Published online Nov 14, 2017. doi: 10.3748/wjg.v23.i42.7531
Peer-review started: May 24, 2017
First decision: July 17, 2017
Revised: July 31, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: November 14, 2017
Processing time: 172 Days and 15.8 Hours
To investigate whether gut maturation could be induced precociously in an athymic T-cell deficient neonatal rat model.
Fourteen day-old athymic (nude) rats (NIH-Foxn1rnu) were gavaged with either phytohaemagglutinin - a lectin from red kidney beans (PHA); trypsin - a protease (Prot); or water - vehicle (control) as a single dose on one day or once a day for 3-day. The nude rats were either nurtured by their mothers or cross-fostered by conventional foster dams of the Sprague-Dawley strain from days 3-5 after birth. At 17 d of age, 72 h after administration of the first treatment, intestinal macromolecular permeability was tested in vivo, prior to euthanasia, after which blood and gut organs were sampled.
Provocation with both, PHA and protease, resulted in increased gut growth and maturation in nude rat pups independent of nursing. Foetal-type enterocytes were replaced by non-vacuolated adult-type enterocytes in the distal small intestine epithelium. Decreased intestinal macromolecular permeability (gut closure) was observed, with reduced permeability markers (BIgG and BSA, P < 0.001) in circulation. Increased pancreatic function, with an increased trypsin to protein ratio in pancreas homogenates, was observed independent of nursing in the nude pups. Immunostaining showed the presence of a few CD3+-cells in the intestinal mucosa of the nude pups. The number of CD3+-cells remained unaltered by provocation and no differences were observed between the nursing sets. Growth and vitality of the nude pups were dependent on nurturing, since cross-fostering by conventional dams increased their macromolecular absorptive capacity (BSA, P < 0.05), as well as their passive immunity (RIgG, P < 0.05).
Precocious gut maturation can be induced by enteral provocation in athymic rat pups, similarly to in euthymic pups, thus showing an independence from thymus-derived T-cells.
Core tip: The rat is born with an immature gut and is thus a suitable model to study gut development. Enteral provocation with phytohaemagglutinin or protease induces precocious gut maturation in conventional (euthymic) rats. It has been suggested that T-lymphocytes are required for gut digestive maturation. The current study showed that precocious gut maturation could be induced by enteral provocation in athymic nude rats similar to that which occurs in euthymic rats. The few intestinal mucosal CD3+-T-cells that were observed in the athymic nude rats appeared to be unaffected by enteral provocation. The intestinal absorptive capacity in nude pups was enhanced when nurtured by conventional (immunocompetent) foster dams.
- Citation: Sureda EA, Gidlund C, Weström B, Prykhodko O. Induction of precocious intestinal maturation in T-cell deficient athymic neonatal rats. World J Gastroenterol 2017; 23(42): 7531-7540
- URL: https://www.wjgnet.com/1007-9327/full/v23/i42/7531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i42.7531
Rodents are an altricial mammalian species that are born highly immature and undergo extensive postnatal development[1,2] making the neonatal rat a suitable model to study gastrointestinal (GI) development and its coordination with changes in the luminal dietary and microfloral milieu[3,4]. During the third postnatal week, the weaning period in the rat, the GI tract shows enhanced growth and undergoes vast maturation[2].
In the distal small intestine (SI), the immature enterocytes with large supranuclear vacuoles, featuring high endocytic and intracellular digestive capacities[5], are replaced by adult-type non-vacuolated enterocytes, lacking these properties. In the proximal SI, the immature enterocytes expressing the neonatal-Fc-receptor (FcRn), involved in transcytosis of milk-borne IgG, are exchanged for low FcRn expression mature enterocytes[6,7]. These SI changes result in a decreased intestinal permeability (gut closure) and absorption of maternal IgG ceases[8]. The gut digestive capacity increases during weaning with characteristic changes in enterocyte brush-border enzymes and pancreatic enzymes secretion[1,9].
The gut immune system is also immature and naïve at birth in the rat[10], and maternal passive immunity is transferred to the young, e.g., milk-borne antibodies and immune cells[11,12]. At weaning, which is apparently coordinated with digestive maturation, dietary and microbial changes stimulate the immune system through the recruitment of immune cells to the gut mucosa[13] and up-regulation of pro-inflammatory cytokines, resulting in what is referred to as controlled “physiological” inflammation[14].
We have previously shown that gut maturation can be induced precociously in suckling rats by enteral provocation with phytohaemagglutinin (PHA) - a lectin[15,16], or proteases[17], mimicking the naturally occuring processes at weaning. Furthermore, provocation with PHA had effects on the thymus and the recruitment of CD3+ T-lymphocytes to the gut mucosa[16]. In fact, it has been suggested that natural GI development at weaning is dependent on T-cell activation in rats[18].
Consequently, the current study aimed to investigate the impact of thymus-derived T-lymphocytes on GI maturation, using an athymic, T-cell deficient (nude), rat strain[19], in combination with our experimental model of induced precocious gut maturation by enteral provocation in suckling rats[15-17].
The experiment was approved by the local Malmö-Lund Ethical Review Committee for Animal Experimentation and conducted in accordance with the European Community regulation concerning the protection of experimental animals (2010/63/EU). The protocol number is M169-14. The experiments were carried out using rats (Rattus norvegicus) of the athymic (nude) strain (NIH-Foxn1rnu, Charles River Laboratories International Inc.) and conventional (euthymic) rats of the Sprague-Dawley (SD) strain (SPRD Han, Taconic M&B, Denmark). The rats were bred and kept in the departmental facility under specific pathogen-free conditions (20 ± 1 °C, 50% ± 10% relative humidity, 12:12 h light-dark cycle). Pregnant dams were moved to individual cages (polycarbonate) with aspen wood bedding (Beekay B & K Universal AB, Sweden), enriched with paper-nesting material (Sizzle-pet, Lillicobiotech). Rats had free access to water and a rodent chow (R36, Lactamin). Parturition date was denominated as day 0 and all nude rat pups litters were kept with the dams during the experiments and a 7 cm wall extender was used to prevent the pups from reaching the chow whilst still nursing from their mothers.
Experiments were performed in a split-litter mode in two different nursing sets of nude rats, either nursed by their mother (Nude/Nude) or cross-fostered from the third postnatal day by conventional dams (Nude/SD). Enteral provocation was performed as previously described[15-17]. In short, 14 day-old nude rats were gavaged via a stomach tube with either porcine pancreatic trypsin (Novo) - a protease (Prot), or with phytohaemagglutinin (PHA) purified from red kidney beans (Phaseolus vulgaris) - a lectin[15]. Both were administered as a single dose (Prot, 1 mg/g b.wt; PHA, 0.1 mg/g b.wt) or once a day for three days (Protx3, 0.6 mg/g b.wt; PHAx3; 0.05 mg/g b.wt). Control pups received the vehicle water in matching volumes (0.01 ml/g b.wt).
At the end of the experiment on day 17, intestinal macromolecular permeability was assessed. A marker cocktail solution containing bovine serum albumin (BSA, Sigma, 1.25 mg/g b.wt) and bovine immunoglobulin G (BIgG, Sigma, 0.25 mg/g b.wt) was administered to the rats via a stomach tube and blood samples were collected 3 h later.
On day 17, the animals were weighed and anesthetized using a subcutaneous injection of a mixture of ketamine (Ketalar®, Pfizer, United States; 0.17 mg/g b.wt.) and azaperone (Stresnil®, Janssen Pharmaceutica, Belgium; 0.03 mg/g b.wt.) prior to sample collection. To ensure deep anaesthesia the rats’ eyelid and withdrawal reflexes were assessed. After opening the thorax, blood was collected by cardiac puncture in EDTA-containing syringes. The blood was then centrifuged at 3000 × g for 15 min at + 4 °Cand plasma was obtained and stored at -20 °C until further analysis. The pancreas was then dissected out, weighed and stored at -70 °C. The SI was divided into proximal and distal halves, the luminal content flushed out with ice-cold saline and SI length and weights were measured. Samples from the middle part of each SI half were fixed in 40 g/L phosphate buffered formaldehyde for 24 h and further embedded in paraffin. Spleen, liver, stomach and caecum were also weighed.
SI sections were deparaffinised and stained with haematoxylin and eosin using standard procedures. Morphometry of at least 20, appropriately oriented, villi and crypts from each rat was analysed using ImageJ software (http://imagej.nih.gov/ij). For immunohistochemical analysis of CD3+-cells, SI sections were deparaffinised and subjected to antigen retrieval by microwaving (2 × 8 min, 750 W) in 10 mmol/L Na-Citrate buffer (pH 6.0). Endogenous peroxidase was blocked with Peroxidized1, while background was reduced with Background Punisher (Biocare Medical, Llc.). Incubation with the primary antibody, rabbit-monoclonal anti-CD3 (1:100, SP7, Abcam), was done overnight at + 4 °C. Detection of CD3+-cells was performed using an HRP-Polymer Detection kit (MACH4 Universal; Biocare Medical, Llc.), according to the manufacturer’s instructions and the sections were counter-stained with haematoxylin. As a negative control for the unspecific binding of the HRP-Polymer detection kit, sections incubated with the antibody diluent, 10 g/L BSA in PBS were included.
The BSA marker concentration was measured by electroimmunoassay[20], using rabbit anti-BSA as the precipitating antibody and purified BSA as the standard, while the BIgG concentration was determined by single radial immunodiffusion using rabbit anti-BIgG as the precipitating antibody (Sigma-Aldrich) and purified BIgG as the standard[21], as previously described[8]. Passive immunity transfer was measured as rat plasma IgG level, which was quantified by single radial immunodiffusion using rabbit anti-rat-IgG (DAKO A/S, Denmark) as the precipitating antibody and purified rat IgG (Miles Laboratories, INC., United States) as the standard[21].
The pancreata were homogenized in ice-cold 0.2 mol/L Tris-HCl buffer + 0.05 mol/L CaCl2, pH 7.8 (1:10 wt/vol) using a glass homogenizer, and then centrifuged at 15000 × g for 20 min at + 4 °C. After activation with enteropeptidase (Sigma-Aldrich), trypsin activity, in the supernatant of the pancreatic homogenates was determined spectrophotometrically using a microplate modification[22] of the method described by Fritz et al[23] and benzoyl-DL-arginine-4-nitroanilide (BAPNA, Sigma-Aldrich) as the substrate. The trypsin activity in units (U) was recalculated as the amount of enzyme that catalyses 1 μmol of substrate per minute. The protein concentration in the supernatants was determined using the Lowry method[24] with a modification for 96-well microplates and using purified BSA (Sigma Chemicals) as the standard.
Statistical analysis was carried out using Prism 7 (http://www.graphpad.com). Comparisons between Nude/Nude treatment and controls were performed using an unpaired t-test and multiple t-test with Sidak post-test. Comparisons within Nude/SD, were performed using a one or two-way ANOVA and Dunnett’s multiple comparison post-test. Comparison of control groups in different nurturing sets were performed either using an unpaired t-test or a one-way ANOVA and Tukey’s multiple comparison post-test. All differences were considered significant when P < 0.05.
Gavage with PHA had no significant effects on the body weight of nude, mother-fed pups (Nude/Nude) 3 d after treatment. Significantly increased body weights were observed in the fostered, nude pups (Nude/SD) that were treated repeatedly with PHA or protease (PHA/Protx3) compared to control littermates gavaged with water (Table 1).
| Nude/Nude | Nude/SD | |||||||||||||
| Control | PHA | Control | PHA | PHAx3 | Prot | Protx3 | ||||||||
| Number of individuals (n)3 | 9-10 | 11 | 5 | 5 | 8 | 4 | 7 | |||||||
| Body weight (g) | 23.03 ± 9.26 | ns4 | 23.64 ± 7.80 | ns5 | 26.62 ± 1.56 | 26.88 ± 0.81 | ns5 | 28.30 ± 1.69 | a5 | 27.78 ± 1.04 | ns5 | 28.63 ± 1.28 | b5 | |
| Organs weight (mg/g2.wt6) | ||||||||||||||
| Stomach | 6.5 ± 0.9 | ns | 7.0 ± 0.6 | ns | 6.1 ± 0.5 | 7.0 ± 0.3 | ns | 6.8 ± 0.5 | ns | 6.3 ± 0.4 | ns | 6.2 ± 0.2 | ns | |
| SI length (cm) | 2.1 ± 0.7 | ns | 2.2 ± 0.5 | ns | 1.8 ± 0.1 | 1.9 ± 0.0 | ns | 1.9 ± 0.1 | ns | 2.0 ± 0.1 | ns | 1.8 ± 0.1 | ns | |
| Proximal SI | 17.0 ± 1.9 | P < 0.05 | 22.0 ± 1.8 | c | 15.0 ± 0.6 | 22.8 ± 1.5 | d | 22.0 ± 1.1 | d | 22.8 ± 1.1 | c1 | 18.7 ± 1.3 | d | |
| Distal SI | 13.9 ± 1.4 | ns | 15.7 ± 1.4 | a | 13.2 ± 0.4 | 16.4 ± 1.0 | d | 18.0 ± 1.2 | d | 17.7 ± 1.1 | d | 16.0 ± 1.3 | d | |
| Caecum | 2.4 ± 0.6 | ns | 3.0 ± 0.4 | ns | 2.0 ± 0.2 | 2.9 ± 0.5 | ns | 2.7 ± 0.3 | ns | 2.8 ± 0.4 | ns | 2.8 ± 0.3 | ns | |
| Spleen | 4.4 ± 0.6 | ns | 4.4 ± 0.8 | ns | 4.4 ± 0.4 | 4.4 ± 0.2 | ns | 4.5 ± 0.2 | ns | 5.4 ± 0.3 | ns | 4.5 ± 0.2 | ns | |
| Liver | 38.8 ± 3.2 | P < 0.05 | 36.5 ± 2.1 | a | 34.9 ± 1.2 | 36.3 ± 1.6 | a | 36.4 ± 1.3 | b | 37.0 ± 0.8 | b | 36.2 ± 1.1 | b | |
| Pancreatic function: | ||||||||||||||
| Pancreas (mg/g b.wt) | 3.3 ± 0.8 | ns | 3.8 ± 0.4 | ns | 2.7 ± 0.3 | 3.3 ± 0.3 | ns | 3.3 ± 0.3 | ns | 3.5 ± 0.3 | ns | 3.3 ± 0.3 | ns | |
| Trypsin (U/mg wet wt7) | 2.8 ± 1.5 | ns | 5.5 ± 1.2 | c | 2.8 ± 1.1 | 8.5 ± 1.7 | d | 6.5 ± 0.9 | d | 6.8 ± 0.9 | d | 3.5 ± 0.9 | ns | |
| Protein (μg/mg wet wt7) | 70.2 ± 24.0 | ns | 64.3 ± 15.5 | ns | 65.4 ± 8.4 | 97.3 ± 10.7 | c | 92.8 ± 16.0 | c | 85.3 ± 6.9 | a | 74.5 ± 5.2 | ns | |
| Trypsin (U/mg Protein) | 43.1 ± 19.1 | ns | 91.8 ± 34.5 | b | 42.3 ± 17.0 | 89.5 ± 25.5 | c | 70.7 ± 10.7 | a | 79.5 ± 8.5 | b | 47.6 ± 13.2 | ns | |
| Morphometry:(n) | -3 | -4 | -5 | -5 | -7 | -4 | -7 | |||||||
| Proximal SI: Villi height | 375 ± 55 | ns | 440 ± 21 | ns | 394 ± 23 | 457 ± 24 | b | 452 ± 20 | a | 394 ± 47 | ns | 418 ± 34 | ns | |
| Villi width | 117 ± 16 | ns | 123 ± 16 | ns | 107 ± 10 | 113 ± 10 | ns | 105 ± 12 | ns | 113 ± 17 | ns | 120 ± 8 | ns | |
| Crypt depth | 70 ± 3 | ns | 83 ± 7.0 | a | 61 ± 4.0 | 81 ± 7.0 | b | 81 ± 13 | b | 64 ± 6 | ns | 68 ± 8 | ns | |
| VH/CD | 5.4 ± 0.6 | ns | 5.3 ± 0.4 | ns | 6.5 ± 0.6 | 5.7 ± 0.8 | ns | 5.7 ± 0.9 | ns | 6.2 ± 0.7 | ns | 6.2 ± 0.6 | ns | |
| Distal SI: Villi height | 362 ± 11 | ns | 356 ± 14 | ns | 365 ± 58 | 370 ± 27 | ns | 426 ± 52 | ns | 374 ± 31 | ns | 379 ± 35 | ns | |
| Villi width | 99 ± 4 | ns | 100 ± 11 | ns | 91 ± 12 | 91 ± 13 | ns | 97 ± 8.0 | ns | 91 ± 9 | ns | 91 ± 7.0 | ns | |
| Crypt depth | 68 ± 5 | ns | 72 ± 3.0 | ns | 56 ± 6.0 | 71 ± 7.0 | b | 72 ± 6.0 | b | 69 ± 7 | a | 69 ± 7.0 | b | |
| VH/CD | 5.3 ± 0.3 | P < 0.05 | 4.9 ± 0.2 | ns | 6.5 ± 0.5 | 5.3 ± 0.7 | a | 6.0 ± 1.0 | ns | 5.5 ± 0.2 | ns | 5.5 ± 0.2 | a | |
Administration of PHA to Nude/Nude pups and PHA or protease to Nude/SD pups stimulated SI growth with increased weights of both SI regions observed compared to that of controls. No significant differences in SI length and stomach and caecum weights were observed in the rats that received PHA and/or protease compared to controls. Significantly increased liver weights (P ≤ 0.03) were observed in treated Nude/SD pups, while liver weights in the Nude/Nude pups were decreased (P = 0.06). Spleen weight was not significantly affected by treatment or nurturing.
Body weights of Nude/Nude pups appeared generally lower than the Nude/SD pups, even though no significance was found, possibly due to the high variation between the Nude/Nude pups. The relative weights of the proximal SI (P = 0.039) and liver (P = 0.0001) were significantly increased in Nude/Nude control pups compared to Nude/SD controls.
Gavage with PHA or protease did not affect pancreatic weight, but increased pancreatic protein and trypsin contents (Table 1). Pancreatic protein and trypsin contents were significantly increased in all treated Nude/SD pups (P ≤ 0.04 and P ≤ 0.0004, respectively) compared to controls, except for in the Nude/SD pups that were repeatedly treated with protease (Protx3), while no effect was observed in the Nude/Nude pups. The relative trypsin to protein pancreatic content was significantly increased in all treated nude pups nurtured either by their mother (Nude/Nude, P = 0.001) or by foster dams (Nude/SD, P ≤ 0.013), except for in the pups that were repeatedly treated with protease (Protx3).
Significantly increased crypt depth (P = 0.036) was observed in the proximal small intestine of PHA-treated Nude/Nude pups, while in the Nude/SD pups both crypt depth (PHA and PHAx3, P ≤ 0.0068) and villi height were significantly increased after PHA treatments (PHA and PHAx3, P ≤ 0.01) (Table1). In the distal SI, only crypt depth was significantly increased by both treatments in Nude/SD rats (P ≤ 0.02), resulting in a significantly decreased villi height to crypt depth ratio in the PHA (P = 0.023) and Protx3 (P = 0.048) groups of Nude/SD rats. No significant effects were observed in villi width.
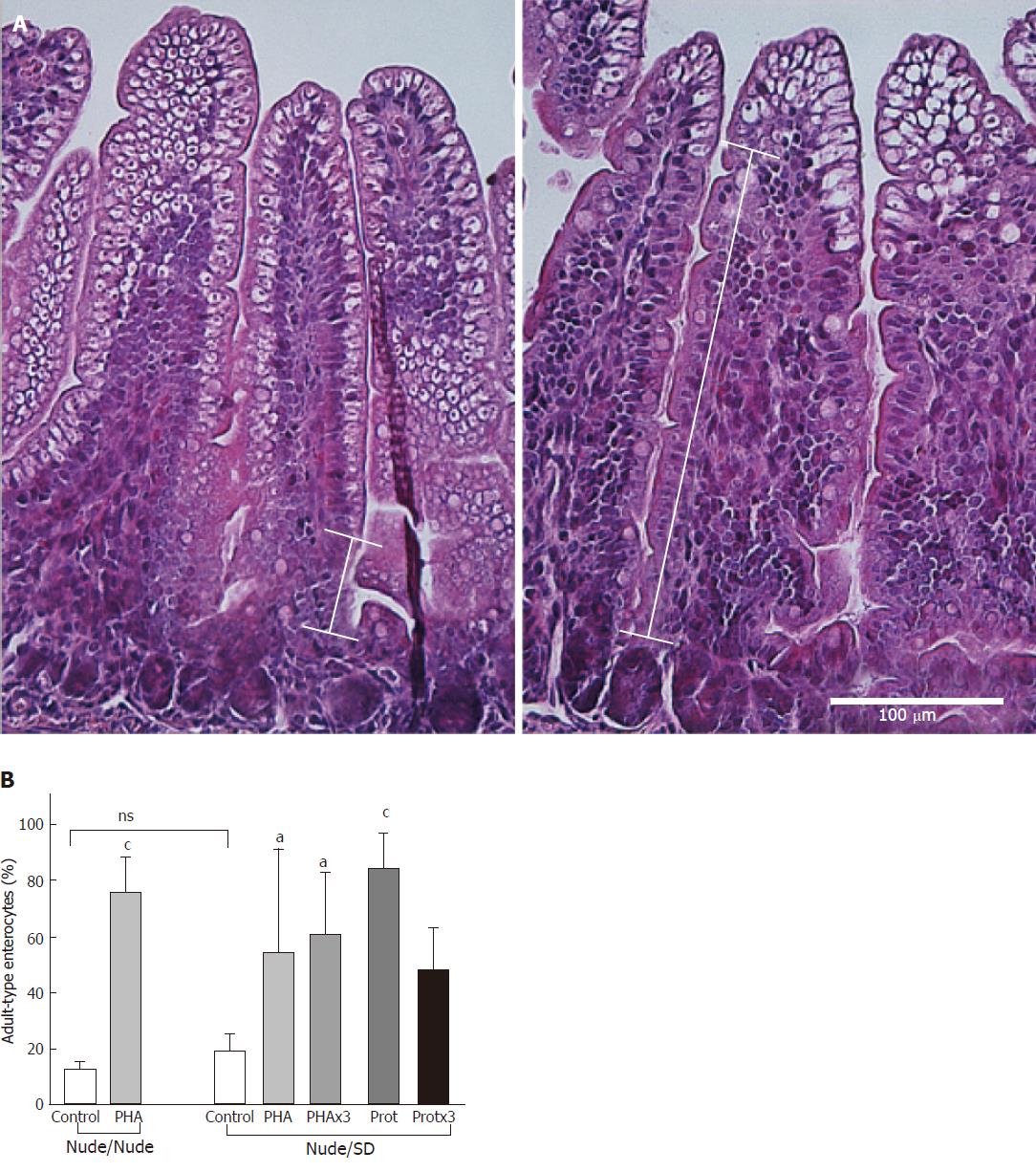
In the distal SI, the proportion of non-vacuolated (adult-type) epithelial cells appearing from the villi base following treatments (Figure 1) was measured to assess maturational status. The proportion of non-vacuolated (adult-type) epithelial cells was significantly increased after all treatments in the nude pups, independently of their nurturing (P ≤ 0.017), except for in the Protx3 group (P = 0.08), compared to control pups.
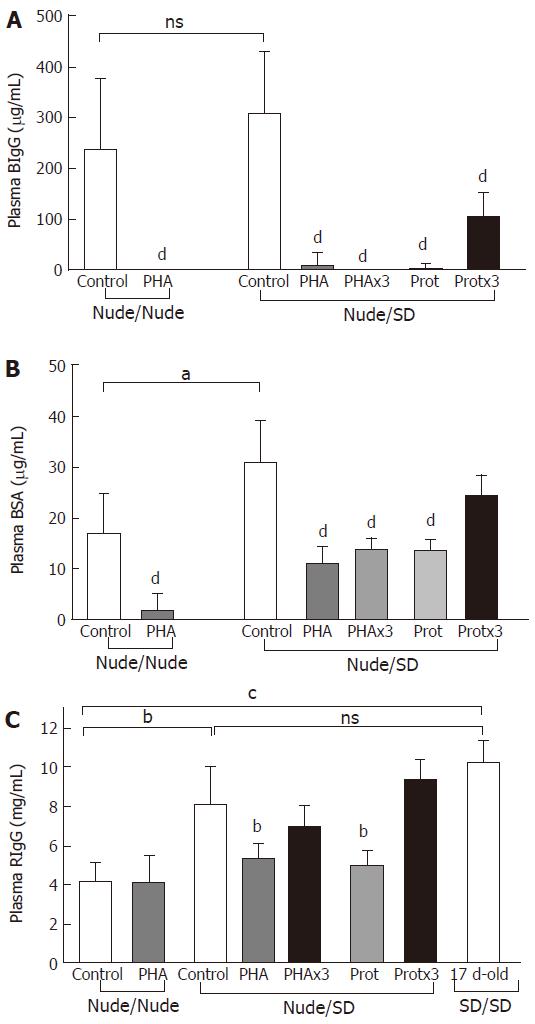
Significantly decreased plasma concentrations of both permeability markers, BIgG and BSA, were observed in all treatment groups compared to controls gavaged with water (P = 0.0001), except for the BSA levels in the Protx3 Nude/SD pups (P = 0.07) (Figure2). The plasma concentration of the cumulative permeability marker of milk-borne IgG absorption, RIgG, was significantly decreased following a single treatment with PHA and protease (P ≤ 0.003) compared to control pups in the Nude/SD group that were gavaged with water (Figure 2C).
The impact of maternal nurturing (athymic-nude vs euthymic-SD) was assessed by comparing the intestinal permeability of the control nude pups. The foster-nurtured (Nude/SD) pups displayed significantly higher BSA permeability compared to those nurtured by their mother (Nude/Nude) (P = 0.01), while no such difference was observed for BIgG permeability (P = 0.39) (Figure 2A and B). The nude pups nurtured by their mother (Nude/Nude) displayed significantly lower plasma RIgG concentrations compared to those nurtured by foster dams (Nude/SD, P = 0.02) or conventional pups (SD/SD, P = 0.0004), while no differences in plasma RIgG concentration were observed between cross-fostered nude rats (Nude/SD) and conventional rat pups (SD/SD) (Figure 2C).
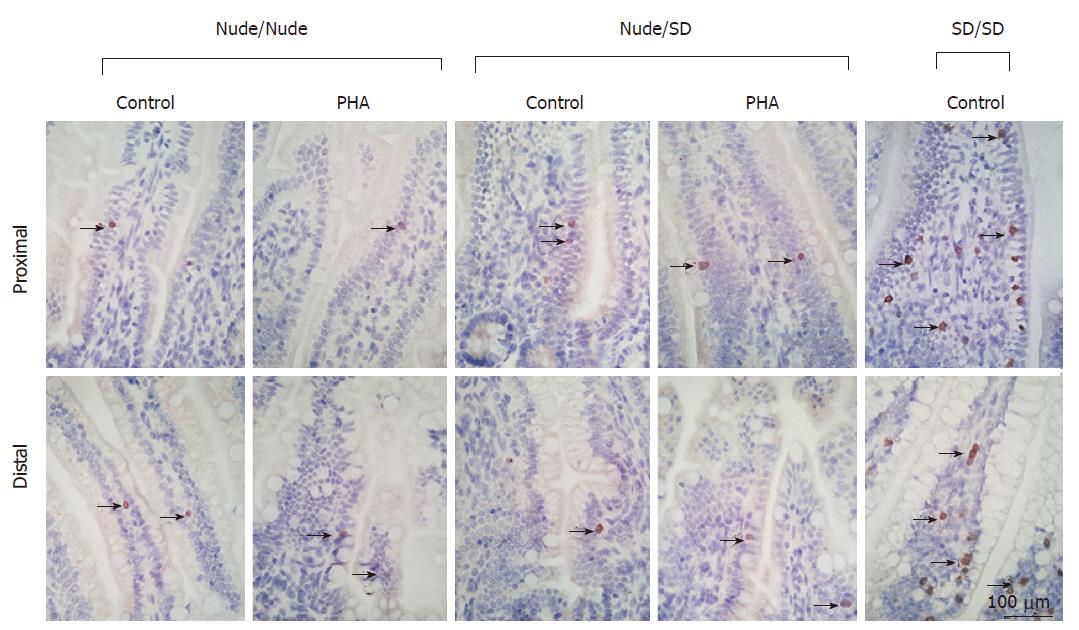
Nude rat pups displayed very few CD3+-cells in the SI mucosa, mainly associated with the epithelium, possibly intraepithelial lymphocytes (IEL), and some rarely scattered as villus lamina propria lymphocytes (LPL) (Figure 3). No differences were observed between PHA-treated, protease-treated and control nude pups (data not shown) or between nursing sets. In contrast, in euthymic SD rats, 7 to 28-d old, increased amounts of CD3+-cells were observed in the SI mucosa (age-matched 17-d old control SD/SD shown in Figure 3).
The current study investigated, whether precocious gut maturation could be induced in nude rat pups inherently lacking a functional thymus (T-cell deficient)[25], as previously shown in the conventional euthymic rat model[15,17].
Gavage of PHA and/or protease had a growth promoting effect in the nude rat pups, resulting in significantly increased body weight, especially in the rat pups that received the treatments more than once. Moreover, increased villi height was observed in the proximal SI segment and increased crypt depth was observed in both the proximal and distal SI segments in the treated nude pups, indicating increased crypt cell proliferation. The villi height/crypt depth ratio decreased in most of the treatment groups, denoting the structural transition which occurs during the maturation process[1]. In addition, SI maturation was confirmed by the change in enterocyte phenotype in the distal SI, with immature vacuolated enterocytes being replaced by adult-type non-vacuolated ones[15,17].
Although no treatment effect was observed with regards to pancreas growth, increased trypsin content was observed after enteral provocation in the nude pups, indicating maturation of the digestive function, which as previously suggested is possibly involved in the initiation of intestinal maturation [17].
The intestinal barrier function was assessed using the in vivo permeability test, which indicated cessation of the absorption of both macromolecular markers (BSA and BIgG) in PHA- and protease-treated nude pups, consistent with “gut closure”[8,15-17]. BSA, which is assumed to be absorbed mainly through nonspecific endocytosis, decreases the intestinal passage coinciding with the replacement of the immature highly endocytotic vacuolated epithelial cells in the distal SI. BIgG is allegedly transported by FcRn-mediated transcytosis in the proximal SI[8] where the epithelial expression of FcRn is reduced during natural and induced maturation in rat pups[6,7].
Provocation with PHA and protease (single dose) in nude pups reared by immunocompetent foster-dams resulted in significantly lower plasma concentrations of the cumulative marker for passive immunity transfer, RIgG, compared to that observed in the corresponding control rat pups. Thus indicating the progressive replacement of the foetal-type gut epithelium by the mature-type gut epithelium with a lowered expression of FcRn over time[7].
Taken together, these results indicate that enteral provocation with PHA or protease can induce precocious gut maturation in athymic neonatal rats resulting in similar changes to those previously shown in euthymic rat pups or seen during natural weaning[15-17,26]. Previous studies in immunodeficient mice have shown that during the peri- and post-weaning periods, recruitment of lymphocytes to the intestinal epithelia elicited a transcriptional response in the enterocytes but did not have effects on crypt proliferation[27]. The treatments used in the current study were administered using two different approaches based on previously published results showing that PHA and Protease induced precocious gut maturation were dose-dependent[15-17]. The lower dose treatment that was repeated (once a day for three days) serves as an experimental simulation of the naturally occurring processes at weaning, where there is a gradual transition from a milk-based to a solid diet. Alternatively, the single administration of a higher treatment dose could be considered similar to an abrupt stressful event, such as maternal separation from the dam, which requires a rapid mechanism of adaptation involving accelerated maturation.
Maternal effects were observed on the overall vitality of the rat pups. Since the nude rats that were nurtured by nude mothers showed a general decrease in growth with signs of undernourishment, they were transferred to immunocompetent foster dams, allowing us to perform experiments in larger sets of littermates. The results showed that nude rat pups nursed by nude mothers displayed lower absorption of the BSA marker, however no effects were observed in the absorption of the BIgG marker. The difference in absorption between the two marker molecules could be due to the different routes of transport, unspecific (BSA) vs receptor-mediated endocytosis (BIgG). The passive immunity transfer, as seen by the accumulation of plasma RIgG, was decreased in nude rats nursed by nude mothers compared to those fostered by conventional dams. Hence, maternal milk from immunocompetent dams provided higher levels of passive immunity (IgG) while simultaneously improving the unspecific intestinal absorptive capacity (BSA) of the nude pups during the suckling period, resulting in better growth and survival. It has previously been shown that immunodeficient mice fostered by wild-type dams had increased pre-weaning mRNA expression of intestinal FcRn[28], despite this we did not observe differences in BIgG absorption.
Consequently, differences in the absorptive capacity due to foster-nursing indicated that milk factors from conventional dams promoted gut growth and delayed gut functional maturation in nude rat pups. Thus, suggesting that the immunocompetence of the dam, maternal milk content and composition, and passive immunity transfer, influence the timing of gut maturation even though it is a genetically programmed process. This would imply that nude dams would provide pups with immunodeficient milk lacking the capacity to supress gut maturation.
In conventional rats mucosal CD3+-cells can be found early after birth and increase in number at weaning[13], whereas they are not present until after weaning in mice[28] and until 4-6 mo of age in the athymic rats[25]. However, the current study showed the presence of low amounts of CD3+ T-cells already in neonatal nude rats. These cells could be of maternal origin, translocated from the milk into the intestinal mucosa[11,12,19], or they could be developed outside of the thymus, in the SI[29]. Since neither cross-fostering, nor enteral provocation as seen in euthymic rats[16] seemed to increase the number of CD3+-cells, the extra-thymic development of these mucosal CD3+-cells would be more probable. Nonetheless, phenotypic differences between intraepithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) have been described[4] and further characterisation of the intestinal CD3+-IELs found in nude rats should be studied.
The recruitment of thymus-derived T-cells to the intestinal mucosa has been shown to be essential for gut maturation[18,19] while kept immature by regulatory T-cells until weaning, as recently shown in mice[14]. The present study shows that gut maturation is independent of thymus-derived T-cells, but since the immune system in neonatal nude rats can adjust to the thymus deficiency[30] further studies are needed to elucidate the possible involvement of extra-thymic CD3+-T-cells, such as δ-T-cells or other immune cells, in gut maturation[31].
In conclusion, the study showed a novel role of maternal milk in the regulation of the macromolecular absorptive capacity in immunodeficient athymic rat pups. Furthermore, despite the hypothesis on the direct role of T-cells in gut development, this study showed that induced gut maturation after enteral provocation does not depend on thymus derived T-cells, although an influence of rare mucosal extra-thymic CD3+-cells cannot be excluded.
In the distal small intestine (SI), the immature enterocytes with large supranuclear vacuoles, featuring high endocytic and intracellular digestive capacities, are replaced by adult-type non-vacuolated enterocytes, lacking these properties. In the proximal SI, the immature enterocytes expressing the neonatal-Fc-receptor (FcRn), involved in transcytosis of milk-borne IgG, are exchanged for low FcRn expression mature enterocytes. These SI changes result in a decreased intestinal permeability (gut closure) and absorption of maternal IgG ceases. The gut digestive capacity increases during weaning with characteristic changes in enterocyte brush-border enzymes and pancreatic enzymes secretion.
The current study investigated, whether precocious gut maturation could be induced in nude rat pups inherently lacking a functional thymus (T-cell deficient), as previously shown in the conventional euthymic rat model.
The study showed a novel role of maternal milk in the regulation of the macromolecular absorptive capacity in immunodeficient athymic rat pups. Furthermore, despite the hypothesis on the direct role of T-cells in gut development, this study showed that induced gut maturation after enteral provocation does not depend on thymus derived T-cells, although an influence of rare mucosal extra-thymic CD3+-cells cannot be excluded.
The study showed a novel role of maternal milk in the regulation of the macromolecular absorptive capacity in immunodeficient athymic rat pups. Furthermore, despite the hypothesis on the direct role of T-cells in gut development, this study showed that induced gut maturation after enteral provocation does not depend on thymus derived T-cells, although an influence of rare mucosal extra-thymic CD3+-cells cannot be excluded.
In the current manuscript, the authors reported that athymic (nude) rats gut maturation could be induced by enteral provocation of PHA and trypsin, and independence from thymus-derived T-cells. This is interesting and would gain our knowledge on intestinal maturation. The study was well designed and the manuscript was well organized. It is preferred to determine the disaccharidase activity of the small intestine for better denoting the gut maturation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jin B, Nagata T S- Editor: Qi Y L- Editor: A E- Editor: Ma YJ
| 1. | Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981;241:G199-G214. [PubMed] |
| 2. | Walthall K, Cappon GD, Hurtt ME, Zoetis T. Postnatal development of the gastrointestinal system: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2005;74:132-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care. 2008;11:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Pérez-Cano FJ, Franch À, Castellote C, Castell M. The suckling rat as a model for immunonutrition studies in early life. Clin Dev Immunol. 2012;2012:537310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Kumagai N, Baba R, Sakuma Y, Arita K, Shinohara M, Kourogi M, Fujimoto S, Fujita M. Origin of the apical transcytic membrane system in jejunal absorptive cells of neonates. Med Mol Morphol. 2011;44:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Martín MG, Wu SV, Walsh JH. Ontogenetic development and distribution of antibody transport and Fc receptor mRNA expression in rat intestine. Dig Dis Sci. 1997;42:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Arévalo Sureda E, Weström B, Pierzynowski SG, Prykhodko O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS One. 2016;11:e0164775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Telemo E, Weström BR, Ekström G, Karlsson BW. Intestinal macromolecular transmission in the young rat: influence of protease inhibitors during development. Biol Neonate. 1987;52:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Rakhimov KR, Karimov OR, Kurbanov AS, Kuchkarova LS. Rearrangement of spectrum of digestive proteases in postnatal ontogenesis of rats. J Evolut Biol Physiol. 2002;38:184-188. [DOI] [Full Text] |
| 10. | Holsapple MP, West LJ, Landreth KS. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Sheldrake RF, Husband AJ. Intestinal uptake of intact maternal lymphocytes by neonatal rats and lambs. Res Vet Sci. 1985;39:10-15. [PubMed] |
| 12. | Cabinian A, Sinsimer D, Tang M, Zumba O, Mehta H, Toma A, Sant’Angelo D, Laouar Y, Laouar A. Transfer of Maternal Immune Cells by Breastfeeding: Maternal Cytotoxic T Lymphocytes Present in Breast Milk Localize in the Peyer’s Patches of the Nursed Infant. PLoS One. 2016;11:e0156762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Pérez-Cano FJ, Castellote C, González-Castro AM, Pelegrí C, Castell M, Franch A. Developmental changes in intraepithelial T lymphocytes and NK cells in the small intestine of neonatal rats. Pediatr Res. 2005;58:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Torow N, Yu K, Hassani K, Freitag J, Schulz O, Basic M, Brennecke A, Sparwasser T, Wagner N, Bleich A. Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period. Nat Commun. 2015;6:7725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Linderoth A, Biernat M, Prykhodko O, Kornilovska I, Pusztai A, Pierzynowski SG, Björn WR. Induced growth and maturation of the gastrointestinal tract after Phaseolus vulgaris lectin exposure in suckling rats. J Pediatr Gastroenterol Nutr. 2005;41:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Prykhod’ko O, Fed’kiv O, Linderoth A, Pierzynowski SG, Weström BR. Precocious gut maturation and immune cell expansion by single dose feeding the lectin phytohaemagglutinin to suckling rats. Br J Nutr. 2009;101:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Prykhodko O, Pierzynowski SG, Nikpey E, Arevalo Sureda E, Fedkiv O, Weström BR. Pancreatic and pancreatic-like microbial proteases accelerate gut maturation in neonatal rats. PLoS One. 2015;10:e0116947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Masjedi M, Tivey DR, Thompson FM, Cummins AG. Activation of the gut-associated lymphoid tissue with expression of interleukin-2 receptors that peaks during weaning in the rat. J Pediatr Gastroenterol Nutr. 1999;29:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Cummins AG, Thompson FM, Mayrhofer G. Mucosal immune activation and maturation of the small intestine at weaning in the hypothymic (nude) rat. J Pediatr Gastroenterol Nutr. 1991;12:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Laurell CB. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1541] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 21. | Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6153] [Cited by in RCA: 6034] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 22. | Pierzynowski SG, Weström BR, Svendsen J, Karlsson BW. Development of exocrine pancreas function in chronically cannulated pigs during 1-13 weeks of postnatal life. J Pediatr Gastroenterol Nutr. 1990;10:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Fritz H, Hartwich G, Werle E. [On protease inhibitors. I. Isolation and characterization of trypsin inhibitors from dog pancreas tissue and pancreas scretion]. Hoppe Seylers Z Physiol Chem. 1966;345:150-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 25. | Helgeland L, Brandtzaeg P, Rolstad B, Vaage JT. Sequential development of intraepithelial γδ and αβ T lymphocytes expressing CD8αβ in neonatal rat intestine: requirement for the thymus. Immunol. 1997;92:447-456. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Cummins AG, Steele TW, LaBrooy JT, Shearman DJ. Maturation of the rat small intestine at weaning: changes in epithelial cell kinetics, bacterial flora, and mucosal immune activity. Gut. 1988;29:1672-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Schjoldager KT, Maltesen HR, Balmer S, Lund LR, Claesson MH, Sjöström H, Troelsen JT, Olsen J. Cellular cross talk in the small intestinal mucosa: postnatal lymphocytic immigration elicits a specific epithelial transcriptional response. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1335-G1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Jenkins SL, Wang J, Vazir M, Vela J, Sahagun O, Gabbay P, Hoang L, Diaz RL, Aranda R, Martín MG. Role of passive and adaptive immunity in influencing enterocyte-specific gene expression. Am J Physiol Gastrointest Liver Physiol. 2003;285:G714-G725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 30. | Schuurman H-J, Hougen HP, van Loveren H. The rnu (Rowett nude) and rnuN (nznu, New Zealand nude) rat: an update. Ilar J. 1992;34:3-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 651] [Article Influence: 65.1] [Reference Citation Analysis (0)] |