to 1.17 million, while the population aged 50 or more has increased from 1.63 million to 2.92 million in Hong Kong[31]. On the other hand, the prevalence of Wilson’s disease has been increasing, from 7.80 to 24.49 per million. This is likely related to the availability of effective treatment. It has been shown that treatment is associated with hepatic and neurological improvements in 82%-90% and 55%-69% of patients, respectively[4,6].
In the current study, the median age at diagnosis was 27.2 years, with the highest incidence rate observed in the 15- to 19-year age group. The proportions of cases in different age strata in our study [age < 20: 58 cases (30.8%); age 20-39: 73 cases (44.5%); age ≥ 40: 39 cases (24.6%)] were consistent with that reported by the Taiwanese population-based study [age < 20: 115 cases (37.5%); age 20-39: 127 cases (41.4%); age ≥ 40: 65 cases (21.2%)][14]. Men and women were equally affected as shown in our study and also previous studies[3,4,14,32]. The proportion of patients with neurological and/or psychiatric involvement was relatively low in our study (n = 49, 23.2%), although some studies also reported a similar rate of 24%-27%[3,33]. As for our center, 25 out of 77 patients (32.5%) had neurological and/or psychiatric involvement. This slightly higher proportion of neurological/psychiatric involvement is likely to be explained by the fact that our center is a tertiary referral hospital and is the only center which provides liver transplantation services in our locality.
However, a significant proportion of patients with hepatic involvement still had liver-related complications, with 21.6% having cirrhosis, 10.8% having cirrhotic complications, 1.7% developing HCC, and 13.6% undergoing liver transplantations. Around 12% of the total cases died during the observation period, with the 5-year and 10-year rates of overall survival being 92.6% and 89.5%, respectively.
Factors that could modify the transplant-free survival included the presence of cirrhosis (HR = 32.16) and concomitant chronic viral hepatitis (HR = 9.97). This highlights the importance of early diagnosis and treatment in reducing the copper load in the body to prevent development of cirrhosis. Future prospective studies with larger sample size are required to ascertain whether early treatment to suppress hepatitis B virus replication and to cure hepatitis C virus infection will be useful in improving the prognosis of patients with concomitant chronic viral hepatitis. The presence of metabolic factors was shown to be associated with lower transplant-free survival by univariate but not multivariate analysis. There are two possible reasons. First, as metabolic factors are associated with increasing age and cirrhosis[17,18,34], the potential risk of metabolic factors per se as shown by univariate analysis was attenuated after adjusting for the effect of age and cirrhosis in multivariate analysis. Second, the present study may be underpowered to detect this potential effect due to the relatively small sample size.
There are some limitations of the current study. First, a small proportion of healthcare services (around 10%) is not covered by the Hospital Authority[19], and hence some patients attending private hospitals will not be captured in the CDARS. As such, the incidence rate and prevalence of Wilson’s disease might be underestimated. Nevertheless, the majority of these patients are likely to be captured in the CDARS eventually, because of preference for long-term follow-up in the public hospitals or having consultation for other illnesses in our locality. Second, information regarding biochemical response to various treatment (D-penicillamine, trientine and zinc) could not be retrieved. Third, whether Wilson’s disease was diagnosed in accordance to the international guidelines (AALSD or EASL guidelines) could not be confirmed in other centers. However, this limitation was unlikely to have significant impact as it is the standard practice that all patients with suspected Wilson’s disease in Hong Kong are referred to hepatologists. As an example, all 77 cases who followed up in our center were diagnosed according to the international guidelines. Fourth, we could not ascertain whether these patients were index cases or were detected by family screening. As for our center, only 8 cases (11.9%) were diagnosed by family screening. In addition, diagnosis of Wilson’s disease in patients with neurological symptoms is usually delayed for a longer time from onset than in patients with hepatic symptoms[35]. The incidence rate of Wilson’s disease in our cohort should therefore be interpreted in this context. Lastly, some of the medications used to treat the neurological and/or psychiatric symptoms (e.g., anticonvulsants) could potentially lead to deranged liver function, which may be wrongly regarded as liver involvement due to Wilson’s disease by simply referring to the ICD coding.
These limitations may be addressed by collaboration between all public and private hospitals in the future. In this way, an even more precise estimate of the epidemiology of Wilson’s disease could be derived, and other potential risk factors for reduced transplant-free survival (e.g., metabolic factors) could be further investigated.
In conclusion, the epidemiology of Wilson’s disease was described in a well defined Chinese population, with identification of risk factors for overall and transplant-free survival. There was a significant increase in the prevalence of Wilson’s disease in Hong Kong. Early diagnosis and treatment as well as control of concomitant chronic viral hepatitis to improve prognosis could potentially reduce the disease complications.
There are few studies on the epidemiology and natural history of Wilson’s disease in the Chinese population. The authors conducted a territory-based study in Hong Kong (HK) with a population of 7.3 million to address this issue.
Epidemiology data are important for recognizing the temporal trend of a particular disease, understanding the natural history and risk factors, as well as for resource allocation.
To investigate the epidemiology and natural history of Wilson’s disease in the Chinese population.
Data were retrieved from the Clinical Data Analysis and Reporting System (CDARS) and Clinical Management System (CMS) of the Hong Kong Hospital Authority. The study observation period was from 2000 to 2016. Cases of Wilson’s disease between 1999 and 2016 were identified from CDARS by the International Classification of Diseases (ICD)-9 code of 275.1. The incidence rate and prevalence of Wilson’s disease between 2000 and 2016 were calculated. Evaluation of the count data and temporal trends was assessed by Poisson regression model. Cox proportional hazards model was used to identify variables that were associated with adverse outcomes. Kaplan-Meier method was used to analyze the adverse outcomes.
The authors identified 211 patients (male-to-female ratio 0.97:1; median age 27.2 years, IQR: 17.1-38.6 years; median follow-up 8.0 years, IQR: 5.0-14.0 years). The average annual incidence rate and prevalence were 1.44 per million person-years and 17.93 per million, respectively. Between 2000 and 2016, the annual incidence rate decreased from 1.65 to 1.23 per million person-years (Poisson P = 0.010), while the annual prevalence increased from 7.80 to 25.20 per million (Poisson P < 0.001). Among the 176 patients with hepatic involvement, 38 (21.6%) had cirrhosis, three (1.7%) developed hepatocellular carcinoma, 24 (13.6%) underwent liver transplantations, and 26 (14.8%) died. Seven patients had concomitant chronic viral hepatitis B or C. The 5- and 10-year overall survival rates were 92.6% and 89.5%, and for transplant-free survival rates 91.8% and 87.4%, respectively. Cirrhosis and possibly chronic viral hepatitis were associated with poorer overall survival.
There was a considerable increase in the prevalence of Wilson’s disease in the Chinese population. The long-term survival was good except in patients with cirrhosis or concomitant viral hepatitis.
The epidemiology of Wilson’s disease was described in a well defined Chinese population, and factors associated with overall survival and transplant-free survival were identified. Future collaboration with the hepatology units from all public and private hospitals is warranted to allow for an even more precise estimate of the epidemiology of Wilson’s disease and to investigate other potential risk factors for reduced transplant-free survival (e.g., metabolic factors).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Codoñer-Franch P, Garcia-Fernandez MI S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010;24:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Seto WK, Mak CM, But D, Hung I, Lam CW, Tam S, Yuen MF, Lai CL. Mutational analysis for Wilson’s disease. Lancet. 2009;374:662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Beinhardt S, Leiss W, Stättermayer AF, Graziadei I, Zoller H, Stauber R, Maieron A, Datz C, Steindl-Munda P, Hofer H. Long-term outcomes of patients with Wilson disease in a large Austrian cohort. Clin Gastroenterol Hepatol. 2014;12:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Bruha R, Marecek Z, Pospisilova L, Nevsimalova S, Vitek L, Martasek P, Nevoral J, Petrtyl J, Urbanek P, Jiraskova A. Long-term follow-up of Wilson disease: natural history, treatment, mutations analysis and phenotypic correlation. Liver Int. 2011;31:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Pfeiffenberger J, Mogler C, Gotthardt DN, Schulze-Bergkamen H, Litwin T, Reuner U, Hefter H, Huster D, Schemmer P, Członkowska A. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015;35:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, Merle U, Ferenci-Foerster D, Maieron A, Stauber R. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028-1035.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Bachmann H, Lössner J, Biesold D. [Wilson’s disease in the German Democratic Republic. I. Genetics and epidemiology]. Z Gesamte Inn Med. 1979;34:744-748. [PubMed] |
