Published online Nov 14, 2017. doi: 10.3748/wjg.v23.i42.7495
Peer-review started: June 8, 2017
First decision: July 13, 2017
Revised: September 14, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: November 14, 2017
Processing time: 158 Days and 15.9 Hours
The main treatment of patients with non-alcoholic fatty liver disease (NAFLD) is life style modification including weight reduction and dietary regimen. Majority of patients are safely treated with this management and pharmacologic interventions are not recommended. However, a subgroup of NAFLD patients with non-alcoholic steatohepatitis (NASH) who cannot achieve goals of life style modification may need pharmacological therapy. One major obstacle is measurement of histological outcome by liver biopsy which is an invasive method and is not recommended routinely in these patients. Several medications, mainly targeting baseline mechanism of NAFLD, have been investigated in clinical trials for treatment of NASH with promising results. At present, only pioglitazone acting as insulin sensitizing agent and vitamin E as an anti-oxidant have been recommended for treatment of NASH by international guidelines. Lipid lowering agents including statins and fibrates, pentoxifylline, angiotensin receptor blockers, ursodeoxycholic acid, probiotics and synbiotics are current agents with beneficial effects for treatment of NASH but have not been approved yet. Several emerging medications are in development for treatment of NASH. Obeticholic acid, liraglutide, elafibranor, cenicriviroc and aramchol have been tested in clinical trials or are completing trials. Here in, current and upcoming medications with promising results in clinical trial for treatment of NAFLD were reviewed.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is an increasing liver disease worldwide. However, most of patients are treated with life style modification including weight loss and dietary regimen. Pharmacologic therapy may be indicated in a group of patients with non-alcoholic steatohepatitis. Here in, the current and emerging medications for treatment of NAFLD was reviewed briefly with regard of their beneficial effects on histological outcomes.
- Citation: Eshraghian A. Current and emerging pharmacological therapy for non-alcoholic fatty liver disease. World J Gastroenterol 2017; 23(42): 7495-7504
- URL: https://www.wjgnet.com/1007-9327/full/v23/i42/7495.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i42.7495
Prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing and NAFLD is probably the most common cause of abnormal liver enzymes worldwide[1]. The spectrum of NAFLD is ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and liver cirrhosis[2]. While simple steatosis is generally considered a benign condition, it may gradually progress to NASH, liver cirrhosis and eventually hepatocellular carcinoma (HCC)[3]. This has been resulted in significant attention of physicians and health care providers toward detection and treatment of NAFLD in recent years.
NASH has been estimated to affect 2%-5% of western population and is associated with 10-fold increase in liver related mortality[4]. In addition to steatosis, NASH is defined by cellular injury and inflammation that may eventuate in liver fibrosis[5]. Hepatocellular injury and fibrosis in NASH is mainly a result of free fatty acid lipotoxicity mediated by Kupffer and hepatic stellate cells causing collagen deposition[6].
The main treatment of NAFLD is life style modification including exercise and weight loss. It has been suggested that more than 7% weight reduction sustained for 48 wk may cause significant improvement in histology of NASH[7]. However, some patients will not achieve goals of lifestyle modification or cannot maintain them for long term period. On the other hand, there are patients with advanced liver disease needing targeted therapy. Most experts suggest pharmacologic therapy for NASH only in individuals with advanced disease or those at high risk of liver cirrhosis[8]. No drug has been approved specifically for treatment of NAFLD yet, however, some drugs are now routinely prescribed or are under trial. Here in, current medications and future drug candidates for treatment of NAFLD are briefly reviewed (Table 1).
| Medication | Mechanism | Effect on histology | Recommended by AASLD/EASL |
| Pioglitazone | PPAR-γ | Improvement of steatosis, lobular inflammation and ballooning | Yes |
| Vitamin E | Anti-oxidant | Improvement of hepatocyte ballooning | Yes |
| Metformin | Amelioration of IR | No beneficial effect | No |
| Statins | HMG-CO A reductase inhibition | No beneficial effects | No |
| Ezetimibe | Inhibition of cholesterol absorption | Improvement of hepatocyte ballooning | No |
| Fibrates | PPAR-α | Improvement of hepatocyte ballooning | No |
| Pentoxifylline | Inhibition of TNF-α and anti-oxidants | Improvement of inflammation and ballooning | No |
| Losartan | ARB | Improvement of steatosis, lobular inflammation, ballooning and fibrosis | No |
| UDCA | Prevention of apoptosis/inflammation | Lacking data | No |
| Synbiotic and probiotics | Modulation of gut microbiota | Lacking data | No |
The pathogenesis of NAFLD is complex including multiple environmental and genetic factors. High calorie dietary regimens rich in carbohydrates and saturated fatty acids causing weight gain; are probably the most important environmental factor. The significant rising prevalence of NAFLD in recent years is attributed to change in life style especially dietary regimen[9]. Insulin resistance (IR) and metabolic syndrome are central in pathogenesis of NAFLD. Several other mechanistic pathways involved in pathogenesis of NAFLD finally result in IR. Metabolic derangements and IR in NAFLD are mediated by dysregulation of metabolic pathways which are naturally regulated by nuclear receptors such as peroxisome proliferator-activated receptors (PPARs), farnesoid X receptors (FXRs) and liver X receptors (LXRs)[10]. Nuclear receptors have been targeted for production of drugs with beneficial effects in NAFLD.
Lipotoxicity, defined as fat induced injury to hepatocytes, oxidative stress, mediated by hypoxia-inducible transcription factors-1α and 2α, and chronic inflammation, through several cytokines and chemokines, are also involved in initiation and progression of NAFLD[11]. These are other targets for medical therapy in NAFLD.
Genetic predisposition to NAFLD has been described in some studies. Patatin-like phospholipase domain containing-3 (PNPLA3) is a gene responsible for encoding a lipase acting for clearance of lipid droplets from the hepatocytes[12]. Loss of function mutation of PNPLA3 results in hepatocytes injury and steatohepatitis. The other important genetic factor is loss of function mutation in trans-membrane 6 superfamily member 2 (TM6SF2) gene that is a predisposing risk for NAFLD[13]. Alteration of adipocytokines and micronutrients[14], thyroid hormone abnormalities[15], and vitamin D deficiency[16] are other proposed contributing factors in pathogenesis of NAFLD. Understanding pathogenesis and epidemiology of NAFLD may help clinicians for estimation of more precise burden of disease in population. While surveillance strategies applied to all individuals is not cost-effective, screening may be suggested for high risk groups such as those with metabolic syndrome, diabetes mellitus and hypertension[17].
Since the main defined mechanism for NAFLD is IR, drugs targeting IR have been implemented as treatment of NAFLD. Peroxisome proliferator-activated receptor (PPAR)-γ is a nuclear receptor and a member of PPAR superfamily that is expressed exclusively in adipose tissue and involved in glucose metabolism and lipogenesis[18]. Thiazolidinediones, agonists of PPAR-γ, are primarily used for treatment of diabetes mellitus and act by improvement of insulin sensitivity[19]. Thiazolidinediones also have anti-inflammatory, ant-fibrotic properties and increase serum adiponectin level[20-22]. The largest clinical trial investigating the effect of thiazolidinedions in NASH patients, PIVENS trial, randomized 247 patients with biopsy proven NASH to receive pioglitazone (30 mg/d), vitamin E (800 IU/d) or placebo for 96 wk[23]. This study showed that pioglitazone could improve steatosis and histological alterations in patients with definite NASH in their liver biopsies. FLIRT trial showed improvement of steatosis and serum aminotransferase level by rosiglitazone in patients with NASH[24]. However, use of rosiglitazone has been prohibited in Europe and highly restricted in United States based on FDA concerns regarding increase in cardiovascular side effects with this drug[25]. Some studies showed beneficial effects of thiazolidinedines in amelioration of hepatic fibrosis[17,26]. American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver (EASL) have suggested use of pioglitazone for treatment of patients with NASH[27,28].
It should be noted that beneficial effects of thiazolidinediones are abrogated after discontinuation and NASH is returned in liver biopsies[29]. Other limitation of thiazolidinedions, restricting their widespread application, is their side effects including weight gain, increased bone loss and fracture risk, deterioration of heart failure and increased risk of bladder cancer with pioglitazone[30-32].
Metformin is used to treat type II diabetes acting through amelioration of IR by decreasing hepatic gluconeogenesis and triglyceride production[33]. Metformin is no longer considered as a treatment for NAFLD. While it had some promising results in animal studies, both pediatric and adult clinical trials failed to demonstrate histological improvement of NASH in human by metformin[34]. AASLD and EASL guidelines do not recommend metformin as a treatment for NAFLD at present[27,28].
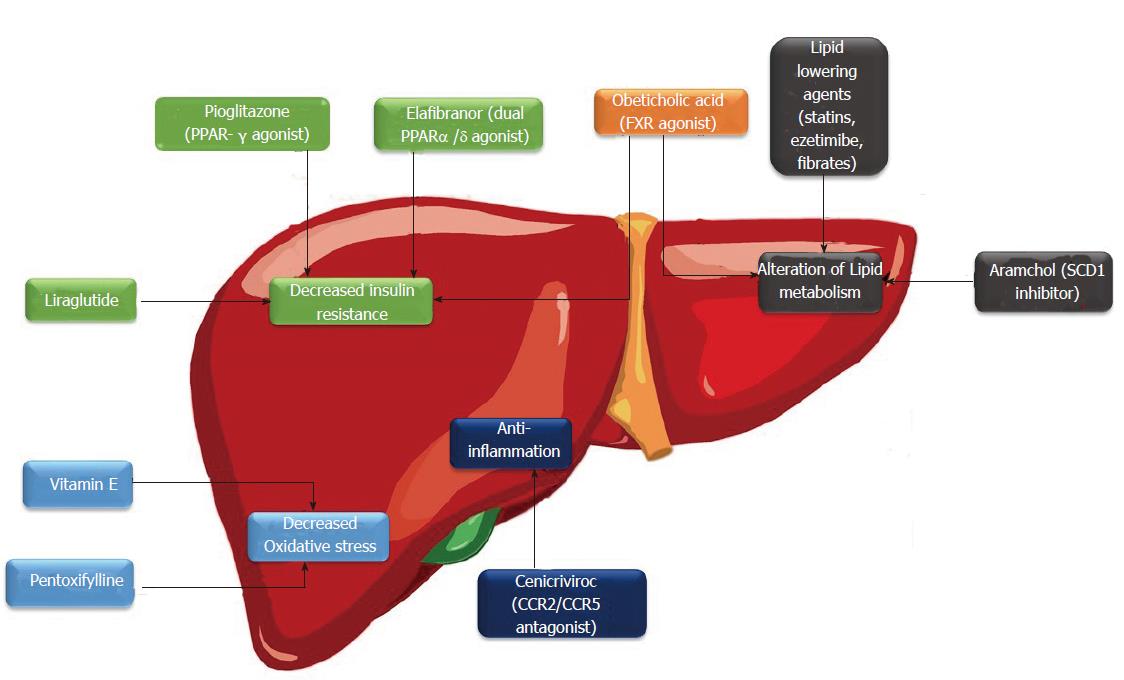
Oxidative stress has a major role in NASH pathogenesis especially activation of hepatic stellate cells (HSCs) and promoting liver fibrosis[35]. Vitamin E is a fat soluble anti-oxidant that is capable of repairing oxidizing radicals and prevent lipid peroxidation[36]. Vitamin E regulates PPAR and transforming growth factor-β1 (TGF-β1) pathways and is involved in inflammation, apoptosis and fibrosis process[37,38]. Vitamin E has been tested in clinical trials to evaluate its efficacy in treatment of patients with NASH. Vitamin E with a dose of 800 IU/d compared to placebo for treatment of patients with NASH in PIVENS trial. After 96 wk of therapy, amelioration of hepatic steatosis, lobular inflammation and hepatocyte ballooning were seen in vitamin E treated group[23]. However, no improvement of fibrosis was observed. The Treatment of NAFLD in Children (TONIC) trial is a randomized trial allocating 800 IU/d vitamin E and metformin (1 g/d) to children with NAFLD. Vitamin E resulted in improvement of hepatocyte ballooning but failed to improve hepatic inflammation, fibrosis and steatosis[39]. Based on promising results of these studies, AASLD and EASL have suggested use of vitamin E for treatment of non-diabetic, non-cirrhotic patients with NASH[27,28]. Safety profile of vitamin E is still controversial. Vitamin E treatment was associated with an increase in all cause related mortality in a meta-analysis[40]. Some studies also showed an increase in hemorrhagic stroke and prostate cancer with high doses and long term use of vitamin E respectively[41,42]. Therefore, it is better to individualize use of vitamin E for treatment of NASH based on these cautions. Medications against NAFLD and their mechanisms of action were outlined in Figure 1.
NAFLD is considered the hepatic manifestation of metabolic syndrome. Many patients with NAFLD have features of metabolic syndrome including diabetes mellitus, dyslipidemia and obesity. Accumulation of free cholesterol in hepatocytes have been also suggested in pathogenesis of NAFLD[43]. Statins that inhibit hydroxyl-methyl-glutaryl-coenzyme A reductase are widely used as cholesterol lowering agents and can be theoretically useful in patients with NAFLD[44]. Athyros et al[45] showed that daily atorvastatin use can improve liver enzymes and reduce cardiovascular morbidity in patients with mild to moderate abnormal liver tests. In an animal model of NASH, simvastatin was associated with amelioration of liver fibrosis by inhibition of hepatic stellate cells via nitric oxide synthase pathway[46]. However, simvastatin therapy was not associated with improvement of liver enzymes, hepatic steatosis and fibrosis in a group of patients with biopsy proven NASH[47]. In a retrospective cohort, statin use was associated with decreased risk of advanced fibrosis in patients at risk for NASH[48]. While there are concerns about risk of using statins in patients with chronic liver disease[49], most recent studies showed that statins are generally safe in patients with NAFLD[50]. Since most of patients with NAFLD have components of metabolic syndrome such as dyslipidemia and DM II, statin use may be considered in this group of patients.
Ezetimibe is a cholesterol absorption inhibitor that is used for treatment of patients with elevated cholesterol level. In a mice model of hepatic steatosis induced by high fat diet, ezetimibe therapy prevented hepatic steatosis and decreased hepatic insulin resistance[51]. In MOZART trial, ezetimibe could not significantly reduce hepatic steatosis as assessed by magnetic resonance imaging-derived proton density fat fraction[52]. In a meta-analysis of 6 studies, Nakeda et al[53] reported that ezetimibe therapy resulted in improvement of liver enzymes and hepatocyte ballooning in patients with NAFLD. Colesvelam is a bile acid sequestrant that is used clinically to decrease low density lipoprotein (LDL). A placebo controlled clinical trial showed that colesvelam was associated with increased liver fat in patients with NASH as assessed by magnetic resonance imaging and magnetic resonance spectroscopy[54].
Fibrates are activator of peroxisome proliferator-activated receptor alpha (PPAR-α) that are used as anti-hyperlipidemic agents and mainly decrease serum triglyceride level. Fibrates also improve insulin resistance, stimulate oxidation of fatty acids and have anti-inflammatory effects[55]. In an animal model of NAFLD, fibrates therapy was associated with resolution of hepatic steatosis, steatohepatitis and fibrosis[56]. A pilot trial showed improvement of metabolic syndrome and glucose metabolism by fenofibrate in patients with NAFLD but with only minimal effects on liver histology[57]. Co-treatment with pentoxifylline plus fenofibrate was effective in reduction of liver stiffness and markers of liver fibrosis in patients with NAFLD[58].
Pentoxifylline is an inhibitor of tumor necrosis factor-alpha (TNF-α) with anti-oxidant properties that initially was known to be effective in treatment of alcoholic hepatitis[59]. Most animal models suggested beneficial effects of pentoxifylline in reducing liver enzymes and hepatic inflammation[60,61]. In a randomized placebo trial, pentoxifylline significantly improved liver fibrosis and decreased NAFLD activity score[62]. They also showed that beneficial effects of pentoxifylline in NASH are mediated by decreasing free-radical- mediated lipid oxidation[63]. Satapathy et al[64] showed that 12 mo pentoxifylline therapy was associated with biochemical and histological improvement in patients with NASH. Two meta-analysis reported beneficial effects of pentoxifylline in terms of reduction of liver enzymes and improvement of histology in NASH patients[65,66]. However, long-term safety and efficacy of pentoxifylline in patients with NASH needs to be investigated as there are some reports of aggravation of fatty liver in mice by pentoxifylline therapy[67].
Angiotensin IIreceptor blockers are a group of medications widely used for treatment of hypertension. AngiotensinIIprobably promotes liver fibrosis via activation of transforming growth factor-β (TGF-β) and toll-like receptor-4 signaling[68,69]. In a series of 7 patients with NASH, 48 mo therapy with an angiotensinIIreceptor blocker, losartan (50 mg/d), resulted in amelioration of necro-inflammatory response and improvement of hepatic fibrosis[70]. These beneficial effects was reported to be mediated via inhibition of HSC in this group of patients[71]. Fogari et al[72] reported that combination of simvastatin and losartan improved hepatic steatosis indices and decreased visceral adipose tissue diameter. In FANTASY trial, telmisartan therapy for 12 mo was associated with decreased serum free fatty acid levels without significant improvement in liver enzymes[73]. Addition of losartan to rosiglitazone had no extra histological benefit than rosiglitazone alone in patients with NASH[74]. In a rat model of typeIIdiabetes, valsartan could reduce hepatic fibrosis and steatosis correlated with reduction of tissue expression of TNF-α and monocyte chemoattractant protein-1 (MCP-1)[75]. Further studies are needed to confirm therapeutic role of angiotensinIIreceptor blockers in treatment of NAFLD.
It has been postulated that ursodeoxycholic acid (UDCA) may prevent progression of NAFLD because of anti-inflammatory and anti-apoptotic properties[76]. In a randomized placebo controlled trial, Ratziu et al[77] showed that high dose UDCA was effective in improvement of serum aminotransferase and markers of liver fibrosis in patients with biopsy proven NASH. A systematic review of 12 trials reported beneficial effects of UDCA for treatment of NASH[78]. However, UDCA is not currently recommended for treatment of NASH in international guidelines due to lacking of well-designed large randomized trials and lack of evidence about histological benefits.
Probiotics are live, human origin, non-pathogenic microorganisms with beneficial effects when consumed adequately. Prebiotics are not live microorganisms but chemicals causing growth of microorganisms. Nutritional supplements composed of probiotics and prebiotics are called synbiotics[79]. The role of gut microbiota has been confirmed in pathogenesis of insulin resistance and NAFLD[80]. Therefore, modulation of gut microbiota using probiotics and synbiotics has been suggested as a treatment option in NAFLD. Several studies with different preparations of probiotics have been conducted among NAFLD patients. A meta-analysis of 4 randomized trials reported that probiotics had beneficial effects on liver enzymes, lipid profile and improved insulin resistance in patients with NAFLD/NASH[81]. A double blind randomized clinical trial showed that Synbiotic + life style modification including physical activity and dietary regimen was superior to life style modification alone in treatment of patients with NAFLD[82]. Beneficial effects of synbiotics was also confirmed in lean NAFLD[83]. Despite promising results, data regarding histologic benefits of synbiotics and probiotics is lacking. Larger studies are needed to further elucidate the issue.
Farnesoid X receptor (FXR) is a nuclear receptor which is expressed in the liver and is involved in bile acid synthesis. Binding of bile acids to FXR results in down-regulation of bile acid synthesis, hepatic lipogenesis, hepatic gluconeogenesis and improved peripheral insulin sensitivity[84]. FXR activation resulted in prevention of weight gain and decreased liver/muscle fat deposition and hepatic steatosis in obese rats[85]. Obeticholic acid (OCA) is a synthetic bile acid derivatives that acts as agonist of FXR and has been shown to be capable of reduction of hepatic steatosis in mice[86]. In a randomized double blind placebo controlled trial, 25 mg or 50 mg OCA was given to patients with type IIdiabetes and NAFLD for 6 wk. After completion of the study, OCA group had significant reduction in liver enzymes and markers of liver fibrosis compared to those in placebo group. However, the histologic features were not evaluated in this study[87]. FLINT trial is a multi-central randomized trial reporting improvement of histological features of NASH with 25 mg daily OCA for 72 h[88]. An unfavorable outcome was a rise in total cholesterol and LDL cholesterol accompanied by a fall in HDL cholesterol[88]. OCA seems to be a promising agent to be included in treatment of NASH in future.
Aramchol is a conjugate molecule composed of two components, cholic and arachidonic acid, which is primarily used for treatment of cholesterol gallstone[89]. Aramchol is an inhibitor of stearoyl CoA desaturase-1 (SCD1) an enzyme which is involved in lipid metabolism and hepatic insulin resistance[89,90]. Administration of 100 or 300 mg aramchol for 3 mo in patients with NAFLD resulted in decreased liver fat content without significant improvement of liver enzymes[91]. Larger clinical trials are needed to further elucidate the role of this agent in treatment of NAFLD.
Peroxisome proliferator-activated receptor alpha (PPAR-α) is a member of PPAR superfamily that is expressed in adipose tissue, liver, skeletal muscle, heart and is involved in regulation of lipid and glucose metabolism. PPAR-α gene expression has been shown to have negative correlation with severity of NASH and visceral adiposity in patients with NAFLD[92]. Activation of another PPAR, PPAR-δ, is associated with improvement of insulin resistance, increase in oxidation of fatty acids and decrease in hepatic gluconeogenesis[93]. Elafibranor, a dual agonist of PPAR α/δ, has improved lipid profile and reduced hepatic fat in animal studies[94]. In a cross-over randomized trial, 80 mg daily administration of elafibranor in obese subjects was associated with improvement of hepatic and peripheral insulin resistance[95]. A recent randomized clinical trial showed that daily oral 120 mg elafibranor for 52 wk was associated with improvement of hepatic steatosis and fibrosis in patients with NASH in a dose dependent manner. Elafibranor therapy was also associated with improvement of systemic inflammation, lipid/glucose profiles and liver enzymes when compared to the placebo group[96]. Authors reported a reversible rise in serum creatinine in elafibranor group, otherwise, the drug was well-tolerated. Elafibranor may be considered as a candidate for treatment of patients with NASH after completion of ongoing phase III trial.
Overexpression of inflammatory chemokines CCL2 (MCP-1) and CCL5 (RANTES) have been established in patients with NASH leading to worsening of hepatic inflammation and fibrosis[97]. CCR2 and CCR5 are chemokine receptors for CCL2 and CCL5 that are inhibited by cenicriviroc. Anti-fibrotic properties of cenicriviroc have been approved in thioacetamide model of hepatic fibrosis in mice[98]. CENTAUR is an ongoing randomized clinical trial evaluating efficacy of cenicriviroc in patients with NASH and hepatic fibrosis[99].
Liraglutide is an incretin mimetic that acts as an agonist of glucagon-like peptide-1 receptor and was primarily used for treatment of type IIdiabetes. In animal model, liraglutide therapy was associated with amelioration of hepatic steatosis in mice fed with high fat/high fructose[100]. In Wistar rats, liraglutide therapy improved insulin resistance and hepatic steatosis by activation of AMP-activated protein kinase[101]. In a randomized trial, addition of liraglutide to insulin glargine was not superior to insulin glargine alone in improvement of glycemia and hepatic steatosis[102]. In a small randomized trial, 1.8 mg liraglutide administered subcutaneously was effective in improvement of histological features in patients with NASH[103]. Table 2 outlined new emerging medications for NAFLD.
| Medication | Mechanism | Histology benefit |
| Obeticholic acid | Farnesoid X receptor agonist | Improvement of steatosis, lobular inflammation, ballooning and fibrosis |
| Aramchol | Inhibition of SCD1 | Lacking data |
| Elafibranor | PPAR α/δ agonist | Improvement of steatosis and fibrosis |
| Cenicriviroc | Inhibition of CCR2/CCR5 | Lacking data |
| Liraglutide | Glucagon-like peptide-1 agonist | Improvements in steatosis and hepatocyte ballooning |
Several medications including thiazolidindiones, metformin, vitamin E, statins, pentoxifylline, losartan, ursodeoxycholic acid, probiotics and synbiotics have been applied for treatment of NAFLD/NASH with promising but conflicting results. Future candidate medications for this purpose with ongoing or completing clinical trials are OCA, elafibranor, aramchol, cenicriviroc and liraglutide targeting different underlying mechanisms in NASH. Some of them may have beneficial effects on histological features of NAFLD/NASH.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de Oliveira C, Lee HC, Lonardo A S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
| 1. | Eshraghian A, Dabbaghmanesh MH, Eshraghian H, Fattahi MR, Omrani GR. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med. 2013;16:584-589. [PubMed] |
| 2. | Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58:3017-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Semin Liver Dis. 2015;35:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 7. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 8. | Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol. 2015;62:S65-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 9. | Eshraghian A. High prevalence of nonalcoholic fatty liver disease in the middle east: Lifestyle and dietary habits. Hepatology. 2017;65:1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The Role of Nuclear Receptors in the Pathophysiology, Natural Course, and Drug Treatment of NAFLD in Humans. Adv Ther. 2016;33:291-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 12. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2597] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 13. | Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, Motta BM, Kaminska D, Rametta R, Grimaudo S. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 418] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 14. | Eshraghian A, Nikeghbalian S, Geramizadeh B, Malek-Hosseini SA. Serum magnesium concentration is independently associated with non-alcoholic fatty liver and non-alcoholic steatohepatitis. United European Gastroenterol J. 2017; Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Eshraghian A, Hamidian Jahromi A. Non-alcoholic fatty liver disease and thyroid dysfunction: a systematic review. World J Gastroenterol. 2014;20:8102-8109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 16. | Eshraghian A. Bone metabolism in non-alcoholic fatty liver disease: vitamin D status and bone mineral density. Minerva Endocrinol. 2017;42:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L, Targher G. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 18. | Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007;50:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Papaetis GS, Orphanidou D, Panagiotou TN. Thiazolidinediones and type 2 diabetes: from cellular targets to cardiovascular benefit. Curr Drug Targets. 2011;12:1498-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Morán-Salvador E, Titos E, Rius B, González-Périz A, García-Alonso V, López-Vicario C, Miquel R, Barak Y, Arroyo V, Clària J. Cell-specific PPARγ deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol. 2013;59:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: A systematic review. Metabolism. 2016;65:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2469] [Article Influence: 164.6] [Reference Citation Analysis (2)] |
| 24. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 25. | Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 582] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 26. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 27. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 28. | European Association for the Study of the Liver (EASL);. European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO).EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 29. | Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, Murad MH. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013;30:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 34. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Oliveira CP, Stefano JT. Genetic polymorphisms and oxidative stress in non-alcoholic steatohepatitis (NASH): A mini review. Clin Res Hepatol Gastroenterol. 2015;39 Suppl 1:S35-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Azzi A, Meydani SN, Meydani M, Zingg JM. The rise, the fall and the renaissance of vitamin E. Arch Biochem Biophys. 2016;595:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Jia YB, Jiang DM, Ren YZ, Liang ZH, Zhao ZQ, Wang YX. Inhibitory effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at early stage of steroid-induced femoral head necrosis. Mol Med Rep. 2017;15:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, Akbari M, Asemi Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can J Diabetes. 2017;41:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 847] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 40. | Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 41. | Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 42. | Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1223] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 43. | Chan J, Sharkey FE, Kushwaha RS, VandeBerg JF, VandeBerg JL. Steatohepatitis in laboratory opossums exhibiting a high lipemic response to dietary cholesterol and fat. Am J Physiol Gastrointest Liver Physiol. 2012;303:G12-G19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Mihos CG, Pineda AM, Santana O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol Res. 2014;88:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 46. | Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PLoS One. 2013;8:e76538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 48. | Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 49. | Eshraghian A, Kamyab AA. Rhabdomyolysis developing secondary to atorvastatin therapy in a patient with liver cirrhosis. Intern Med. 2013;52:823-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Tziomalos K, Athyros VG, Paschos P, Karagiannis A. Nonalcoholic fatty liver disease and statins. Metabolism. 2015;64:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Ushio M, Nishio Y, Sekine O, Nagai Y, Maeno Y, Ugi S, Yoshizaki T, Morino K, Kume S, Kashiwagi A. Ezetimibe prevents hepatic steatosis induced by a high-fat but not a high-fructose diet. Am J Physiol Endocrinol Metab. 2013;305:E293-E304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, Hooker J, Kono Y, Bhatt A, Hernandez L, Nguyen P, Noureddin M, Haufe W, Hooker C, Yin M, Ehman R, Lin GY, Valasek MA, Brenner DA, Richards L; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 53. | Nakade Y, Murotani K, Inoue T, Kobayashi Y, Yamamoto T, Ishii N, Ohashi T, Ito K, Fukuzawa Y, Yoneda M. Ezetimibe for the treatment of non-alcoholic fatty liver disease: A meta-analysis. Hepatol Res. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, Brenner D, Sirlin C, Loomba R; San Diego Integrated NAFLD Research Consortium (SINC). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 55. | Idzior-Walus B, Sieradzki J, Rostworowski W, Zdzienicka A, Kawalec E, Wójcik J, Zarnecki A, Blane G. Effects of comicronised fenofibrate on lipid and insulin sensitivity in patients with polymetabolic syndrome X. Eur J Clin Invest. 2000;30:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C, Benhamed F. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 57. | Fernández-Miranda C, Pérez-Carreras M, Colina F, López-Alonso G, Vargas C, Solís-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 58. | El-Haggar SM, Mostafa TM. Comparative clinical study between the effect of fenofibrate alone and its combination with pentoxifylline on biochemical parameters and liver stiffness in patients with non-alcoholic fatty liver disease. Hepatol Int. 2015;9:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 60. | Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Ye JH, Chao J, Chang ML, Peng WH, Cheng HY, Liao JW, Pao LH. Pentoxifylline ameliorates non-alcoholic fatty liver disease in hyperglycaemic and dyslipidaemic mice by upregulating fatty acid β-oxidation. Sci Rep. 2016;6:33102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 63. | Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634-638. [PubMed] |
| 65. | Du J, Ma YY, Yu CH, Li YM. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Zeng T, Zhang CL, Zhao XL, Xie KQ. Pentoxifylline for the treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized double-blind, placebo-controlled studies. Eur J Gastroenterol Hepatol. 2014;26:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 67. | Massart J, Robin MA, Noury F, Fautrel A, Lettéron P, Bado A, Eliat PA, Fromenty B. Pentoxifylline aggravates fatty liver in obese and diabetic ob/ob mice by increasing intestinal glucose absorption and activating hepatic lipogenesis. Br J Pharmacol. 2012;165:1361-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Li YS, Ni SY, Meng Y, Shi XL, Zhao XW, Luo HH, Li X. Angiotensin II facilitates fibrogenic effect of TGF-β1 through enhancing the down-regulation of BAMBI caused by LPS: a new pro-fibrotic mechanism of angiotensin II. PLoS One. 2013;8:e76289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Shirai Y, Yoshiji H, Noguchi R, Kaji K, Aihara Y, Douhara A, Moriya K, Namisaki T, Kawaratani H, Fukui H. Cross talk between toll-like receptor-4 signaling and angiotensin-II in liver fibrosis development in the rat model of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2013;28:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 71. | Yokohama S, Tokusashi Y, Nakamura K, Tamaki Y, Okamoto S, Okada M, Aso K, Hasegawa T, Aoshima M, Miyokawa N. Inhibitory effect of angiotensin II receptor antagonist on hepatic stellate cell activation in non-alcoholic steatohepatitis. World J Gastroenterol. 2006;12:322-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Fogari R, Maffioli P, Mugellini A, Zoppi A, Lazzari P, Derosa G. Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol. 2012;24:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Hirata T, Tomita K, Kawai T, Yokoyama H, Shimada A, Kikuchi M, Hirose H, Ebinuma H, Irie J, Ojiro K. Effect of Telmisartan or Losartan for Treatment of Nonalcoholic Fatty Liver Disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int J Endocrinol. 2013;2013:587140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Qiang G, Zhang L, Yang X, Xuan Q, Shi L, Zhang H, Chen B, Li X, Zu M, Zhou D. Effect of valsartan on the pathological progression of hepatic fibrosis in rats with type 2 diabetes. Eur J Pharmacol. 2012;685:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Steinacher D, Claudel T, Trauner M. Therapeutic Mechanisms of Bile Acids and Nor-Ursodeoxycholic Acid in Non-Alcoholic Fatty Liver Disease. Dig Dis. 2017;35:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 78. | Xiang Z, Chen YP, Ma KF, Ye YF, Zheng L, Yang YD, Li YM, Jin X. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: a systematic review. BMC Gastroenterol. 2013;13:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 79. | Buss C, Valle-Tovo C, Miozzo S, Alves de Mattos A. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann Hepatol. 2014;13:482-488. [PubMed] |
| 80. | Quigley EM, Monsour HP. The Gut Microbiota and Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 280] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 82. | Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 83. | Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 84. | De Magalhaes Filho CD, Downes M, Evans RM. Farnesoid X Receptor an Emerging Target to Combat Obesity. Dig Dis. 2017;35:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 86. | Kunne C, Acco A, Duijst S, de Waart DR, Paulusma CC, Gaemers I, Oude Elferink RP. FXR-dependent reduction of hepatic steatosis in a bile salt deficient mouse model. Biochim Biophys Acta. 2014;1842:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-582.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 736] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 88. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1796] [Article Influence: 179.6] [Reference Citation Analysis (3)] |
| 89. | Ratziu V. Novel Pharmacotherapy Options for NASH. Dig Dis Sci. 2016;61:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Gutiérrez-Juárez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 91. | Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, Oren R; FLORA Group. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2085-2091.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 92. | Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, Taskinen MR, Van Hul W, Mertens I. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 93. | Bojic LA, Huff MW. Peroxisome proliferator-activated receptor δ: a multifaceted metabolic player. Curr Opin Lipidol. 2013;24:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 95. | Cariou B, Hanf R, Lambert-Porcheron S, Zaïr Y, Sauvinet V, Noël B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923-2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 96. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 97. | Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 98. | Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS One. 2016;11:e0158156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 99. | Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 100. | Mells JE, Fu PP, Sharma S, Olson D, Cheng L, Handy JA, Saxena NK, Sorescu D, Anania FA. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225-G235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 101. | Yamazaki S, Satoh H, Watanabe T. Liraglutide enhances insulin sensitivity by activating AMP-activated protein kinase in male Wistar rats. Endocrinology. 2014;155:3288-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Tang A, Rabasa-Lhoret R, Castel H, Wartelle-Bladou C, Gilbert G, Massicotte-Tisluck K, Chartrand G, Olivié D, Julien AS, de Guise J. Effects of Insulin Glargine and Liraglutide Therapy on Liver Fat as Measured by Magnetic Resonance in Patients With Type 2 Diabetes: A Randomized Trial. Diabetes Care. 2015;38:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 103. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |