INTRODUCTION
Functional dyspepsia (FD) is a common gastrointestinal disorder that is characterized by persistent or recurrent upper abdominal pain or discomfort in the absence of any structural, morphological or known organic abnormality, often accompanied by psychosocial disturbance. Gastric hypersensitivity (GHS) is one of the characteristic pathogeneses of FD and represents a cardinal pathophysiological change in FD[1-4]. GHS is closely associated with not only postprandial epigastric pain but also some other symptoms such as early satiety, nausea or vomiting[5,6]. Clinical studies have shown that approximately 35%-65% of FD patients suffer from GHS[7], and among them, 10%-25% have been confirmed to have GHS-related postprandial epigastric pain[8,9]. However, although researchers have focused on GHS in the past, its molecular mechanisms and etiology remain largely unclear.
Anxiety is a common psycho-social disturbance[10] and troubles 40%-90% of the FD patients in the clinic[11]. Various studies have suggested that anxiety may influence gastric sensitivity, gastrointestinal movement, gastric emptying and gut neuroendocrine regulation through the hypothalamic-pituitary-adrenal axis (HPA-axis), autonomic nervous system and endogenous pain regulation system[12-14]. Unfortunately, although anxiety has been identified as an important factor triggering or aggravating FD, the mechanisms by which affect the development of FD and the relationship between them are still unknown. In part, this knowledge gap is due to a lack of both available visceral tissue from FD patients and normal human subjects and suitable animal models of FD with anxiety[15]. Therefore, to elucidate the pathogenesis of FD and to develop new drugs for use FD treatment, the creation of a novel animal model of FD with anxiety-like GHS is of vital importance.
Animal experiments have shown that acute mild gastric irritation or maternal separation (MS) in the neonatal period can induce hypersensitivity to gastric distention in adult rats[16,17]. MS, especially, can have long-lasting influences on emotionality[18], stress responsiveness[19], neurotransmitters in the central nervous system and enteric nervous system[20,21], the HPA-axis[22] and visceral sensitivity[23]. Clinical studies have also demonstrated that adverse physiological or psychological experiences in early life are linked to the development of FD and acute stress in adulthood[24,25]. Meanwhile, some neuromodulators such as 5-hydroxytryptamine (5-HT)[26-28], γ-aminobutyric acid (GABA)[29,30], brain-derived neurotrophic factor (BDNF)[15,31] and nesfatin-1[32,33] are involved in the regulation of anxiety, depression and other psychosocial activities as well as visceral sensations. These anxiety-related brain-gut modulators are neurobiochemical regulatory substances that are shared by the brain and gut of FD patients. Generally, a decrease in 5HT, GABA and BDNF levels or an increase in the nesfatin-1 content in the brain, stomach and plasma may induce or aggravate anxiety-like symptoms and behavior while increasing visceral sensitivity. Based on these findings, the present study was designed to expose animals to novel sequential stress, MS and acute gastric irritation (AGI), early in life followed by exposure to restraint stress (RS) in adulthood, in the hopes of creating a new anxiety-like GHS rat model of FD and exploring the alterations in 5-HT, GABA, BDNF, and nesfatin-1 in the hippocampus, plasma and mucosa of the gastric fundus and the 5-HT1A receptor (5-HT1AR) in the hippocampal CA1 subfield to more deeply understand the molecular mechanisms of these complex clinical disorders from the perspective of brain-blood-stomach axis.
MATERIALS AND METHODS
Animals and reagents
Male Sprague-Dawley rat pups were used in this study and housed with their mothers in cages lined with sterilized bedding materials. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were kept under a constant temperature of 24 °C ± 1 °C and relative humidity of 55% ± 5% with a 12-h light: 12-h dark cycle (lights on at 7:00 a.m.). The animals had access to regular chow diet and water ad libitum. For the implementation of anesthesia, an intraperitoneal injection of 50 mg/kg of sodium pentobarbital was used. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection. All experiments were performed according to the guidelines established by the European Community for the Care and Use of Laboratory Animals and were approved by the Experimental Animal Care and Use Committee of the Xi’an Jiao Tong University.
Iodoacetamide (IA) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). The enzyme-linked immunosorbent assay (ELISA) kits for myeloperoxidase (MPO), 5-HT, GABA, BDNF and nesfatin-1 were purchased from Shanghai Hong Ju Biological company (Shanghai, China). The 5-HT1AR antibody was purchased from Abcam company (Cambridge, United Kingdom). They were used following the manufacturer’s instructions. All other reagents were of analytical grade and purchased from Guo Yao Group Co., Ltd. (Shanghai, China).
Experimental design
Animals were randomly divided into two groups: Control rats and sequential- stress-treated rats. The rat pups in the sequential-stress-treated group received MS from postnatal day 2 (PND 2) to PND 21, during which AGI was given to them from PND 10 to PND 16. Meanwhile, the pups in the control group were still reared undisturbed with their mothers without undergoing MS or AGI. All animals, including the controls and the sequential-stress-treated ones, were weaned at PND 22, reared up to 8 wk, and then the sequential-stress-treated rats were forced to undergo RS for 7 d, while the control rats were housed freely. From the 9th postnatal week, the control and sequential-stress-treated rats began to undergo the operation for the implantation of the gastric balloon and electrodes for behavioral and electromyographic (EMG) testing. One week after surgery, the rats were first tested on the elevated plus maze (EPM). Then, on the day following the EPM, the open field (OF) experiment was performed on the same rats. Twenty-four hours after the OF test, abdominal withdrawal reflex (AWR) testing, EMG recordings, and blood collection were performed on the rats. According to previously published studies[34,35], the hippocampus is one of the important brain regions closely related to the regulation of mood such as anxiety and depression, while the gastric fundus is modulated mainly by serotonergic mechanisms. Therefore, the present study chose the hippocampus and gastric fundus of the animal as two of its research targets. After blood collection, some of the rats were perfused transcardially and immediately with ice-cold formalin (40 g/L) solution, and the brain and stomach were quickly removed for immunohistochemistry (IHC) measurements. Others of the rats were sacrificed by rapid decapitation and the hippocampus and gastric fundus were isolated carefully and dropped in liquid nitrogen immediately and stored at -80 °C for ELISA or Western blot (WB) measurements. Meanwhile, a different group of sequential-stress-treated and control rats was used only for the gastric emptying experiment.
Maternal separation
Maternal separation (MS) was performed as previously described[36]. Briefly, the rat pups that were randomly assigned to the sequential-stress-treated group were taken away from their maternity cages, placed in a smaller cage and housed individually in a separate room for 3 h daily (9:00 am-12:00 pm) from PND 2 to PND 21. After separation, the pups were then returned to their home cage. Pups in the control group were reared undisturbed with their mothers. At PND 22, all of the pups were weaned and maintained to 8 wk of age.
Acute gastric irritation
The PND 10 rat pups in the sequential-stress-treated group were administered 0.2 mL 1 g/L IA in 20 g/L sucrose daily for 6 consecutive days by oral gavages. Pups in the control group received 0.2 mL 20 g/L sucrose[16].
Restraint stress
The rats in the sequential-stress-treated group were restrained in cylindrical and well-ventilated tubes for 90 min daily for 1 wk beginning at 8 wk of age. They were returned to their rearing cages immediately after restraint[37].
Implantation of the gastric balloon and electrodes
After being fasted overnight prior to surgery, rats were anesthetized with an intraperitoneal injection of 50 mg/kg of sodium pentobarbital[17]. Balloons (2.5 cm long) made from latex gloves were fixed to a long catheter (PE-240). A left lateral epigastric incision was made, and the balloon was placed in the stomach through a small hole made at the tip of the fundus. The pylorus was not obstructed so that there was no blockage of gastric emptying. The hole was then tied tightly to avoid leakage of gastric fluid into the peritoneum. The catheter was exteriorized at the back of the neck along with the EMG electrode leads. The electrodes were implanted into the acromiotrapezius muscle and externalized at the back of the neck. One week later, the behavioral and EMG tests were performed.
AWR and EMG recordings
AWR to graded gastric distention (20-100 mmHg) was tested on day 6 after the implantation of the gastric balloon. Each pressure was achieved by inflating the balloon for 20 s followed by a 5-min interval[15]. The AWR was graded as previously reported[16]: 0, no behavioral response to gastric distention (GD); 1, brief head movement followed by immobility; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching, lifting of pelvic structures and stretching of body. Visceromotor responses (VMRs) to GD were recorded simultaneously along with measuring the AWR with a BL-420S biological signal collecting and processing system (Techman Software CO., Chengdu, China). EMG activity (including baseline and during every pressure distention of the stomach) was then rectified and quantified by calculating the area under the curve (AUC). VMRs to GD were expressed as a percentage increase over baseline.
EPM
A standard PMT-100 rat EPM (Techman Software CO., Chengdu, China) was used in this experiment. The maze consists of four arms (two open without walls and two enclosed by 30-cm-high walls) 50 cm long and 10 cm wide. Each arm was elevated 50 cm off the floor. The light intensity was set at 300 lux. The rat’s movements were automatically recorded by a video-tracking system (Techman Software CO., Chengdu, China) to collect the behavioral data. Every procedure was started by placing the rat at the junction of the open and closed arms, facing the open arm opposite to where the experimenter was standing and lasted 5 min[38]. The maze was cleaned with alcohol between each test animal. The EPM test is based on the rat’s avoidance of open spaces. This avoidance is thought to be one of the anxiety-like behavioral characteristics, namely, thigmotaxis, which implies an aversion for open areas by the rats’ tendency to remain in enclosed spaces. The increased amount of time spent in the closed arms usually indicates high anxiety-like behavior.
OF testing
The OF consisted of a square arena (100 cm × 100 cm with twenty-five 20 cm × 20 cm virtual grids and four 40-cm-high walls) was divided into a peripheral zone and a central zone (60 cm × 60 cm). Rats were positioned in the center of the OF and allowed to freely explore for 5 min. The light intensity was also set at 300 lux. The time spent in the central area, number of virtual grids climbed and the velocity of the rat’s movement were examined[39].
Gastric emptying testing
In another group of sequential-stress-treated and control rats, the gastric emptying of a solid meal was investigated as demonstrated previously[40]. Rats that had been fasted overnight were allowed to freely consume water and a preweighed amount of solid food for 3 h. After that, the water and food were removed, and the rats rested for 3 h. The rats were then killed, and the stomach was removed and emptied thoroughly. The rate of gastric emptying was calculated by the following formula: Gastric emptying (%) = 100 - (gastric content/food intake) × 100.
Histology
Gastric tips cut from along the greater curvature of the killed rats were immersed in 10% formaldehyde for at least 72 h. Then, four-micrometer sections from paraffin-embedded tissue specimens were processed for HE staining for histological measurement.
Assay of MPO activity and levels of 5-HT, GABA, BDNF, nesfatin-1 and 5-HT1AR
Under anesthesia with sodium pentobarbital (50 mg/kg, intraperitoneally) following the AWR and EMG testing, 1 mL blood was collected from the rat tail vein using a syringe (1 mL), smoothly injected into a 1.5 mL centrifugal tube and centrifuge 3000 rpm for 15 min. Then, 300 μL supernatant (plasma) was stored in a vial and kept at -80 °C until analysis. Once the blood collection was finished, some of the rats were perfused transcardially and immediately with ice-cold formalin (40 g/L) solution, and the brain and stomach were quickly removed and post-fixed by immersing in 40 g/L formalin solution at 4 °C overnight for immunohistochemistry (IHC) measurements[45]. Others of the rats were sacrificed by rapid decapitation and the hippocampus and gastric fundus (by another operator) were then isolated carefully, wrapped in foil and dropped in liquid nitrogen immediately and stored at -80 °C for ELISA or Western blot (WB) measurements[46]. The ELISA assays of MPO activity and the levels of 5-HT, GABA, BDNF and nesfatin-1 were analyzed as described by the instructions of the ELISA kits and the methods described previously[32,41-44]. The IHC and WB tests of the levels of 5-HT1AR in the hippocampal CA1 subfield or the mucosa of the gastric fundus were also performed using the methods reported previously[45,46].
Statistical analysis
Data were expressed as the mean ± SEM. Student’s t-test or 2-way repeated measures ANOVA were used for comparisons. Post hoc comparisons were made using the Student-Newman-Keuls test. Statistical analysis was performed using SPSS 17.0 software kits. A value of P < 0.05 was considered significant.
RESULTS
EPM
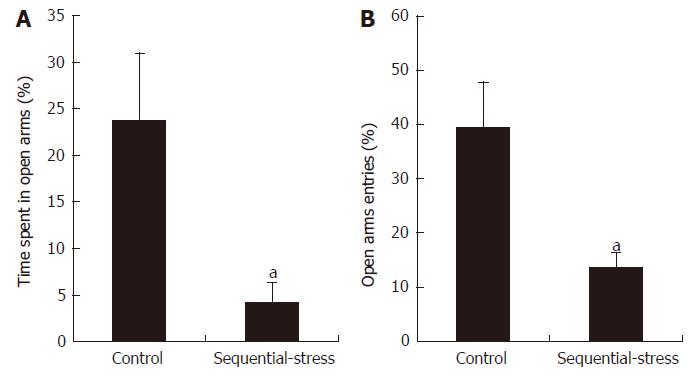
The EPM experiment showed that the sequential-stress-treated rats spent less time in the open arms than the control rats (4.26 ± 2.10 s vs 23.76 ± 7.24 s, P < 0.05, Figure 1A; n = 8 in each group). They also entered into the open arms fewer times than the control group (13.67 ± 2.77 vs 39.49 ± 8.31, P < 0.05, Figure 1B).
Figure 1 Elevated plus maze examination showed the sequential-stress-treated rats demonstrated anxiety-like behavior.
A: They spent less time in the open arms; B: They made fewer entries into the open arms. Data were expressed as the mean ± SEM of the percentage of time spent in open arms and open arms entries (n = 8 in each group). aP < 0.05 vs control.
OF testing
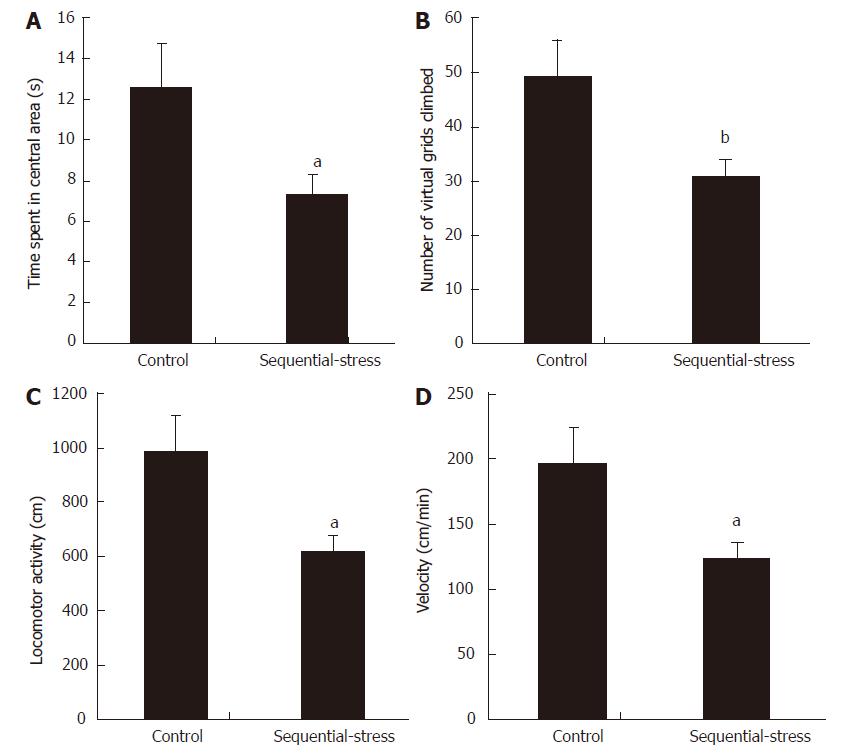
In the OF test, animals showing anxiety-like behavior usually demonstrate a decreased amount of time spent in the central area and decreased number of virtual grids climbed. Our results were in accordance with these phenomena (Time spent in the central area: 7.27 ± 1.00 s vs 12.53 ± 2.16 s, P < 0.05; Figure 2A; Number of virtual grids climbed: 30.86 ± 2.96 vs 49.14 ± 6.88, P < 0.01; Figure 2B for the sequential-stress-treated and control groups, respectively; n = 8 in each group). The two groups also showed significant differences in the total exploring distance (Locomotor activity: 617.14 ± 59.11 vs 982.86 ± 137.56, P < 0.05; Figure 2C) and mean velocity in the OF test (Velocity: 123.43 ± 11.82 vs 196.57 ± 27.51, P < 0.05; Figure 2D).
Figure 2 OF test showed the sequential-stress-treated rats demonstrated anxiety-like behavior.
A: They spent less time in central area; B: They climbed fewer number of virtual grids in OF box; C: Their total exploring distance was shorter; D: Their mean moving speed was slower. Data were expressed as the mean ± SEM of the time spent in central area, number of virtual grids climbed, total exploring distance and mean moving speed (n = 8 in each group). aP < 0.05, bP < 0.01 vs control group.
GHS
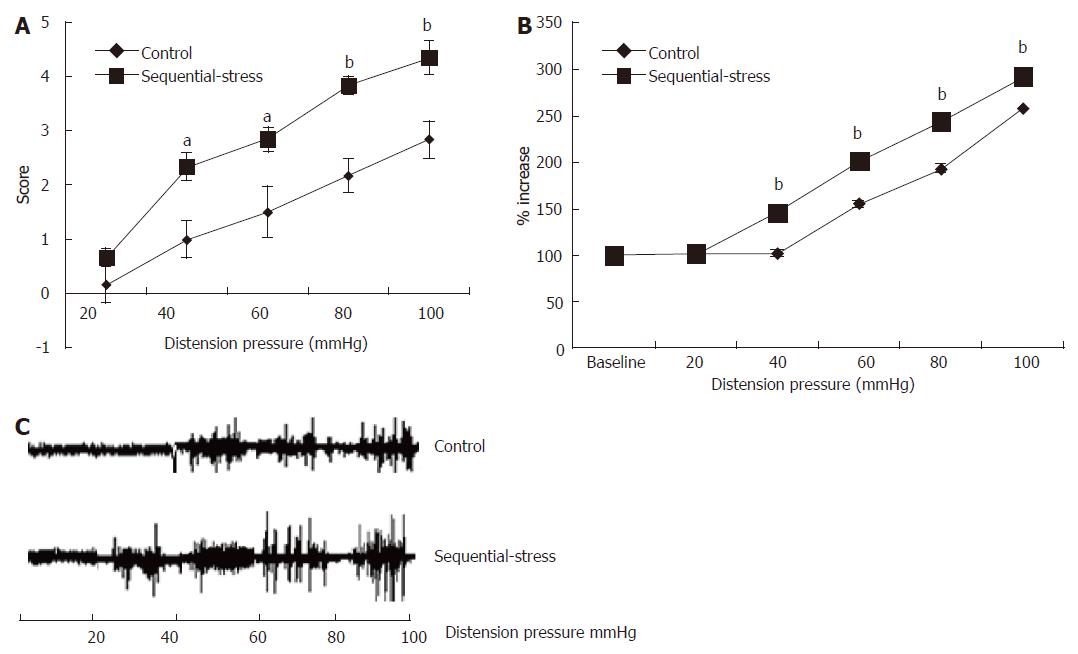
At 8 wk, the effects of gastric distention (GD) on the rats were examined. The AWR experiments showed that the behavioral scores in response to GD in the sequential-stress-treated rats were significantly higher than in the control group (20 mmHg: 0.67 ± 0.33 vs 0.17 ± 0.17, P > 0.05; 40 mmHg: 2.33 ± 0.33 vs 1.00 ± 0.26, P < 0.05; 60 mmHg: 2.83 ± 0.48 vs 1.50 ± 0.22, P < 0.05; 80 mmHg: 3.84 ± 0.31 vs 2.17 ± 0.17, P < 0.01; 100 mmHg: 4.33 ± 0.33 vs 2.82 ± 0.30, P < 0.01; Figure 3A, n = 8 in each group). Statistical analysis indicated significant differences in the AWR scores at 40, 60, 80 and 100 mmHg.
Figure 3 Abdominal withdrawal reflex and electromyographic tests showed the sequential-stress-treated rats exhibited gastric hypersensitivity to gastric distention.
A: AWR to GD of the sequential-stress-treated rats was significantly higher at the distention pressure 40, 60, 80 and 100 mmHg; B: EMG responses of the sequential-stress-treated rats were greater at the distention pressure 40, 60, 80 and 100 mmHg; C: The representative EMG response from both an adult sequential-stress-treated rat and control rat to GD. Data were expressed as the mean ± SEM of the AWR score and the percentage of EMG derived AUC increased (n = 8 in each group). aP < 0.05, bP < 0.01 vs control group.
These results were verified by computing the AUCs of the EMG recordings of the acromiotrapezius, which were used to quantify the VMRs to GD. Rats in the sequential-stress-treated group exhibited significantly greater EMG responses and significantly higher AUCs of the EMG activity than those in the control group at GD pressures 40, 60, 80 and 100 mmHg (146.23 ± 2.95 vs 102.97 ± 0.59, 202.39 ± 3.73 vs 156.00 ± 2.24, 243.79 ± 3.10 vs 193.93 ± 1.22, 293.05 ± 4.75 vs 258.17 ± 0.54, respectively, P < 0.01; Figure 3B, n = 8 in each group). A representative EMG response from both an adult sequential stress-treated rat and control rat to GD is shown in Figure 3C.
Changes in the levels of 5-HT, GABA, BDNF and nesfatin-1 in the hippocampus
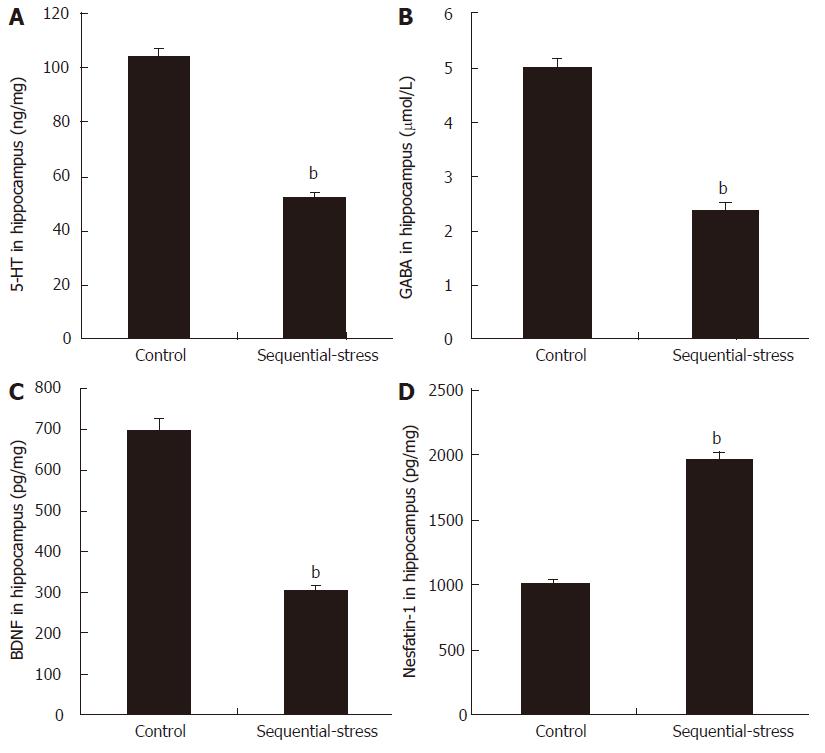
The ELISA results showed that compared with the levels in the control group, sequential stress significantly decreased the levels of 5-HT (51.91 ± 1.88 vs 104.21 ± 2.88, P < 0.01, Figure 4A), GABA (2.38 ± 0.16 vs 5.01 ± 0.13, P < 0.01; Figure 4B) and BDNF (304.40 ± 10.16 vs 698.17 ± 27.91, P < 0.01; Figure 4C) in the hippocampus but obviously increased the content of nesfatin-1 in the same site (1961.38 ± 56.89 vs 1007.50 ± 33.05, P < 0.01; Figure 4D; n = 8 in each group).
Figure 4 Influence of sequential stress on the levels of 5-hydroxytryptamine, γ-aminobutyric acid, brain-derived neurotrophic factor and nesfatin-1 in hippocampus.
A: 5-hydroxytryptamine (5-HT); B: γ-aminobutyric acid (GABA); and C: brain-derived neurotrophic factor (BDNF) were significantly down-regulated; D: Nesfatin-1 was significantly up-regulated. Data were expressed as the mean ± SEM of the levels of 5-HT, GABA, BDNF and Nesfatin-1 in Hippocampus (n = 8 in each group). bP < 0.01 vs control group.
Changes in the levels of 5-HT, BDNF and nesfatin-1 in the plasma
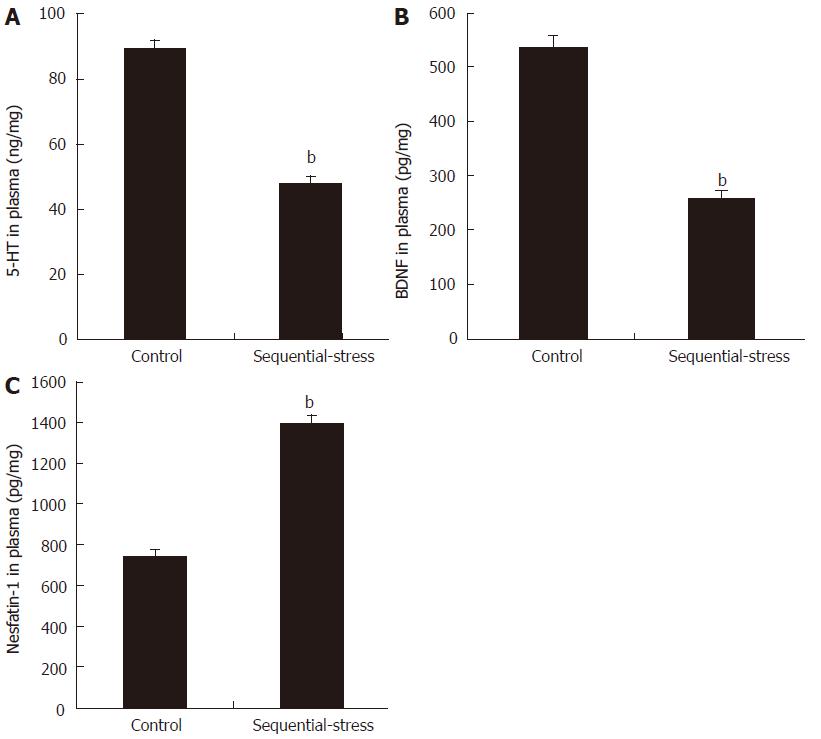
Compared with the levels in the control group, the levels of 5-HT (47.82 ± 2.29 vs 89.45 ± 2.61, P < 0.01; Figure 5A) and BDNF (257.05 ± 12.89 vs 536.71 ± 20.73, P < 0.01; Figure 5B) in the plasma of the sequential-stress-treated rats decreased significantly, but the content of nesfatin-1 in the plasma of the sequential-stress-treated rats was obviously increased (1391.75 ± 42.77 vs 737.88 ± 33.15, P < 0.01, Figure 5C; n = 8 in each group).
Figure 5 Influence of sequential stress on the levels of 5-hydroxytryptamine, brain-derived neurotrophic factor and nesfatin-1 in plasma.
A: 5-hydroxytryptamine (5-HT) and B: Brain-derived neurotrophic factor (BDNF) was significantly down-regulated; C: Nesfatin-1 was significantly up-regulated. Data were expressed as the mean ± SEM of the levels of 5-HT, BDNF and Nesfatin-1 in plasma (n = 8 in each group). bP < 0.01 vs control group.
Changes in the levels of 5-HT, BDNF and nesfatin-1 in the gastric fundus
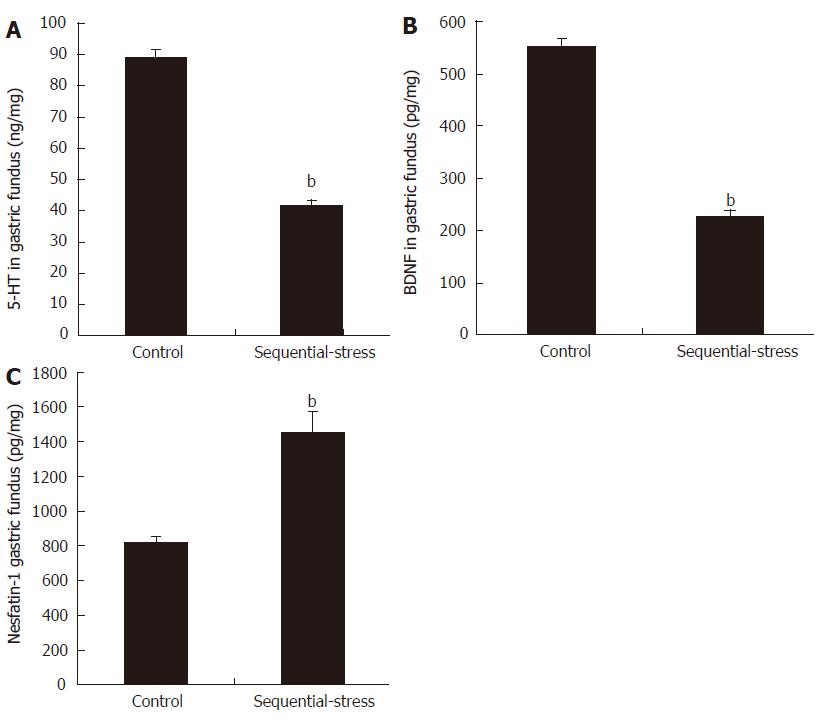
Compared with the levels in the control group, the levels of 5-HT (41.15 ± 1.81 vs 89.17 ± 2.31, P < 0.01; Figure 6A) and BDNF (226.49 ± 12.10 vs 551.36 ± 16.47, P < 0.01; Figure 6B) in the gastric fundus of the sequential-stress-treated rats decreased significantly, but the content of nesfatin-1 in the gastric fundus of the sequential-stress-treated rats was obviously increased (1534.75 ± 38.52 vs 819.63 ± 38.04, P < 0.01; Figure 6C; n = 8 in each group).
Figure 6 Influence of sequential stress on the levels of 5-hydroxytryptamine, brain-derived neurotrophic factor and nesfatin-1 in gastric fundus.
A: 5-hydroxytryptamine (5-HT) and B: Brain-derived neurotrophic factor (BDNF) were significantly down-regulated; C: Nesfatin-1 was significantly up-regulated. Data were expressed as the mean ± SEM of the levels of 5-HT, BDNF and Nesfatin-1 in gastric fundus (n = 8 in each group). bP < 0.01 vs control group.
Changes in the levels of 5-HT1AR in the hippocampal CA1 subfield and mucosa of gastric fundus by IHC
The IHC results revealed that, compared with the levels in the controls, the levels of 5-HT1AR expression in the hippocampal CA1 subfield (Figure 7A) and the mucosa of the gastric fundus (Figure 7A). Aof the sequential-stress-treated rats were both down-regulated, while the levels of 5-HT were reduced in these same sites (Optical Density value: Figure 7B hippocampus 15253.50 ± 760.35 vs 21149.75 ± 834.13 and Figure 7C gastric fundus 15865.25 ± 521.24 vs 23865.75 ± 1868.60; P < 0.05, respectively; n = 8 in each group).
Figure 7 Presentation of 5-hydroxytryptamine 1A receptor expression in the hippocampal CA1 subfield and the mucosa of gastric fundus of each group by immunohistochemistry.
A: Magnification, hippocampus, × 400 and gastric fundus, × 100; B: Level of 5-hydroxytryptamine 1A receptor (5-HT1AR) in hippocampus; C: Level of 5-HT1AR in gastric fundus. Data were expressed as the mean ± SEM of the levels of 5HT1AR (n = 8 in each group). aP < 0.05 vs control group.
Changes in the levels of 5-HT1AR in the hippocampal CA1 subfield by WB
The WB assay of the relative quantitative level of 5-HT1AR in the hippocampal CA1 subfield demonstrated that the expression of 5-HT1AR in this region was significantly lower in the sequential-stress-treated rats than in the control group (0.38 ± 0.01 vs 0. 57 ± 0.03, P < 0.01; Figure 8; n = 8 in each group).
Figure 8 Western blot detection showed a significant decrease of the level of 5-hydroxytryptamine 1A receptor in the hippocampal CA1 subfield of the sequential-stress-treated rats.
Data were expressed as the mean ± SEM of the levels of 5-hydroxytryptamine 1A receptor (5-HT1AR) (n = 8 in each group). bP < 0.01 vs control group.
Lack of an inflammatory response in the gastric wall of FD-like rats
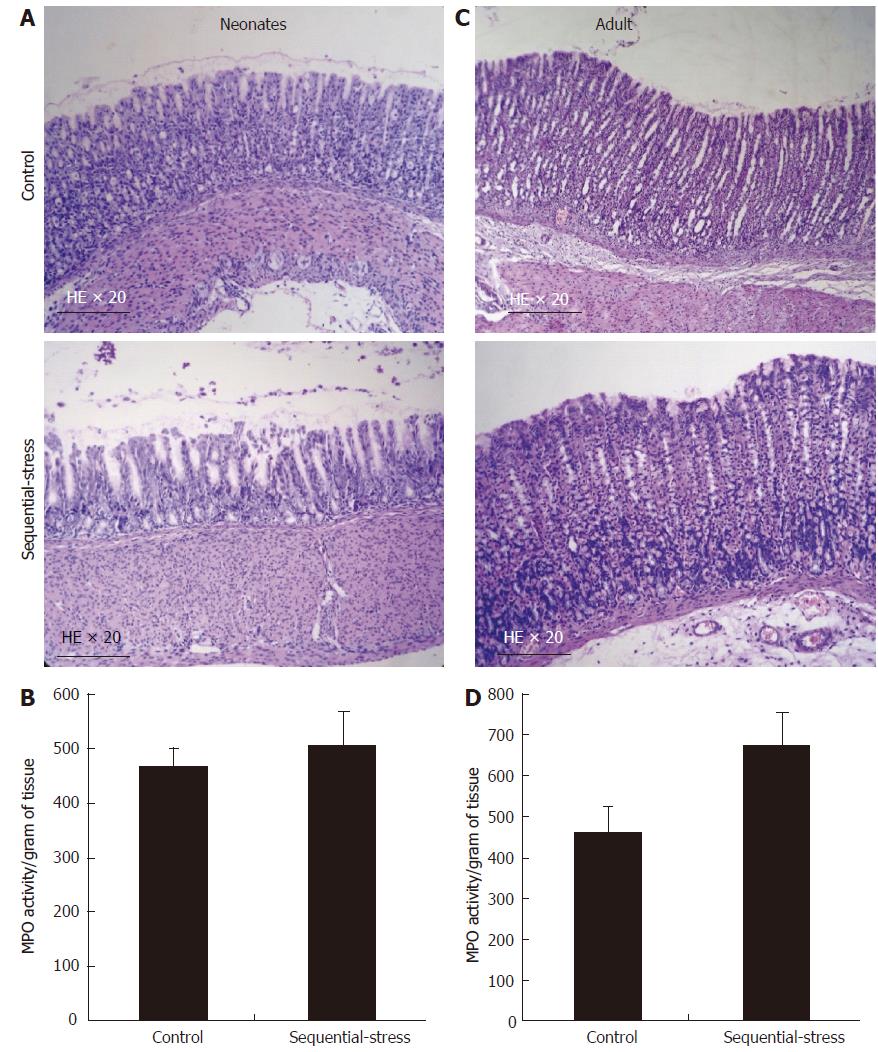
In the sequential-stress-treated group, gastric histology only showed superficial sloughing of the mucosa on day 6, and no deeper injury or inflammation was revealed compared with that in the control group (Figure 9A; n = 8 in each group). At the same time, the myeloperoxidase (MPO) activity assay demonstrated no significant difference between the two groups (505.28 ± 64.10 vs 467.00 ± 34.33, P > 0.05, Figure 9B; n = 8 in each group). In another set of experiment, sequential-stress-treated rats were followed to adulthood and treated with RS as described before. On day 56 post-treatment, neither histology (Figure 9C) nor the MPO activity test (670.91 ± 79.80 vs 462.30 ± 60.07, P > 0.05, Figure 9D; n = 8 per group) showed a lesion or abnormality in the gastric mucosa of either group.
Figure 9 Influence of the sequential stress on inflammatory response of gastric mucosa.
A: On day 6 in early life only superficial sloughing of the mucosa was observed in the sequential-stress-treated group; B: Myeloperoxidase (MPO) activity assay at the same time demonstrated no statistical significance between two groups; C and D: after RS treatment, both histology and MPO activity test display no lesion or abnormality in the gastric mucosa of the two groups. MPO activity is represented as activity per unit of dry weight. Data were expressed as the mean ± SEM of the MPO activity (n = 8 in each group).
Delayed gastric emptying
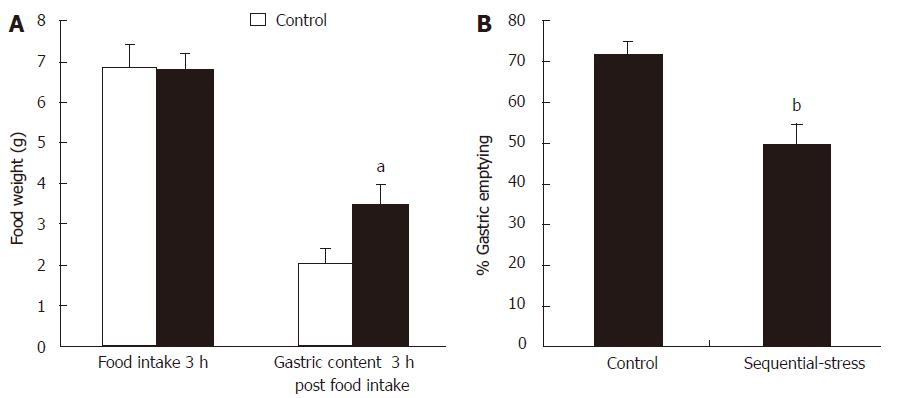
After 18 h of fasting, the adult rats of the sequential-stress-treated and control groups consumed similar amount of solid food in a 3-h period (6.52 ± 0.34 g vs 6.75 ± 0.51 g, P > 0.05, respectively; Figure 10A, n = 8 in each group). However, compared with that observed in the controls, the sequential-stress-treated rats exhibited a significant increase in the gastric contents 3 h post food intake (3.50 ± 0.51 vs 2.02 ± 0.38, P < 0.05; Figure 10A) and decrease in the rate of gastric emptying of the food ingested (49.55 ± 5.11 vs 71.37 ± 3.36, P < 0.05; Figure 10B).
Figure 10 Influence of sequential stress on the rate of gastric emptying.
A: Although the food intake in 3 hours after 18 h fast were similar between the two groups, the gastric contents 3 h post food intake in sequential-stress-treated rats increased; B: the rate of gastric emptying of the food ingested in sequential-stress-treated rats decreased. Data were expressed as the mean ± SEM of the food weight and the rate of gastric emptying (n = 8 in each group). aP < 0.05, bP < 0 .01 vs control group.
DISCUSSION
Anxiety is one of the most common symptoms among FD patients and an important cause of refractory FD, with a high incidence of 40%-90% in the clinic[11]. As previously described, anxiety may induce a functional disturbance of the HPA-axis and neuroendocrine regulation system, increase gastric sensitivity, and further promote the development of FD[14,47]. However, the cellular and molecular mechanisms underlying these actions are still unknown. One of the primary reasons for this gap in knowledge is that researchers lack mature animal models mimicking anxiety-like FD with or without GHS.
Previous studies have suggested that neonatal or adolescent adverse physiological or psychological experiences and adult acute stress are all factors associated with the development of FD[24,25]. Acute mild gastric irritation in the neonatal period may be one of the causes of chronic GHS and gut dysfunction[16]. As a negative stimulus in childhood, MS has been proven to induce anxiety-like behavior[48,49], affect the regulation from the hypothalamus to the adrenal gland and the retro-regulation from the adrenal gland to hypothalamus of the HPA-axis, and result in an anxiety-like psychological stress reaction[50]. MS can also have long-lasting influences on emotionality[18], stress responsiveness[19], neurotransmitters in the central nervous system and enteric nervous system[20,21], and visceral sensitivity[23]. Acute stress in adulthood has similar effects on the HPA-axis as MS and has a certain impact on gastric motility and gastric accommodation by influencing the secretion of ghrelin[35,51].
However, although studies published previously have reported some FD-like GHS animal models that have been successfully created using MS, acute stress or neonatal gastric irritation[15-17], few of them focused on the psychological stress responses such as anxiety and depression that the stimuli caused in the experimental animals and the interaction or mechanisms between the psychological stress responses and the GHS in FD from the perspective of brain-blood-stomach axis.
Our study showed that although AGI combined with MS and RS could induce the superficial sloughing of the gastric mucosa in the initiation stage, this change could recover rapidly in a short period, and the histological and MPO tests of the gastric mucosa were completely normal at 8 weeks postnatal, which agrees with the definition of FD. Compared with that in the controls, there was a significant decrease in the rate of gastric emptying in the sequential-stress-treated rats but no significant difference in the food intake amount between the two groups. These findings are in accordance with the clinical observations that up to 40% of patients with FD suffer from a delay in gastric emptying[52-54]. In addition, the AWR and EMG tests of our study also successfully mimicked the GHS in FD, which has been thought to exist in 35%-65% of patients with FD and to be a main cause for refractory FD[1,2,7].
Anxiety is another characteristic of FD. Our study found that the sequential-stress-treated rats spent less time and showed fewer entries into the open arms of EPM than the control rats. Similarly, in the OF test, the sequential-stress-treated rats seemed to avoid staying in the central area, moved a shorter distance, climbed fewer grids, and walked slower than the control animals. The sequential-stress-treated rats demonstrated typical anxiety-like behaviors. These results showed that we have successfully developed a potential anxiety-like GHS rat model of FD.
5-HT is thought to be closely related to both anxiety and the function of the gut system, but the mechanism of its effects is still controversial. In the brain, some reports have found that inhibiting the reuptake of 5-HT by the presynaptic membrane, activating the 5-HT1AR, or up-regulating the expression of the 5-HT1AR protein may produce anxiolytic effects[26,55-57]. In the gut, there are five types of 5-HT receptors, the 5-HT1, 2, 3, 4, and 7 receptor, distributed throughout the gastrointestinal wall. The different types of receptors play different roles in the regulation of gastrointestinal function. The 5-HT1AR and 5-HT7 receptor in the stomach mainly mediate the gastric accommodative relaxation and the delay of gastric emptying[58-60], while the 5-HT2, 3, and 4 receptors mainly participate in the contractive regulation of the gastrointestinal smooth muscle[27]. The present study found that the levels of 5-HT and the expression of 5-HT1AR in the hippocampus and gastric fundus of the sequential-stress-treated rats were synchronously diminished compared with the levels in the control rats, in agreement with the changes observed in the behavioral experiments. These results implied that the 5-HT and 5-HT1AR in the hippocampus and gastric fundus participate in not only the regulation of anxiety-like responses but also the development of FD symptoms.
GABA is another important inhibitory neurotransmitter in the central nervous system. When it is deficient, animals will manifest obvious psychological responses such as anxiety, insomnia, instable mood and so on[61]. There are known to be two subtypes of the GABA receptor, GABAA and GABAB, and both of them have anxiolytic effects when activated[29,62]. In addition, a recent study has reported a novel finding that the GABABR agonist baclofen produced an analgesic effect on the visceral pain of FD rats. This result indicated that in an anxious state, the deficiency of GABA or GABABR in the hippocampus could down-regulate the threshold of visceral pain and elevate the sensitivity of visceral perception[30]. In our current study, the concentration of GABA in the hippocampus of the sequential-stress-treated rats was significantly lower, but the anxiety-like behaviors and the gastric sensitivity were greatly higher than in the control rats. These findings, along with those cited above, suggest that GABA takes part in the formation of anxiety-like GHS in FD.
BDNF is the most prominent neurotrophic factor throughout the mammalian brain including the hippocampus and plays an important role in the regulation of the development, plasticity and survival of dopaminergic, cholinergic, serotonergic and GABAergic neurons[63-64]. BDNF is also related to the formation of anxiety[65]. When the BDNF gene is knocked out or the expression of BDNF is down-regulated in the hippocampus, animals will demonstrate an anxious mood and behavior; however, after BDNF is injected into the cerebral region, these changes are completely alleviated[31,66]. In addition, some research has discovered that changes in the levels of BDNF in certain parts of the body are correlated with gut sensitivity[15,67]. Our study found that the content of BDNF in the hippocampus, plasma and gastric fundus was greatly reduced in the sequential-stress-treated rats, implying that BDNF participated in the regulation of anxiety-like behavior and gastric sensitivity. The changes in BDNF were in line with those of 5-HT, 5-HT1AR and GABA.
Nesfatin-1 is a peptide hormone comprised of 82 amino acids. Nesfatin-1 has been considered to be able to reduce food intake and induce an anxiety-like emotional reaction[68,69]. Evidence has shown that in anorexia nervosa patients with anxiety the plasma levels of nesfatin-1 are elevated, and injection of nesfatin-1 into the cerebral ventricle may delay gastric emptying[70]. Nesfatin-1 could also influence visceral perception and increase visceral sensitivity[33]. The present study found that the levels of nesfatin-1 in the hippocampus, plasma and gastric fundus were significantly elevated in the sequential-stress-treated rats, which was in accordance with the anxiety-like behavior and the delay in gastric emptying. From our study together with previous findings, nesfatin-1 can be inferred to potentially be one of the most important regulatory hormones in anxiety-like GHS in FD.
In brief, sequential using a combination of MS, AGI and RS, the present study provided a potential anxiety-like GHS rat model of FD. This model demonstrated the complex behavioral characteristics of anxiety and GHS. Based on this model, our study initially observed the complicated alterations in some anxiety-related neurobiochemical modulators in brain-blood-stomach axis such as 5-HT, GABA, BDNF and nesfatin-1 in the hippocampus, plasma and gastric fundus of this model. The results indicated that more than one biological regulatory factor is involved in the modulation of FD patients with GHS and anxiety. Furthermore, this study supplied a possible tool for the development of new drugs and the exploration of the mechanisms of FD.
ARTICLE HIGHLIGHTS
Research background
Functional dyspepsia (FD) is a common gastrointestinal disorder that is characterized by persistent or recurrent upper abdominal pain or discomfort in the absence of any structural, morphological or known organic abnormality, often accompanied by psychosocial disturbance. Gastric hypersensitivity (GHS) is one of the characteristic pathogeneses of FD and represents a cardinal pathophysiological change in FD. Clinical studies have shown that approximately 35%-65% of FD patients suffer from GHS, and among them, 10%-25% have been confirmed to have GHS-related postprandial epigastric pain. Anxiety is a common psycho-social disturbance and troubles 40%-90% of the FD patients in clinic. Various studies have suggested that anxiety may influence gastric sensitivity, gastrointestinal movement, gastric emptying and gut neuroendocrine regulation. However, although GHS and anxiety have been identified as important factors triggering or aggravating FD, the mechanisms by which affect the development of FD and the relationship between them are still unknown. In part, this is due to a lack of both available visceral tissue from FD patients and normal human subjects and suitable animal models of FD with anxiety. Therefore, in order to elucidate the pathogenesis of FD and develop new drugs for the treatment of FD, creating a novel animal model of FD with anxiety-like GHS is of vital importance.
Research motivation
The research motivation came from the reality that there are no defined approaches in rat models that simultaneously mimic anxiety-like behaviors and GHS of FD in a standardized way yet. The authors tried to solve this problem by making rats undergo stress early in life and late in adulthood. The authors believed that the establishment of this rat model would be useful to explore the pathogenesis of FD and to develop new drugs for use FD treatment in the future.
Research objectives
The main objective of this study was to develop a new rat model of anxiety-like GHS of FD by the method of novel sequential stress. The results showed that the authors have realized this objective initially. Its significance lies in that researchers will have a new choice in the study of the pathogenesis of FD and in the development of new drugs.
Research methods
In order to create this rat model successfully, the authors took a method of novel sequential stress. It includes maternal separation (MS) and acute gastric irritation (AGI) early in life and restraint stress (RS) in adulthood. Furthermore, the authors used the elevated plus maze, open field experiment, abdominal withdrawal reflex testing and electromyographic recordings to evaluate the rat’s behavioral characteristics. Finally, the alterations of several anxiety-related brain-stomach modulators including 5-hydroxytryptamine (5-HT), γ-aminobutyric acid (GABA), brain-derived neurotrophic factor (BDNF) and nesfatin-1 in the rat hippocampus, plasma and gastric fundus and the 5-HT1A receptor (5-HT1AR) in the hippocampal CA1 subfield and the mucosa of the gastric fundus were examined.
Research results
The results showed that the sequential-stress-treated rats simultaneously demonstrated anxiety-like behaviors and GHS compared with the control group. Their rate of gastric emptying was also lower than the control group. Furthermore, sequential stress could significantly decrease the levels of 5-HT, GABA and BDNF in the hippocampus but increase the content of nesfatin-1 in the same site; significantly decrease the levels of 5-HT and BDNF in the plasma but increase the content of nesfatin-1 in it; significantly decrease the levels of 5-HT and BDNF in the gastric fundus but increase the content of nesfatin-1 in the same site. The expressions of 5-HT1AR in the hippocampal CA1 subfield and the mucosa of the gastric fundus were down-regulated. The contribution of this study, as is thought by the authors, is that it provided a novel rat model of FD with anxiety-like GHS to the research in this field and partly explored the molecular mechanisms of this important disease. However, there are still many complicated problems such as the relationship between anxiety-like GHS of FD and hypothalamic-pituitary-adrenal axis to be solved in the future.
Research conclusions
A method of sequential stress, namely, MS and AGI early in life followed by RS in adulthood, were firstly adopted in creating a rat model of anxiety-like GHS of FD, which could mimic the phenomenon observed in clinic that adverse physiological or psychosocial experiences in early life and acute stress in adulthood are linked to the development of FD. The results also showed the alterations of several anxiety-related neurobiochemical modulators in brain-blood-stomach axis such as 5-HT, GABA, BDNF and nesfatin-1, and thus further deepened the understanding of the molecular mechanisms of FD. Besides, this study supplied a possible tool for the development of new drugs in the treatment of FD.
Research perspectives
While doing this study the operation of the implantation of gastric balloon and electrodes is very important in creating this novel rat model of FD with anxiety-like GHS. The direction of the future research in this field lies in the investigation of the molecular mechanisms of the psychological and pathophysiological abnormalities in brain-gut interaction of this important clinical problem and the development of new drugs using this model.