| 8. | Reilly M, Daly L, Hutchinson M. An epidemiological study of Wilson’s disease in the Republic of Ireland. J Neurol Neurosurg Psychiatry. 1993;56:298-300. [PubMed] |
| 9. | Park RH, McCabe P, Fell GS, Russell RI. Wilson’s disease in Scotland. Gut. 1991;32:1541-1545. [PubMed] |
| 10. | Lössner J, Bachmann H, Siegemund R, Kühn HJ, Günther K. [Wilson’s disease in East Germany: in retrospect and perspectives -- an evaluation]. Psychiatr Neurol Med Psychol (Leipz). 1990;42:585-600. [PubMed] |
| 11. | Frydman M. Genetic aspects of Wilson’s disease. J Gastroenterol Hepatol. 1990;5:483-490. [PubMed] |
| 12. | Poujois A, Woimant F, Samson S, Chaine P, Girardot-Tinant N, Tuppin P. Characteristics and prevalence of Wilson’s disease: A 2013 observational population-based study in France. Clin Res Hepatol Gastroenterol. 2017; [Epub ahead of print]. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Coffey AJ, Durkie M, Hague S, McLay K, Emmerson J, Lo C, Klaffke S, Joyce CJ, Dhawan A, Hadzic N. A genetic study of Wilson’s disease in the United Kingdom. Brain. 2013;136:1476-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Lai CH, Tseng HF. Population-based epidemiologic study of Wilson’s disease in Taiwan. Eur J Neurol. 2010;17:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1998] [Article Influence: 199.8] [Reference Citation Analysis (4)] |
| 16. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 17. | Seto WK, Fung J, Cheung KS, Mak LY, Hui RW, Liu KS, Lai CL, Yuen MF. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2016;44:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Kasmari AJ, Welch A, Liu G, Leslie D, McGarrity T, Riley T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med. 2017;130:746.e1-746.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | The Hospital Authority. Hospital Authority statistical report 2012-2013. Available at: http://www.ha.org.hk/haho/ho/stat/HASR1415_2.pdf. Accessed: January 12 2017; . |
| 20. | The Hospital Authority. Introduction. Available at: http://www.qeh.org.hk/visitor/ha_visitor_index.asp?Content_ID=10008Lang=ENDimension=100. Accessed: January 12 2017; . |
| 21. | Wong CP. Health informatics development in the hospital authority. Available at: http://www.ha.org. hk/haconvention/Accessed: January 12 2017; . |
| 22. | Man KKC, Ip P, Hsia Y, Chan EW, Chui CSL, Lam MPS, Wong WHS, Chow CB, Yung A, Wong ICK. ADHD Drug Prescribing Trend Is Increasing Among Children and Adolescents in Hong Kong. J Atten Disord. 2017;21:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Chan EW, Lau WC, Leung WK, Mok MT, He Y, Tong TS, Wong IC. Prevention of Dabigatran-Related Gastrointestinal Bleeding With Gastroprotective Agents: A Population-Based Study. Gastroenterology. 2015;149:586-595.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 25. | Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin Transl Gastroenterol. 2017;8:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Man KK, Chan EW, Coghill D, Douglas I, Ip P, Leung LP, Tsui MS, Wong WH, Wong IC. Methylphenidate and the risk of trauma. Pediatrics. 2015;135:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Wong AY, Root A, Douglas IJ, Chui CS, Chan EW, Ghebremichael-Weldeselassie Y, Siu CW, Smeeth L, Wong IC. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (1)] |
| 29. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8266] [Article Influence: 393.6] [Reference Citation Analysis (0)] |
| 30. | Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI. American Association Of Clinical Endocrinologists And American College Of Endocrinology Guidelines For Management Of Dyslipidemia And Prevention Of Cardiovascular Disease. Endocr Pract. 2017;23:1-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 31. | Census and Statistics Department. Hong Kong Statistics. Available at: http://www.censtatd.gov.hk/hkstat/sub/sp150.jsp?tableID=002ID=0pr ductType8. Accessed: March 12 2017; . |
| 32. | Lau JY, Lai CL, Wu PC, Pan HY, Lin HJ, Todd D. Wilson’s disease: 35 years’ experience. Q J Med. 1990;75:597-605. [PubMed] |
| 33. | Asadi Pooya AA, Eslami NS, Haghighat M. Wilson disease in southern Iran. Turk J Gastroenterol. 2005;16:71-74. [PubMed] |
| 34. | Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427-436. [PubMed] |
| 35. | Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007;56:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |